Selective Recovery of Copper from a Synthetic Metalliferous Waste Stream Using the Thiourea-Functionalized Ion Exchange Resin Puromet MTS9140
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solution Preparation
2.2. Resin Preconditioning
2.3. Static Equilibrium Experiments
2.4. Fixed-Bed (Dynamic) Experiments
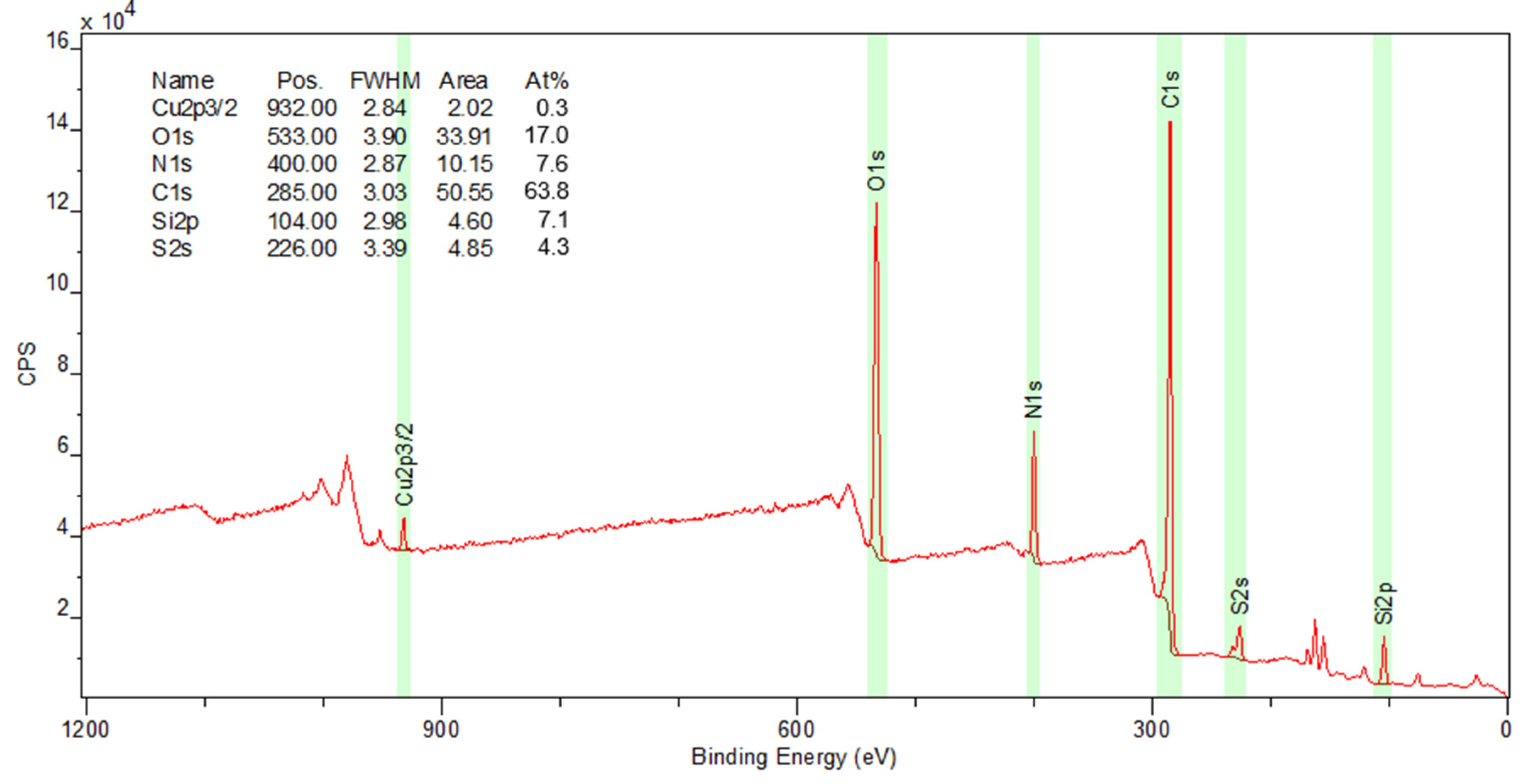
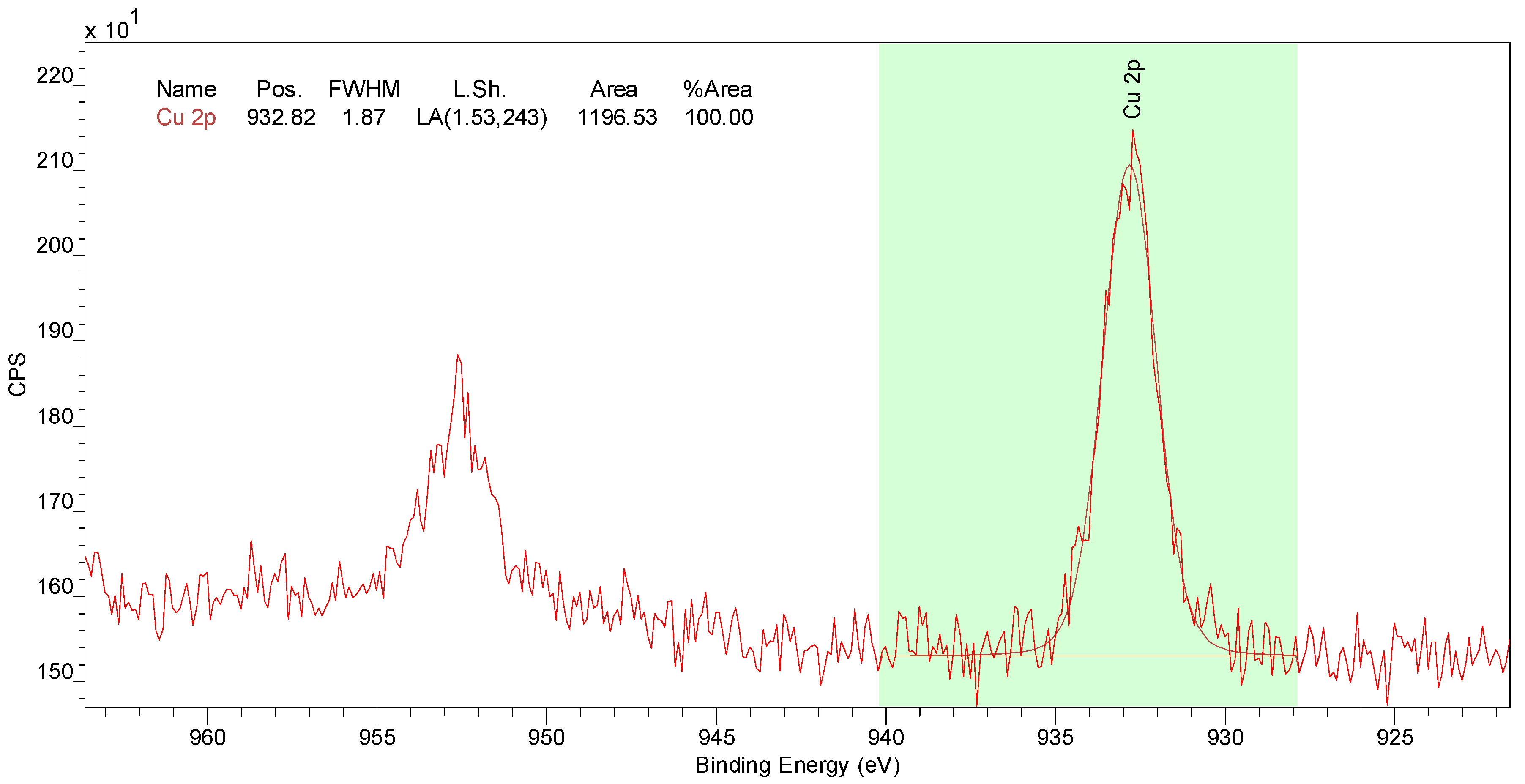
2.5. Solid-State Analysis
2.6. Breakthrough Modelling
3. Results and Discussion
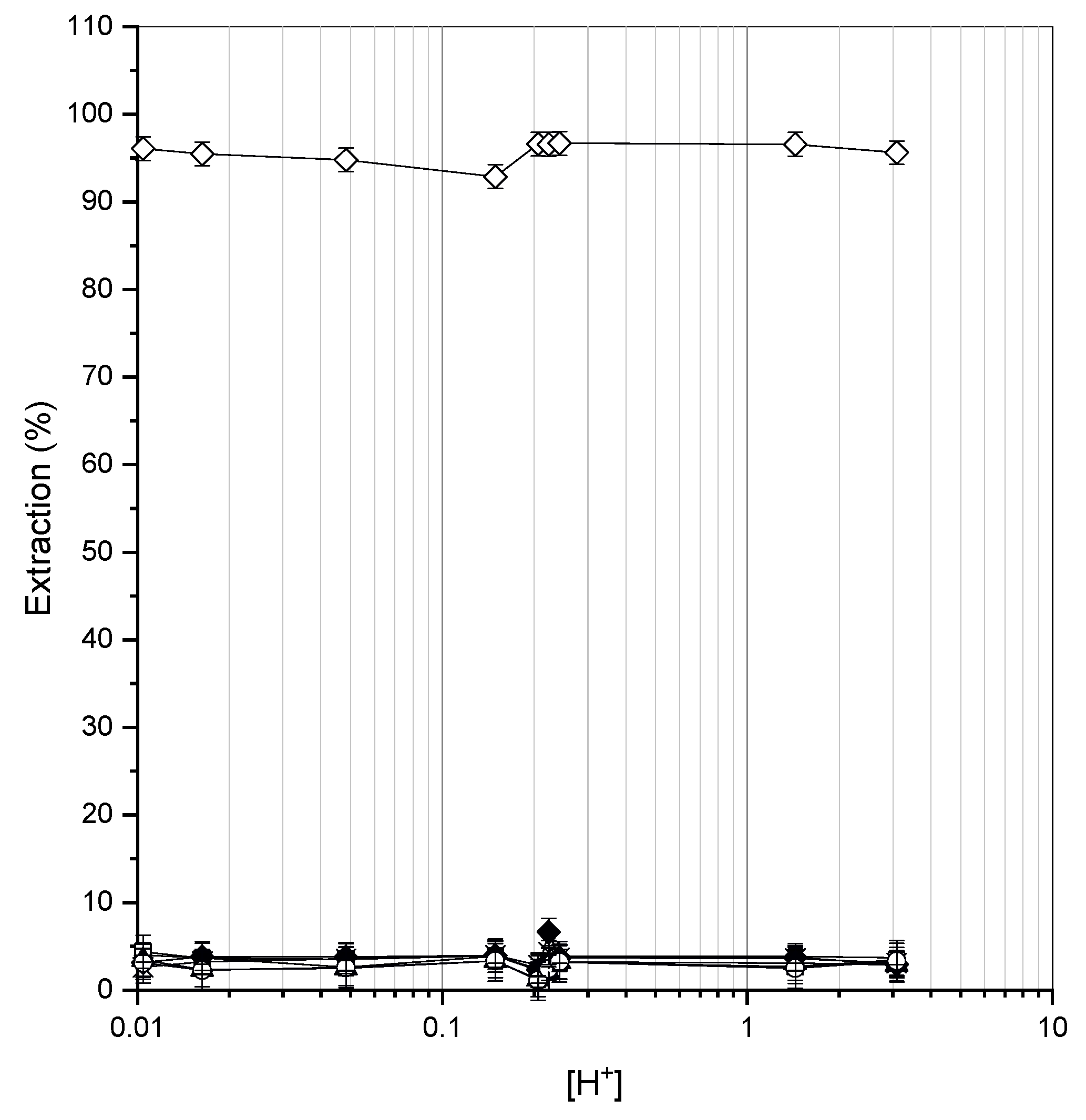
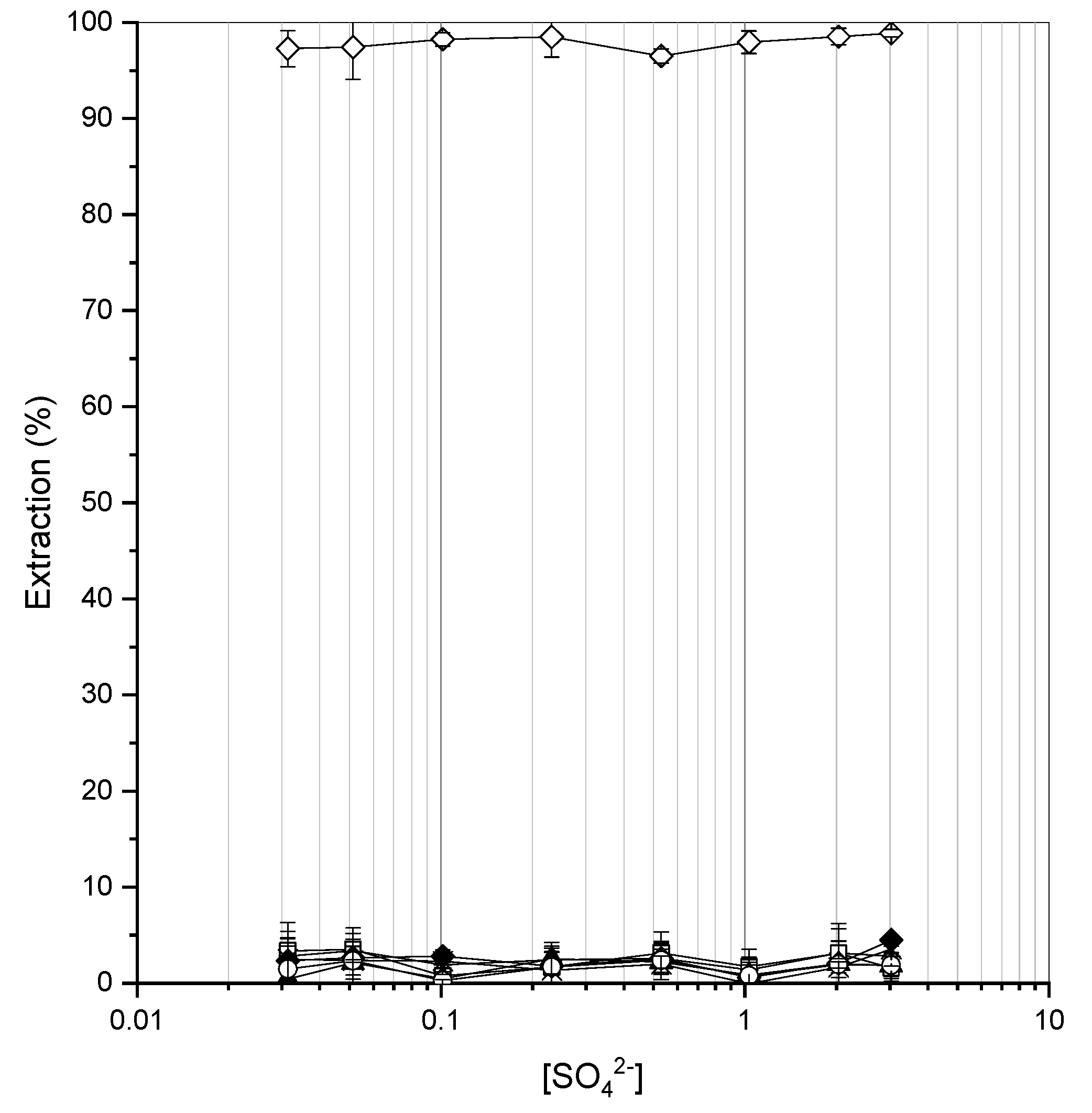
3.1. Static (Batch) Extraction
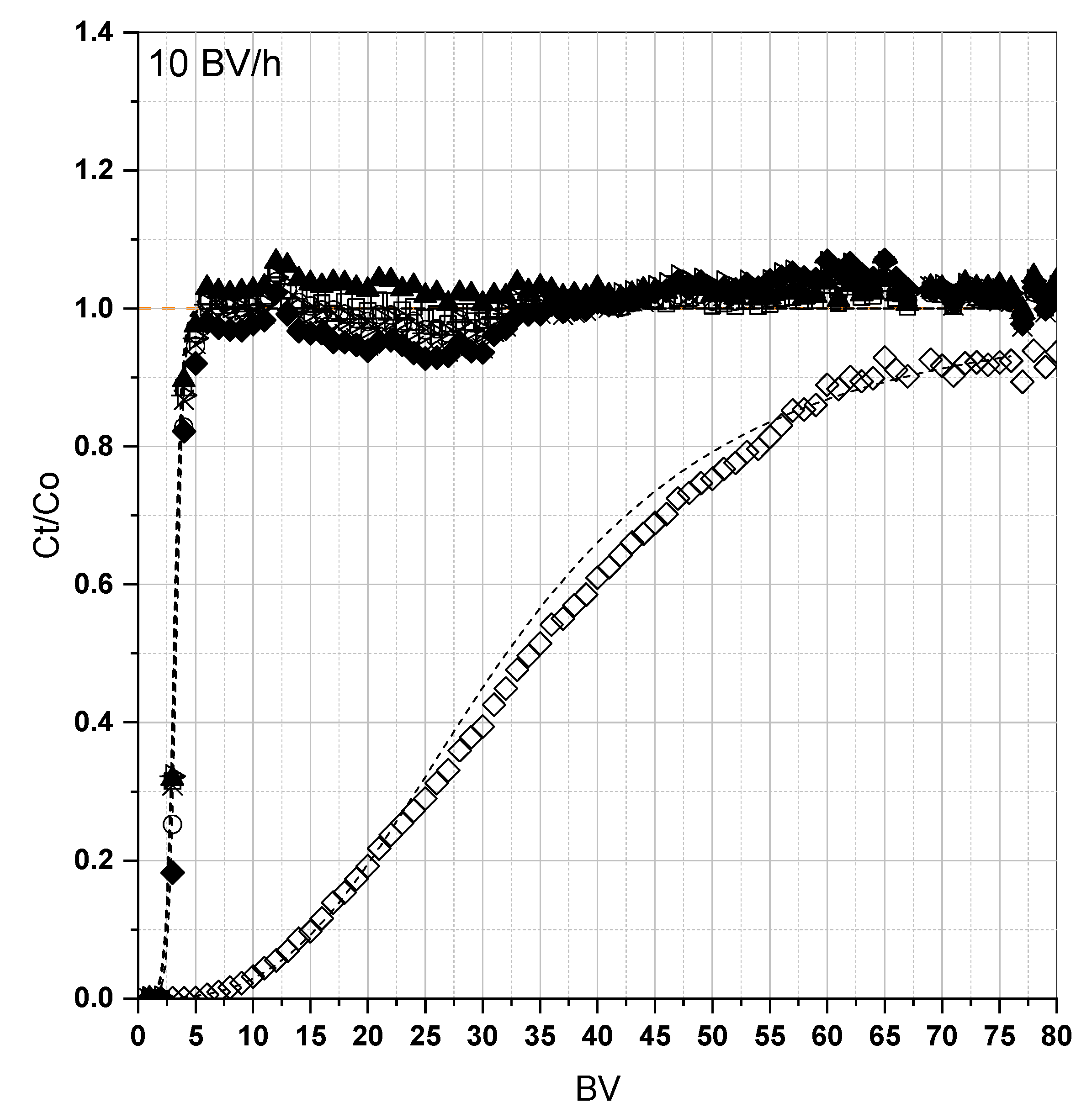
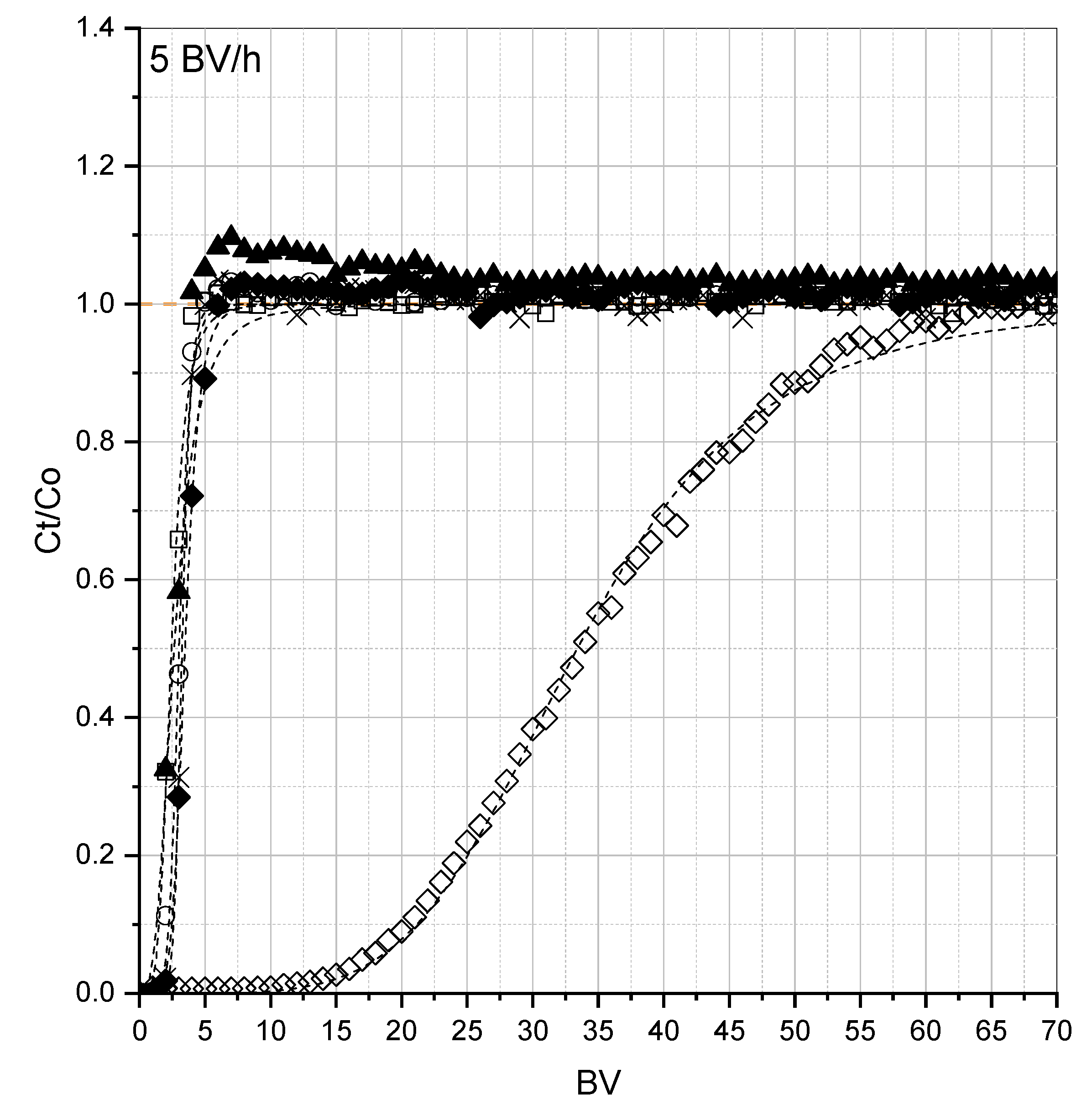
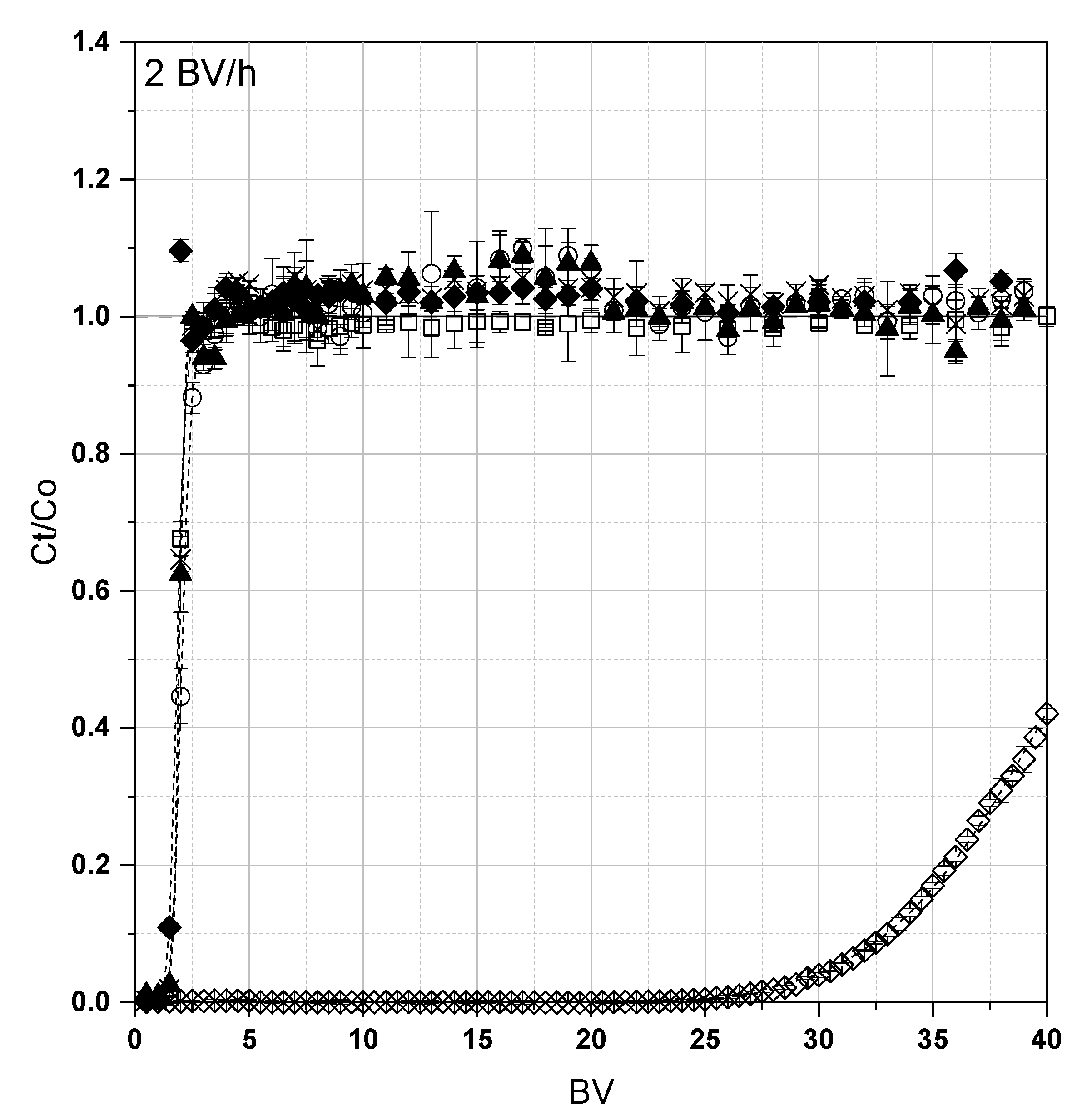
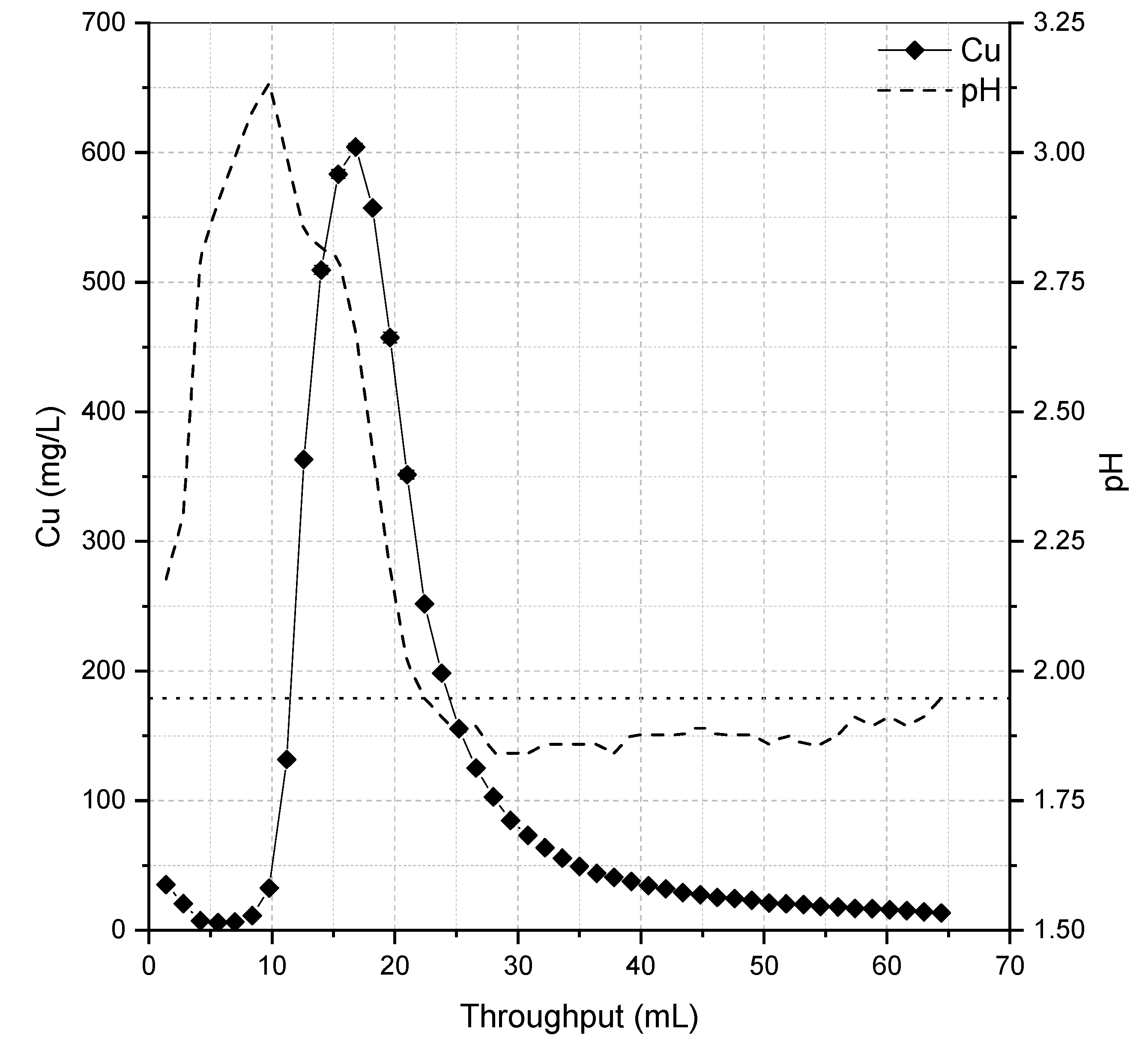
3.2. Fixed-Bed Adsorption
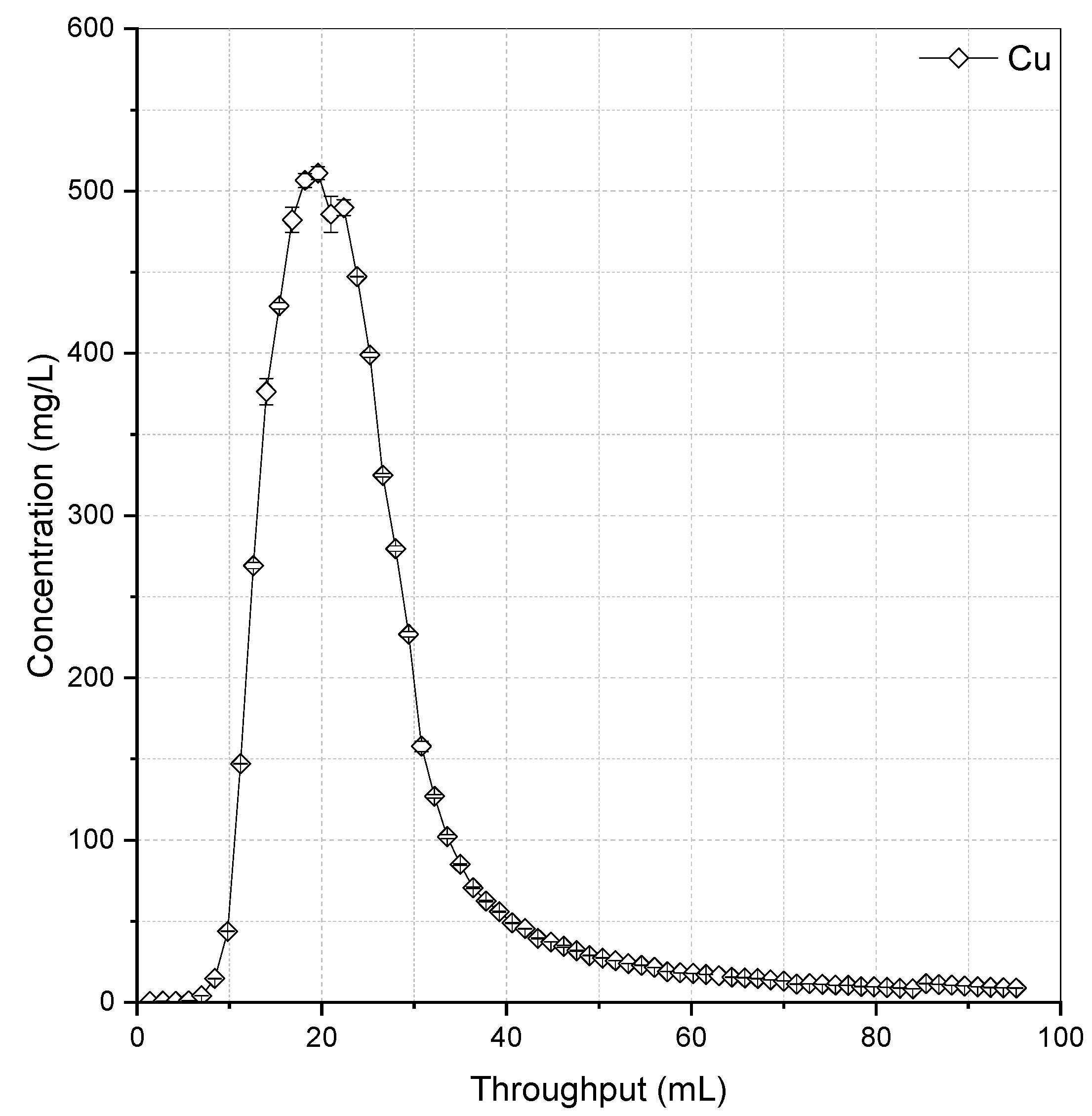
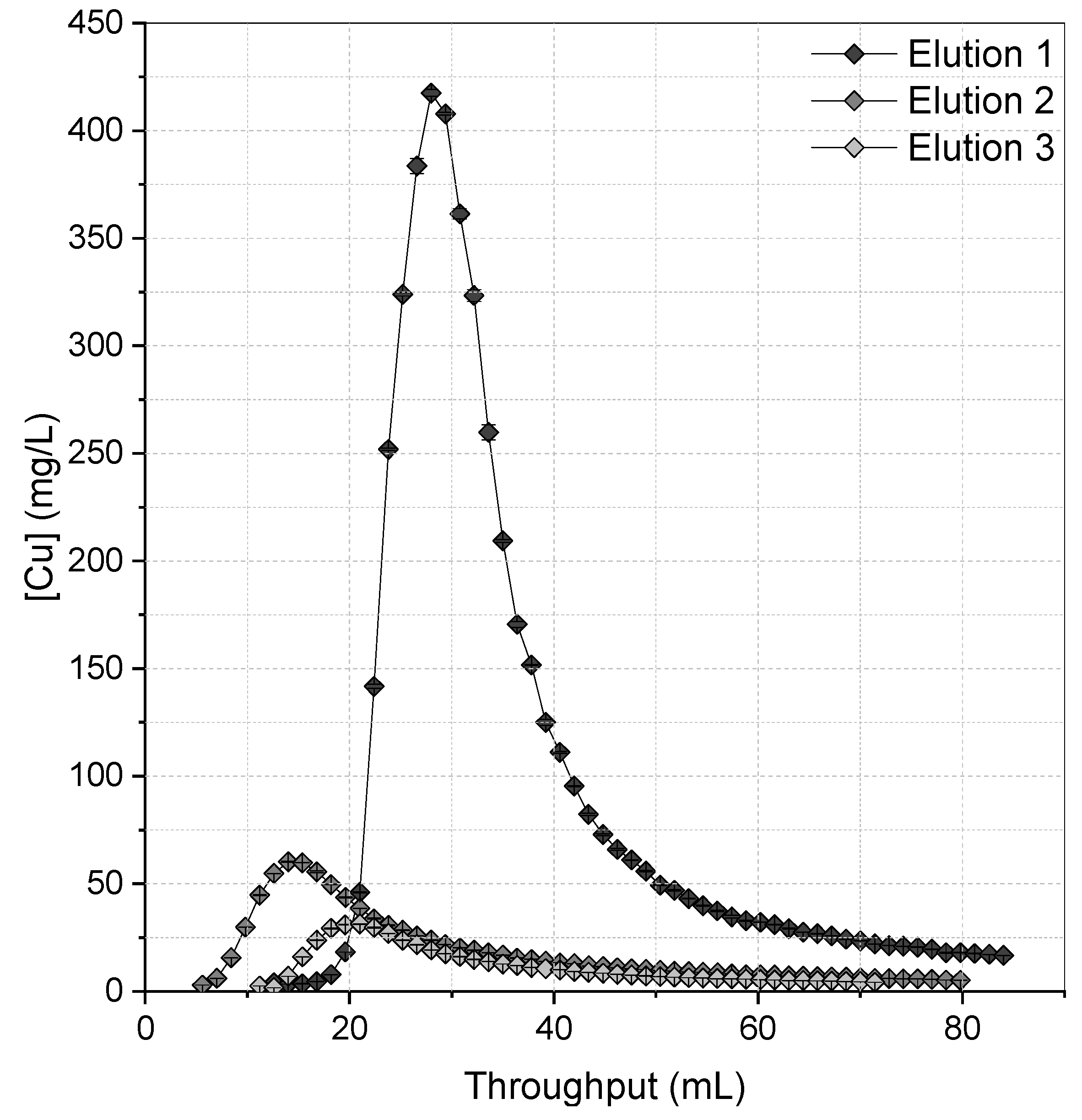
3.3. Resin Elution
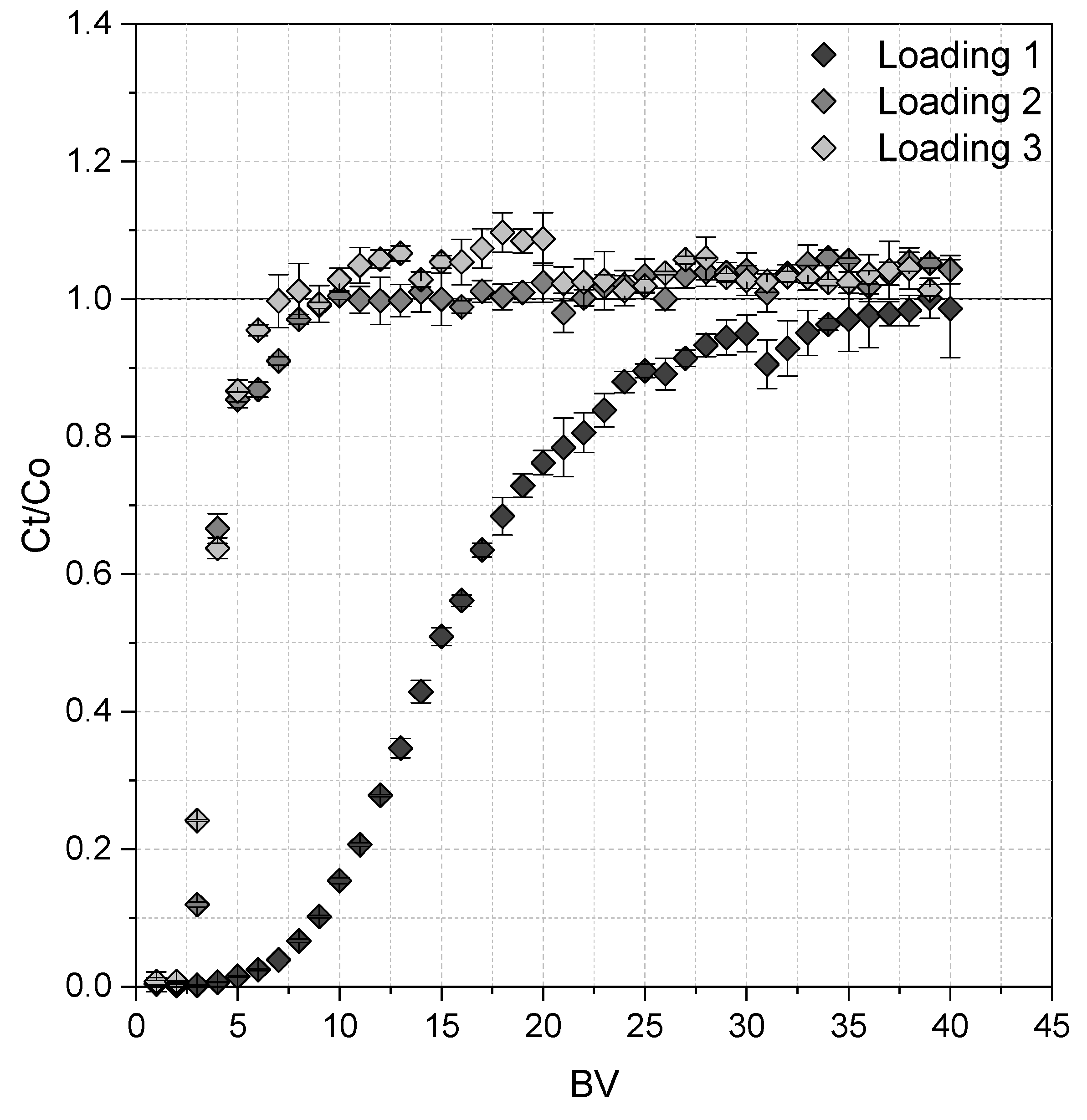
3.4. Resin Reusability
3.5. Functionality Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Northey, S.; Haque, N.; Mudd, G. Using sustainability reporting to assess the environmental footprint of copper mining. J. Clean. Prod. 2013, 40, 118–128. [Google Scholar] [CrossRef]
- Han, B.; Altansukh, B.; Haga, K.; Stevanović, Z.; Jonović, R.; Avramović, L.; Urosević, D.; Takasaki, Y.; Masuda, N.; Ishiyama, D.; et al. Development of copper recovery process from flotation tailings by a combined method of high‒pressure leaching‒solvent extraction. J. Hazard. Mater. 2018, 352, 192–203. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Ji, J.; Xu, B. Bioleaching of two different genetic types of chalcopyrite and their comparative mineralogical assessment. Anal. Bioanal. Chem. 2018, 410, 1725–1733. [Google Scholar] [CrossRef]
- Owusu, C.; Abreu, S.B.; Skinner, W.; Addai-Mensah, J.; Zanin, M. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures. Miner. Eng. 2014, 55, 87–95. [Google Scholar] [CrossRef]
- Gouvea, L.R.; Morais, C.A. Development of a process for the separation of zinc and copper from sulfuric liquor obtained from the leaching of an industrial residue by solvent extraction. Miner. Eng. 2010, 23, 492–497. [Google Scholar]
- Crane, R.A.; Sapsford, D.J. Towards Greener Lixiviants in Value Recovery from Mine Wastes: Efficacy of Organic Acids for the Dissolution of Copper and Arsenic from Legacy Mine Tailings. Minerals 2018, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Bezzina, J.P.; Ruder, L.R.; Dawson, R.; Ogden, M. Ion exchange removal of Cu(II), Fe(II), Pb(II) and Zn(II) from acid extracted sewage sludge—Resin screening in weak acid media. Water Res. 2019, 158, 257–267. [Google Scholar] [CrossRef]
- Shuva, M.; Rhamdhani, M.; Brooks, G.; Masood, S.; Reuter, M. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean. Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- Min, X.; Luo, X.; Deng, F.; Shao, P.; Wu, X.; Dionysiou, D.D. Combination of multi-oxidation process and electrolysis for pretreatment of PCB industry wastewater and recovery of highly-purified copper. Chem. Eng. J. 2018, 354, 228–236. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, F.; Ma, Y. Ultrasonic recivery of copper and iron through the simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011, 185, 155–161. [Google Scholar]
- Hu, S.-H.; Hu, S.-C.; Fu, Y.-P. Resource Recovery of Copper-Contaminated Sludge with Jarosite Process and Selective Precipitation. Environ. Prog. Sustain. Energy 2012, 31, 379–385. [Google Scholar]
- Arshadi, M.; Mousavi, S.; Rasoulnia, P. Enhancement of simultaneous gold and copper recovery from discarded mobile phone PCBs using Bacillus megaterium: RSM based optimization of effective factors and evaluation of their interactions. Waste Manag. 2016, 57, 158–167. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A review of gold leaching in acid thiourea solutions. Miner. Process. Extr. Met. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Yang, X.; Moats, M.S.; Miller, J.D.; Wang, X.; Shi, X.; Xu, H. Thiourea–thiocyanate leaching system for gold. Hydrometallurgy 2011, 106, 58–63. [Google Scholar] [CrossRef]
- Jing-Ying, L.; Xiu-Li, X.; Wen-Quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.R.; Porter, C.P.; Robshaw, T.J.; Bezzina, J.P.; Shields, V.R.; Hides, A.; Bruce, R.; Ogden, M.D. An alternative to cyanide leaching of waste activated carbon ash for gold and silver recovery via synergistic dual-lixiviant treatment. J. Ind. Eng. Chem. 2020, 92, 120–130. [Google Scholar] [CrossRef]
- Silva, R.A.; Hawboldt, K.; Zhang, Y. Application of resins with functional groups in the separation of metal ions/species—A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 395–413. [Google Scholar] [CrossRef]
- Harland, C.E. Ion Exchange: Theory and Practice, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 1994. [Google Scholar]
- Amphlett, J.; Ogden, M.; Foster, R.I.; Syna, N.; Soldenhoff, K.; Sharrad, C. Polyamine functionalised ion exchange resins: Synthesis, characterisation and uranyl uptake. Chem. Eng. J. 2018, 334, 1361–1370. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the Behaviour of Charcoal with Respect to Chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar]
- Hamdaoui, O. Removal of copper(II) from aqueous phase by Purolite C100-MB cation exchange resin in fixed bed columns: Modeling. J. Hazard. Mater. 2009, 161, 737–746. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martín-Lara, M. Study of Cr (III) biosorption in a fixed-bed column. J. Hazard. Mater. 2009, 171, 886–893. [Google Scholar] [CrossRef]
- ThermoScientific. XPS: Copper. 2020. Available online: https://xpssimplified.com/elements/copper.php (accessed on 2 February 2020).
- Hayez, V.; Franquet, A.; Hubin, A.; Terryn, H. XPS study of the atmospheric corrosion of copper alloys of archaeological interest. Surf. Interface Anal. 2004, 36, 876–879. [Google Scholar] [CrossRef]
- Krzewska, S.; Podsiadly, H.; Pajdowski, L. Studies on the Reaction of Copper(II) with thiourea—III: Equilibrium and Stability Constants in Cu(II)-Thiourea-HClO4 Redox System. J. Inorg. Nucl. Chem. 1980, 42, 89–94. [Google Scholar]
- Gaur, A.; Shrivastava, B.D.; Srivastava, K.; Prasad, J.; Raghuwanshi, V.S. X-ray absorption fine structure study of multinuclear copper(I) thiourea mixed ligand complexes. J. Chem. Phys. 2013, 139, 034303. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Huang, X.; Cao, X.; Wang, W.; Zhong, H.; Cao, Z. Preparation of a novel resin with acyl and thiourea groups and its properties for Cu(II) removal from aqueous solution. J. Environ. Manag. 2017, 204, 264–271. [Google Scholar] [CrossRef]
- Vanýsek, P. Electrochemical Series. In CRC Handbook of Chemistry and Physics; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 20–29. [Google Scholar]
- Korak, J.A.; Huggins, R.; Arias-Paic, M. Regeneration of pilot-scale ion exchange columns for hexavalent chromium removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef]
- Jeffrey, M.; Hewitt, D.; Dai, X.; Brunt, S. Ion exchange adsorption and elution for recovering gold thiosulphate from leach solutions. Hydrometallurgy 2010, 100, 136–143. [Google Scholar] [CrossRef]
- Tavakoli, H.; Sepehrian, H.; Semnani, F.; Samadfam, M. Recovery of uranium from UCF liquid waste by anion exchange resin CG-400: Breakthrough curves, elution behavior and modeling studies. Ann. Nucl. Energy 2013, 54, 149–153. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Brites, L.M.; Bustamante, M.C.C.; Parpot, P.; Teixeira, J.A.; Mussatto, S.I.; Barboza, M. Fixed-Bed Column Process as a Strategy for Separation and Purification of Cephamycin C from Fermented Broth. Ind. Eng. Chem. Res. 2015, 54, 3018–3026. [Google Scholar] [CrossRef] [Green Version]
- Sahu, S.; Sahoo, P.R.; Patel, S.; Mishra, B.K. Oxidation of thiourea and substituted thioureas: A review. J. Sulfur Chem. 2011, 32, 171–197. [Google Scholar] [CrossRef]
- Pharmafile. Copper Could Replace Precious Metals in Pharma Production. 2012. Available online: http://www.pharmafile.com/news/175878/copper-could-replace-precious-metals-pharma-production (accessed on 1 November 2021).
- Tandon, P.; Singh, S. Catalytic applications of copper species in organic transformations: A review. J. Catal. Catal. 2014, 1, 21–34. [Google Scholar]
- Rahman, A.; Uahengo, V.; Daniel, L.S. Green chemistry concept: Applications of catalysis in pharmacuetical industry. Glob. Drugs Ther. 2017, 2, 1–6. [Google Scholar]
- Amphlett, J.; Pepper, S.; Riley, A.; Harwood, L.; Cowell, J.; Whittle, K.; Lee, T.; Ogden, M. Impact of copper(II) on activation product removal from reactor decommissioning effluents in South Korea. J. Ind. Eng. Chem. 2019, 82, 261–268. [Google Scholar] [CrossRef]
- Pepper, S.E.; Whittle, K.R.; Harwood, L.M.; Cowell, J.; Lee, T.S.; Ogden, M.D. Cobalt and nickel uptake by silica-based extractants. Sep. Sci. Technol. 2018, 53, 1552–1562. [Google Scholar] [CrossRef]


| Functional Group | Capacity (eq/L) | Polymer Matrix | Moisture (%) | Particle Size (µm) | Density (g/mL) |
|---|---|---|---|---|---|
| Thiourea | 1 | PS-DVB 1 | 50–56 | 300–1200 | 0.308 |
| Modified Dose Response | Bohart–Adams | Thomas | Yoon–Nelson | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | Qo | R2 | Ka | W | R2 | Kt | Qo | R2 | Kyn | t50 | R2 | |
| Al | 9.15 | 15.45 | 2.10 | 0.996 | 0.14 | 3.27 | 0.996 | 0.14 | 2.12 | 0.996 | 0.49 | 18.22 | 0.998 |
| Co | 9.64 | 15.44 | 2.15 | 0.999 | 0.14 | 3.34 | 0.999 | 0.14 | 2.17 | 0.999 | 0.52 | 18.20 | 0.996 |
| Cu | 3.01 | 160.26 | 19.84 | 0.998 | 0.005 | 33.07 | 0.989 | 0.005 | 21.20 | 0.989 | 0.02 | 199.98 | 0.989 |
| Fe | 9.97 | 16.48 | 2.13 | 0.980 | 0.16 | 3.31 | 0.979 | 0.16 | 2.15 | 0.979 | 0.53 | 19.44 | 0.995 |
| Mn | 9.06 | 15.57 | 2.02 | 0.986 | 0.15 | 3.14 | 0.985 | 0.15 | 2.04 | 0.985 | 0.49 | 18.36 | 0.997 |
| Ni | 10.07 | 15.37 | 2.10 | 0.999 | 0.15 | 3.26 | 0.999 | 0.15 | 2.11 | 0.999 | 0.54 | 18.11 | 0.997 |
| Zn | 10.07 | 15.37 | 2.01 | 0.999 | 0.14 | 3.27 | 0.997 | 0.14 | 2.12 | 0.997 | 0.48 | 18.96 | 0.999 |
| Modified Dose Response | Bohart-Adams | Thomas | Yoon-Nelson | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | Qo | R2 | Ka | W | R2 | Kt | Qo | R2 | Kyn | t50 | R2 | |
| Co | 4.49 | 12.15 | 1.69 | 0.994 | 0.05 | 2.72 | 0.995 | 0.05 | 1.76 | 0.995 | 0.18 | 26.52 | 0.992 |
| Cu | 4.81 | 167.02 | 20.68 | 0.998 | 0.004 | 35.13 | 0.998 | 0.004 | 22.77 | 0.998 | 0.01 | 360.37 | 0.998 |
| Fe | 6.45 | 17.32 | 2.23 | 0.999 | 0.05 | 3.50 | 0.997 | 0.05 | 2.27 | 0.997 | 0.18 | 36.91 | 0.996 |
| Mn | 10.29 | 16.18 | 2.10 | 0.999 | 0.09 | 3.26 | 0.999 | 0.09 | 2.12 | 0.999 | 0.28 | 34.22 | 0.998 |
| Ni | 3.09 | 13.20 | 1.80 | 0.987 | 0.04 | 2.85 | 0.956 | 0.04 | 1.84 | 0.956 | 0.17 | 27.37 | 0.992 |
| Zn | 7.35 | 15.07 | 1.87 | 0.995 | 0.07 | 3.05 | 0.999 | 0.07 | 1.98 | 0.999 | 0.23 | 31.79 | 0.998 |
| Modified Dose Response | Bohart-Adams | Thomas | Yoon-Nelson | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | Qo | R2 | Ka | W | R2 | Kt | Qo | R2 | Kyn | t50 | R2 | |
| Co | 16.67 | 9.56 | 1.46 | 0.996 | 0.09 | 2.25 | 0.997 | 0.09 | 1.46 | 0.997 | 0.333 | 57.52 | 0.999 |
| Cu | 9.74 | 206.45 | 32.04 | 0.999 | 0.002 | 48.75 | 0.997 | 0.002 | 31.66 | 0.997 | 0.01 | 1223.9 | 0.997 |
| Fe | 10.46 | 9.16 | 0.94 | 0.999 | 0.07 | 1.48 | 0.999 | 0.07 | 0.96 | 0.999 | NA | NA | NA |
| Mn | 16.14 | 9.63 | 1.08 | 0.999 | 0.10 | 1.66 | 0.999 | 0.10 | 1.08 | 0.999 | 0.30 | 57.72 | 0.999 |
| Ni | 16.57 | 9.69 | 1.18 | 0.999 | 0.09 | 1.81 | 0.999 | 0.09 | 1.18 | 0.999 | 0.29 | 57.94 | 0.999 |
| Zn | 9.97 | 10.25 | 1.13 | 0.996 | 0.06 | 1.73 | 0.996 | 0.06 | 1.13 | 0.996 | 0.17 | 61.52 | 0.996 |
| [NaClO3] | Cu Loaded (mg/mL) | Bed Volume (mL) | Total Cu on Bed (mg) | Cu Recovered (mg) | FWHM (mL) | Recovery Efficiency (%) |
|---|---|---|---|---|---|---|
| 0.5 M | 8.64 | 1.4 | 12.10 | 9.55 | 16.2 | 78.91 |
| 1.0 M | 8.36 | 1.4 | 11.70 | 9.58 | 13.2 | 81.86 |
| Cycle | Loading (mg) | Elution (mg) | Recovery (%) |
|---|---|---|---|
| 1 | 7.95 | 6.79 | 85.34 |
| 2 | 1.55 | 1.37 | 88.46 |
| 3 | 1.55 | 0.72 | 46.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riley, A.L.; Porter, C.P.; Ogden, M.D. Selective Recovery of Copper from a Synthetic Metalliferous Waste Stream Using the Thiourea-Functionalized Ion Exchange Resin Puromet MTS9140. Eng 2021, 2, 512-530. https://doi.org/10.3390/eng2040033
Riley AL, Porter CP, Ogden MD. Selective Recovery of Copper from a Synthetic Metalliferous Waste Stream Using the Thiourea-Functionalized Ion Exchange Resin Puromet MTS9140. Eng. 2021; 2(4):512-530. https://doi.org/10.3390/eng2040033
Chicago/Turabian StyleRiley, Alex L., Christopher P. Porter, and Mark D. Ogden. 2021. "Selective Recovery of Copper from a Synthetic Metalliferous Waste Stream Using the Thiourea-Functionalized Ion Exchange Resin Puromet MTS9140" Eng 2, no. 4: 512-530. https://doi.org/10.3390/eng2040033






