The Effects of Multistage Fuel-Oxidation Chemistry, Soot Radiation, and Real Gas Properties on the Operation Process of Compression Ignition Engines
Abstract
:1. Introduction
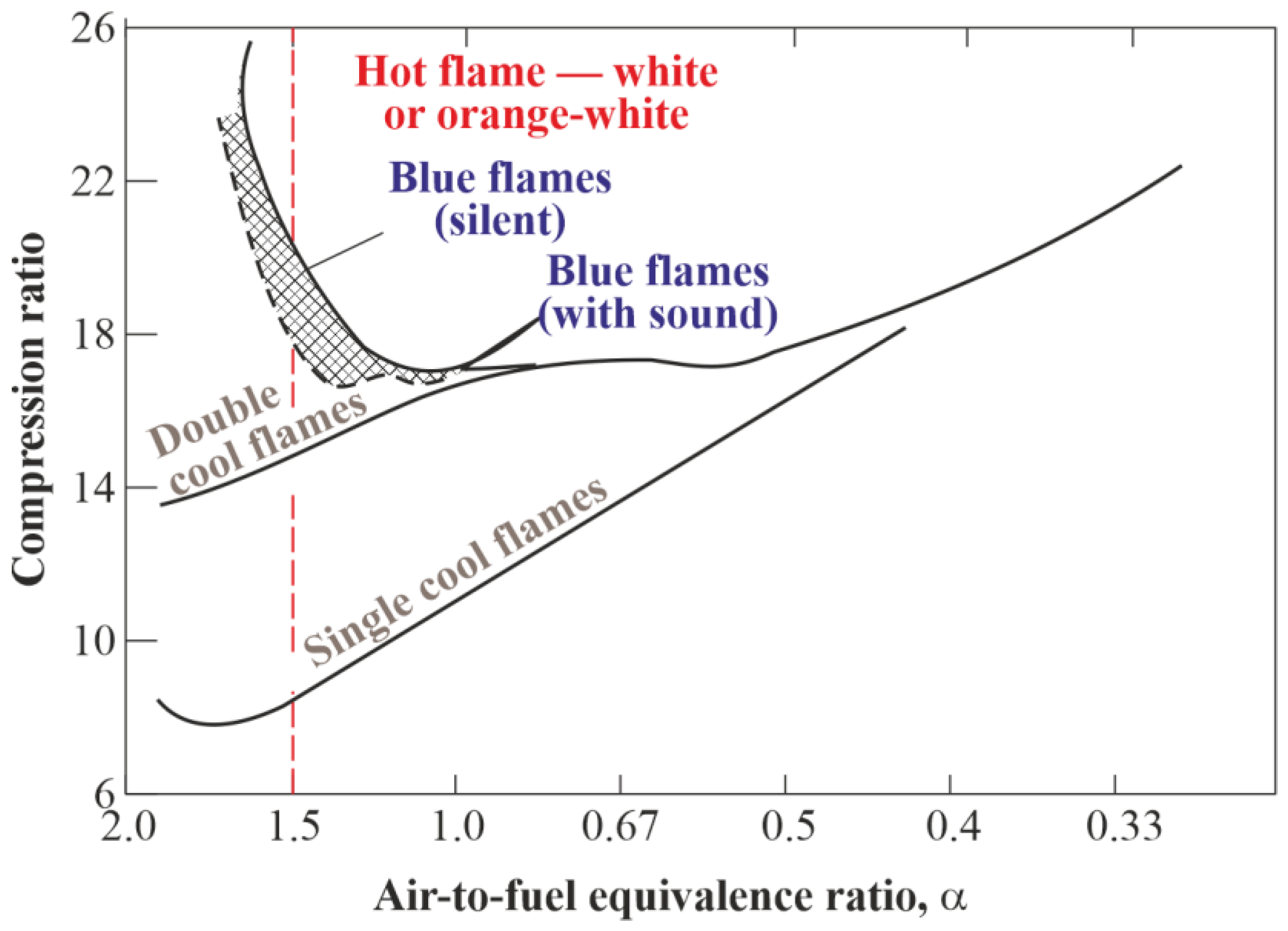
1.1. Multistage Autoignition
1.2. Soot Formation and Radiation
1.3. Real Gas Effects
2. Materials and Methods
2.1. Reaction Mechanism
2.2. Droplet Autoignition and Combustion
2.3. Real Gas Equation of State
2.4. Solution Procedure of the Zero-Dimensional Problem
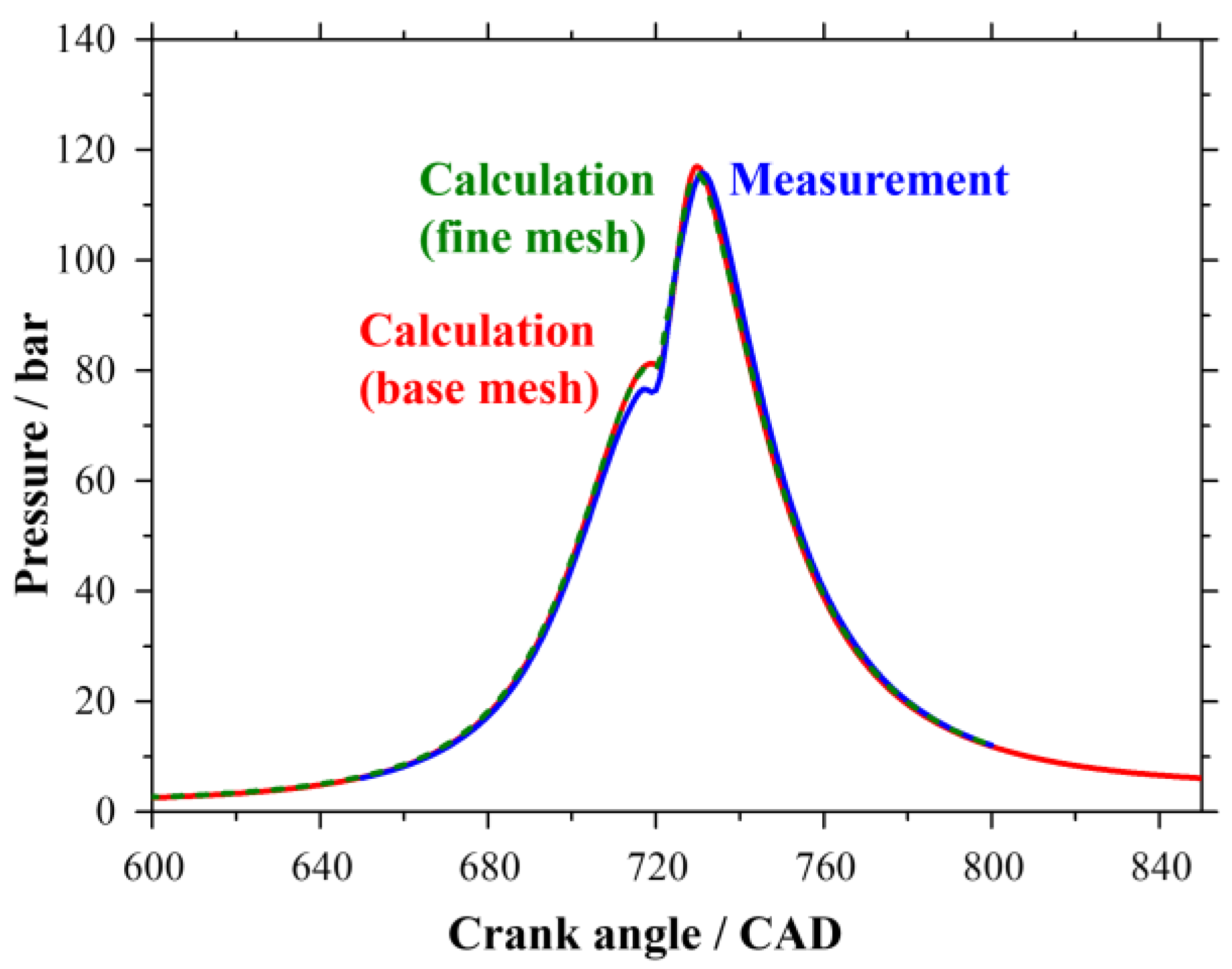
2.5. Solution Procedure of the One-Dimensional Problem
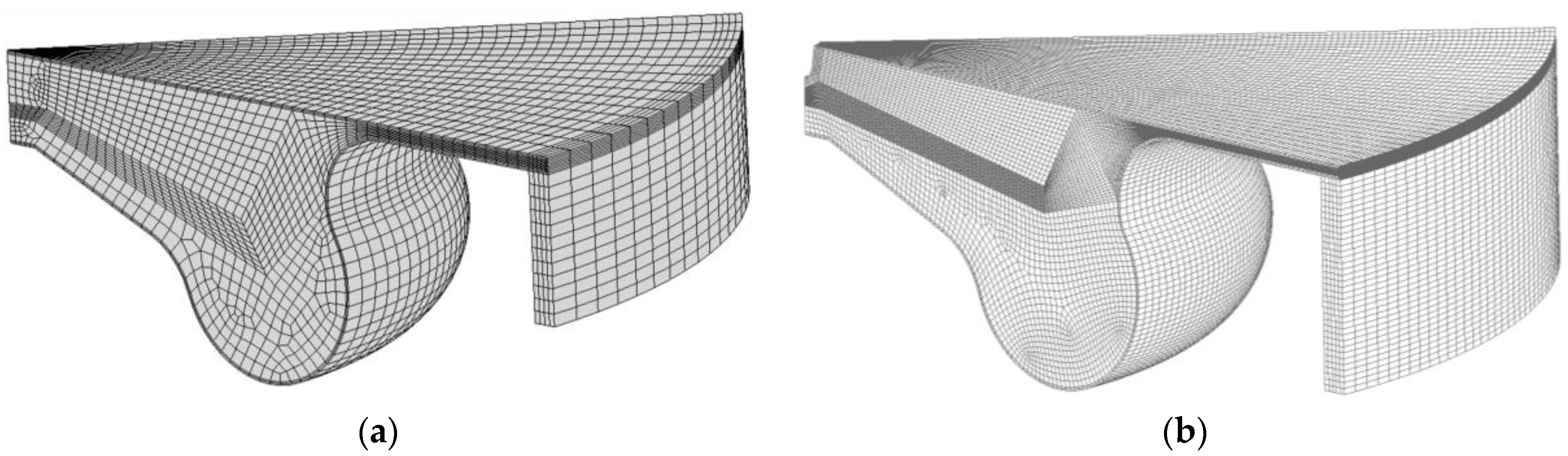
2.6. Solution Procedure of the Three-Dimensional Problem
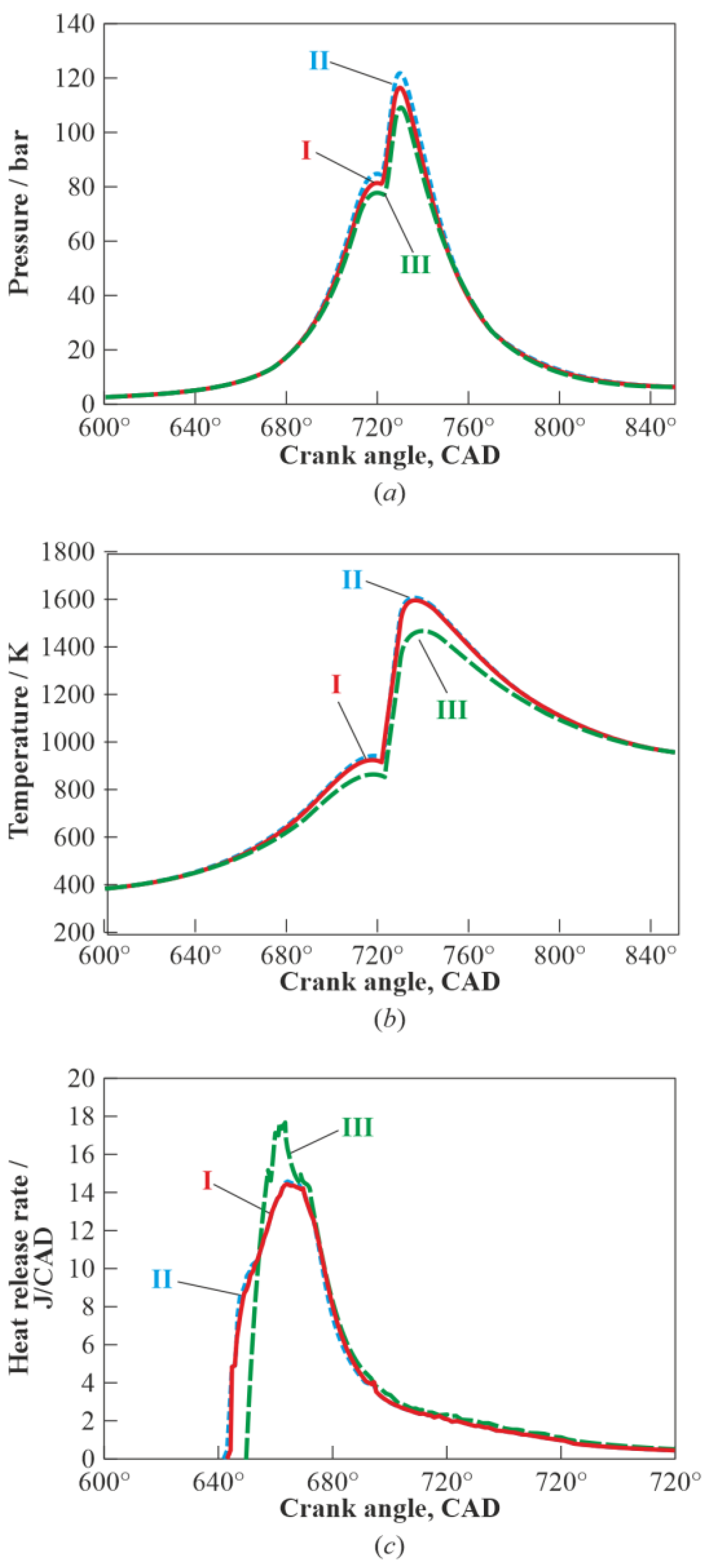
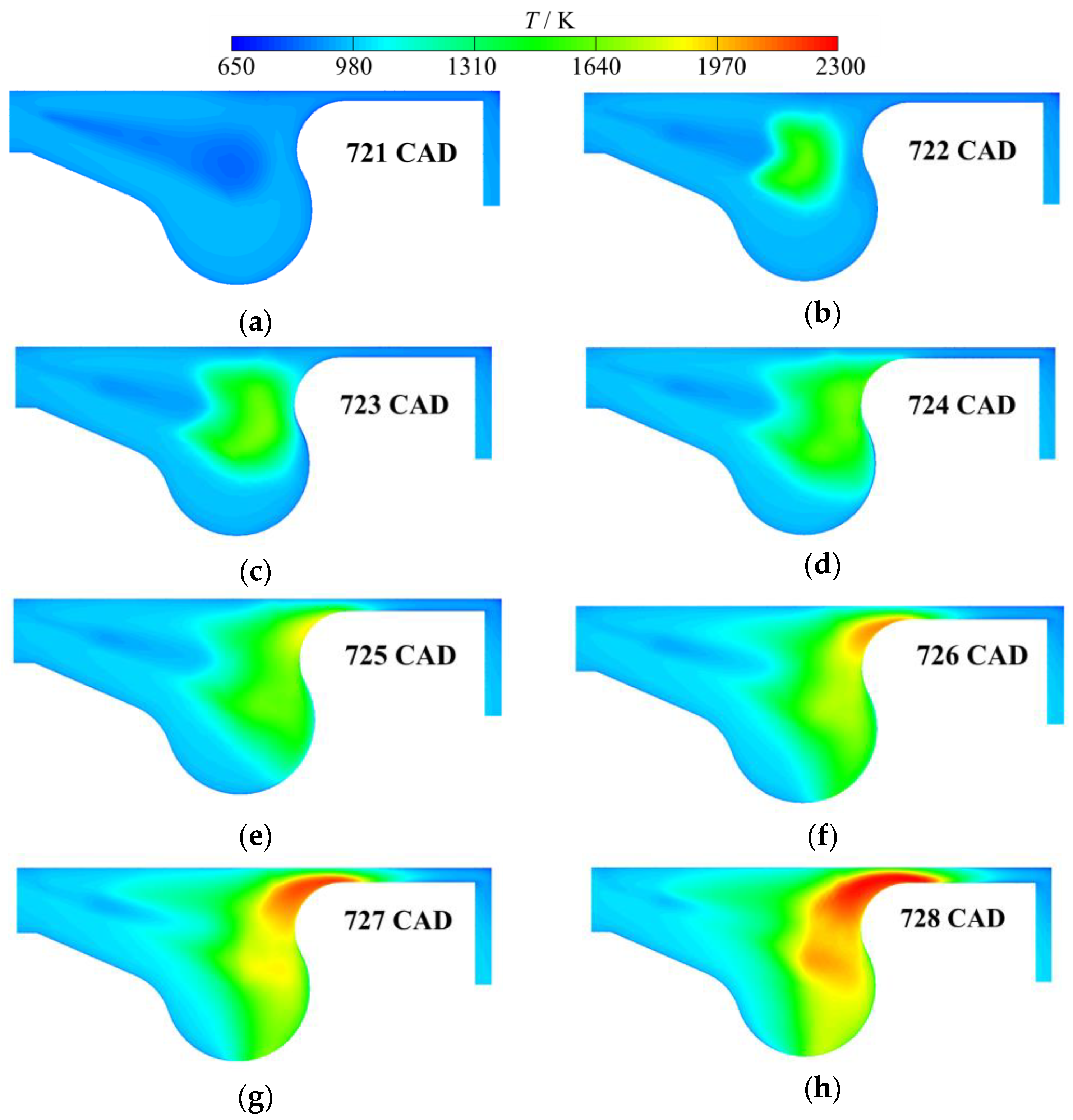
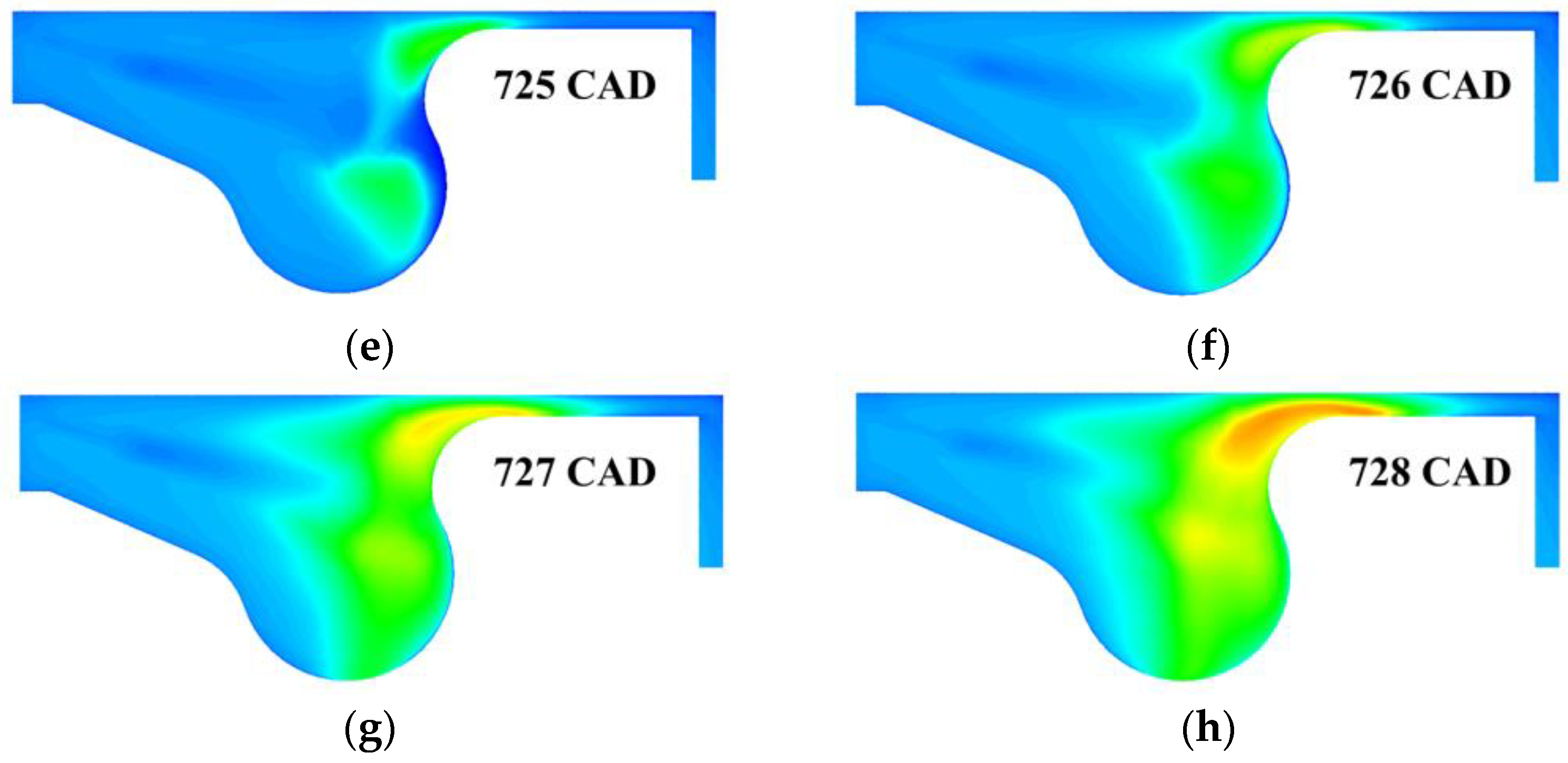
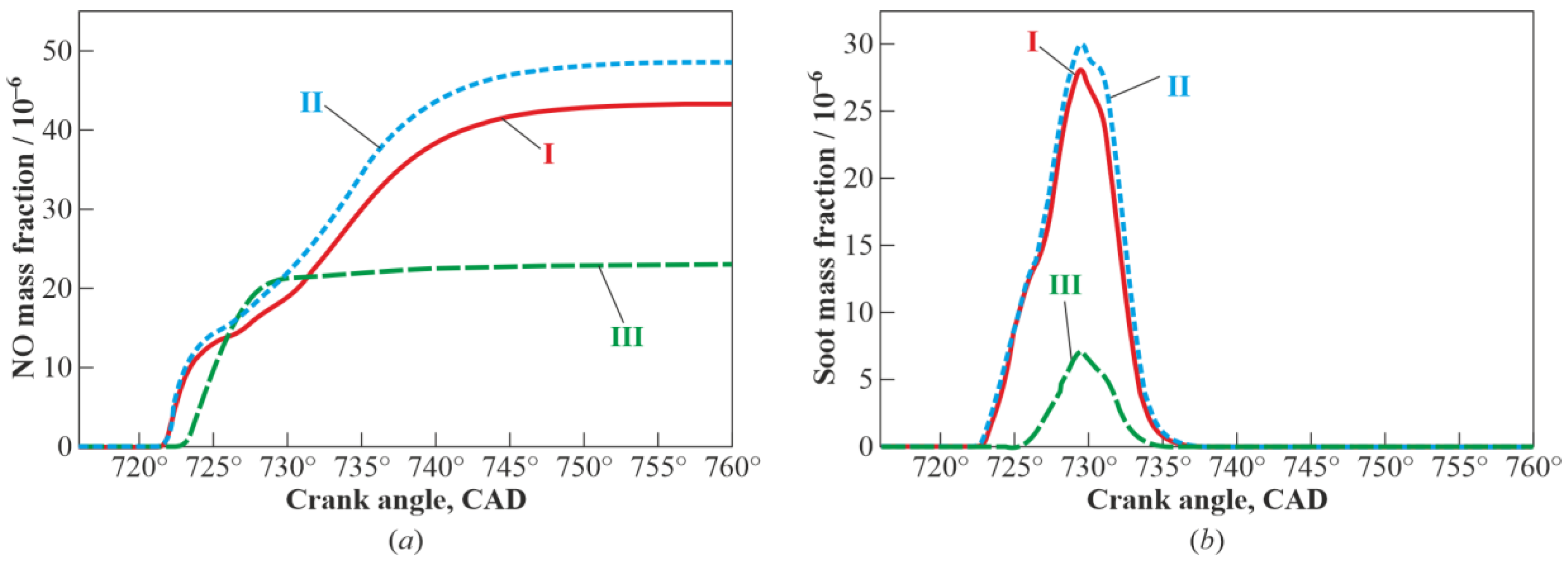
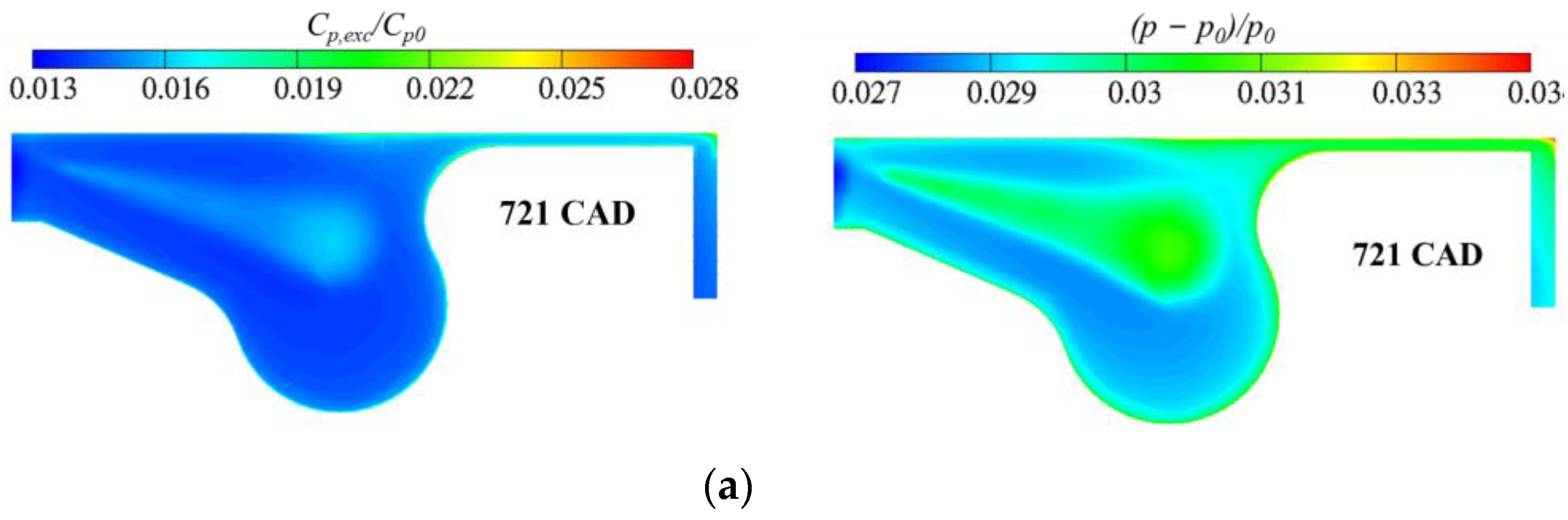
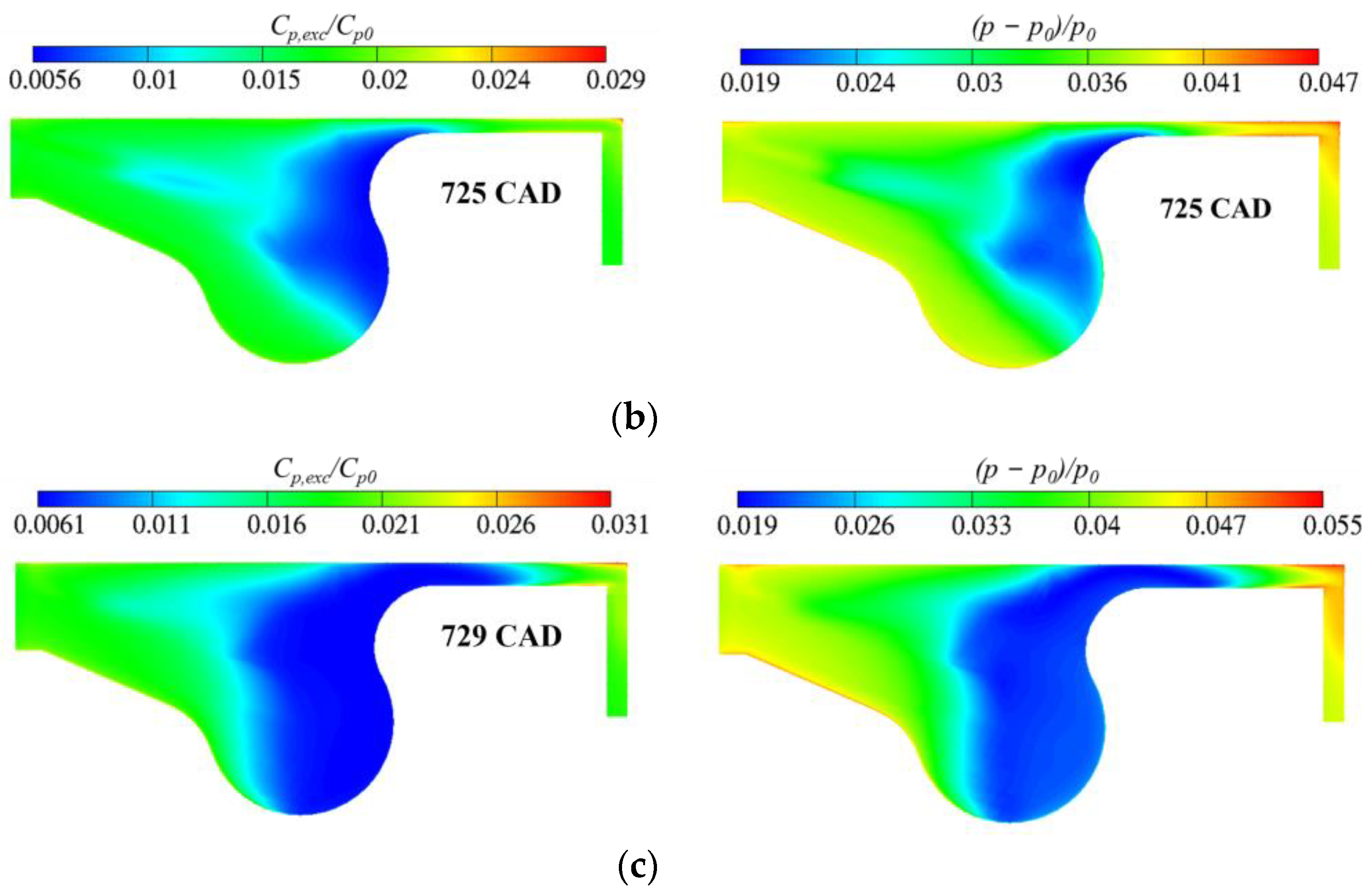
3. Results and Discussion
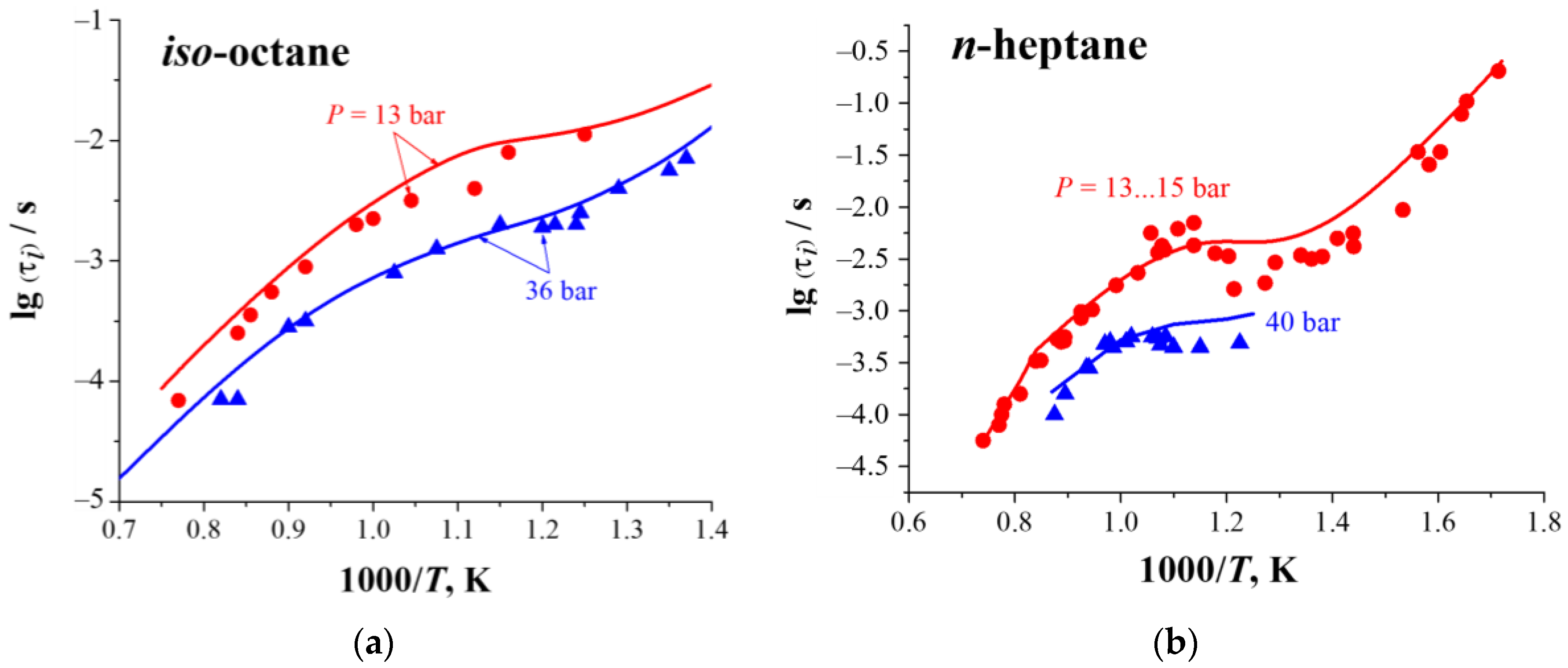
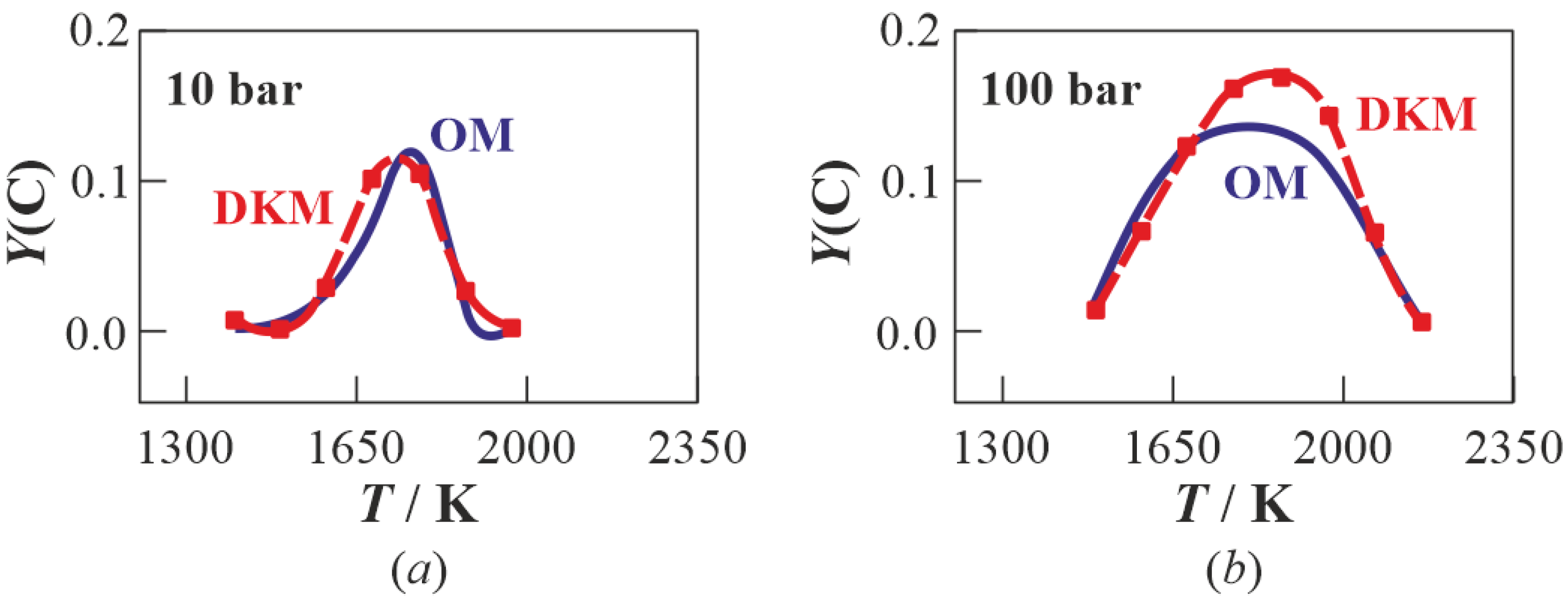
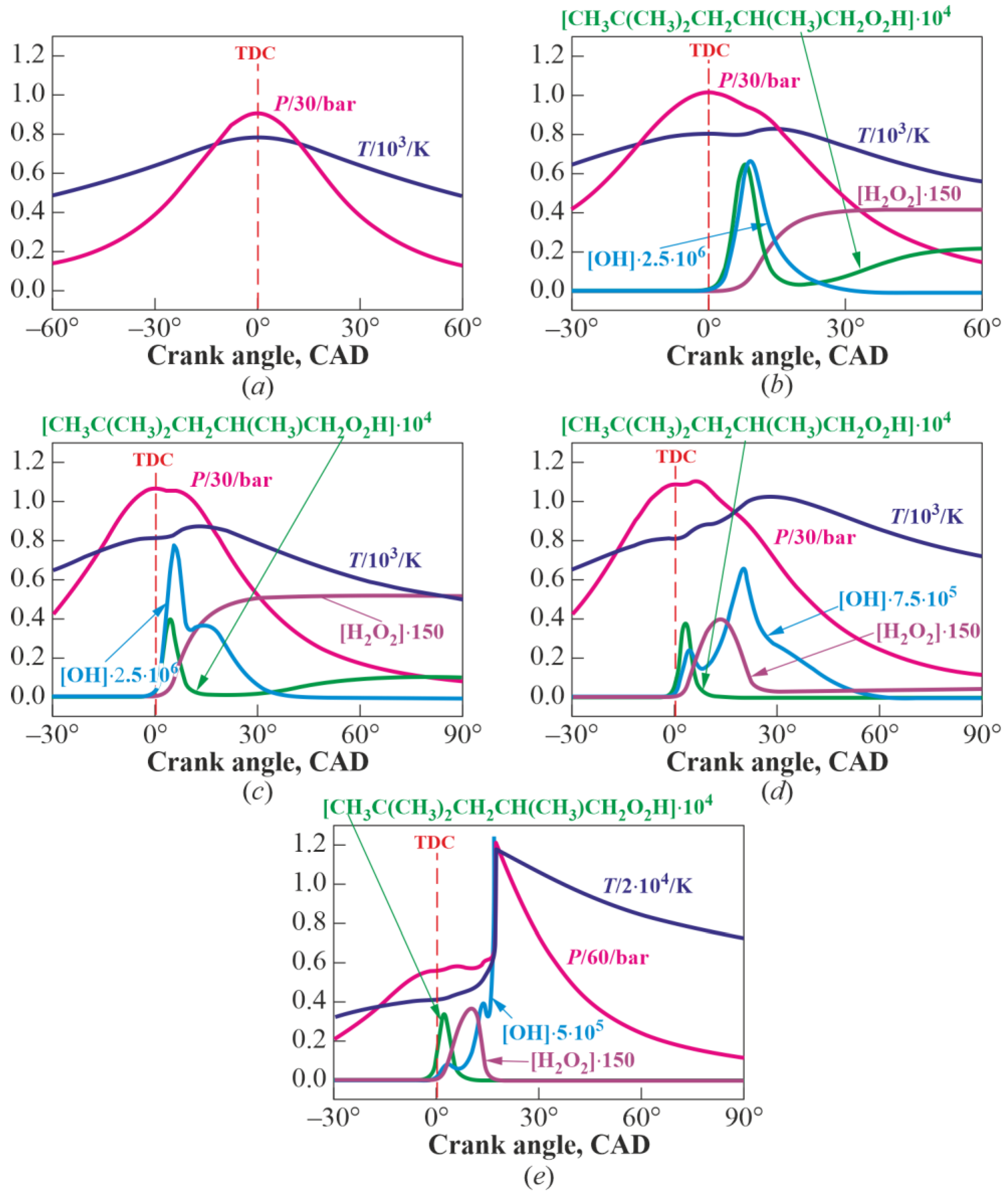
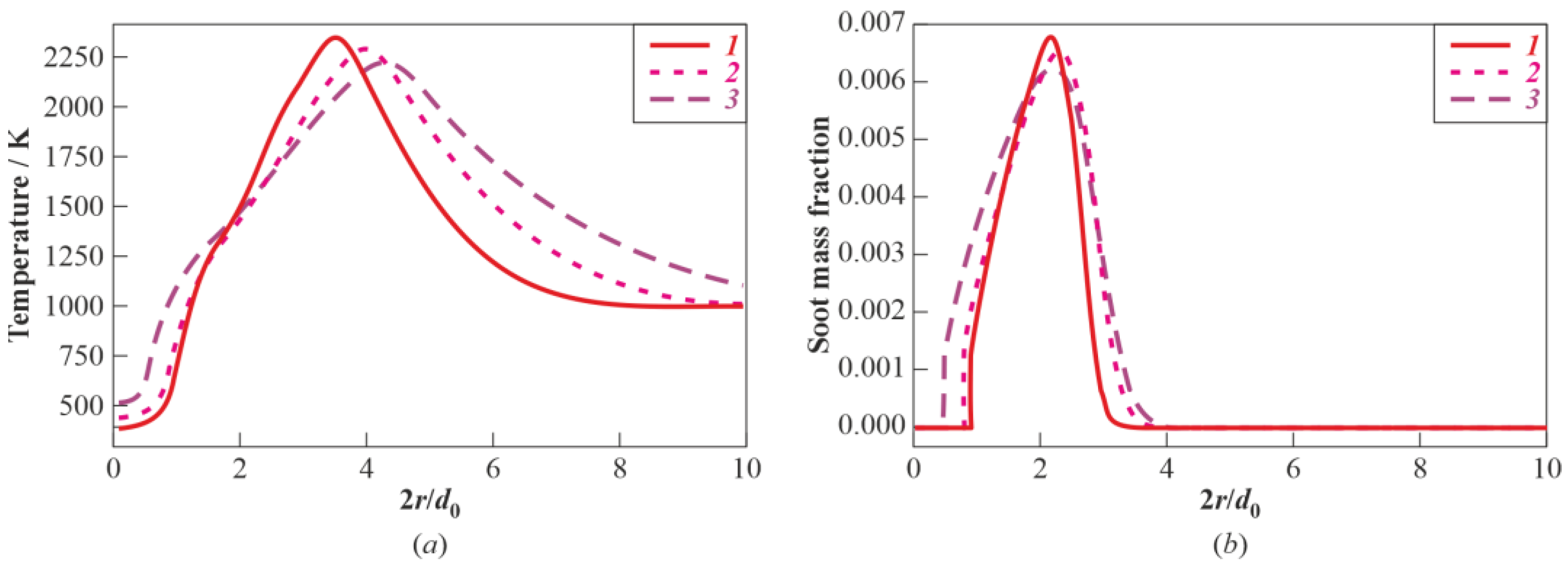
3.1. Multistage Fuel-Oxidation Chemistry
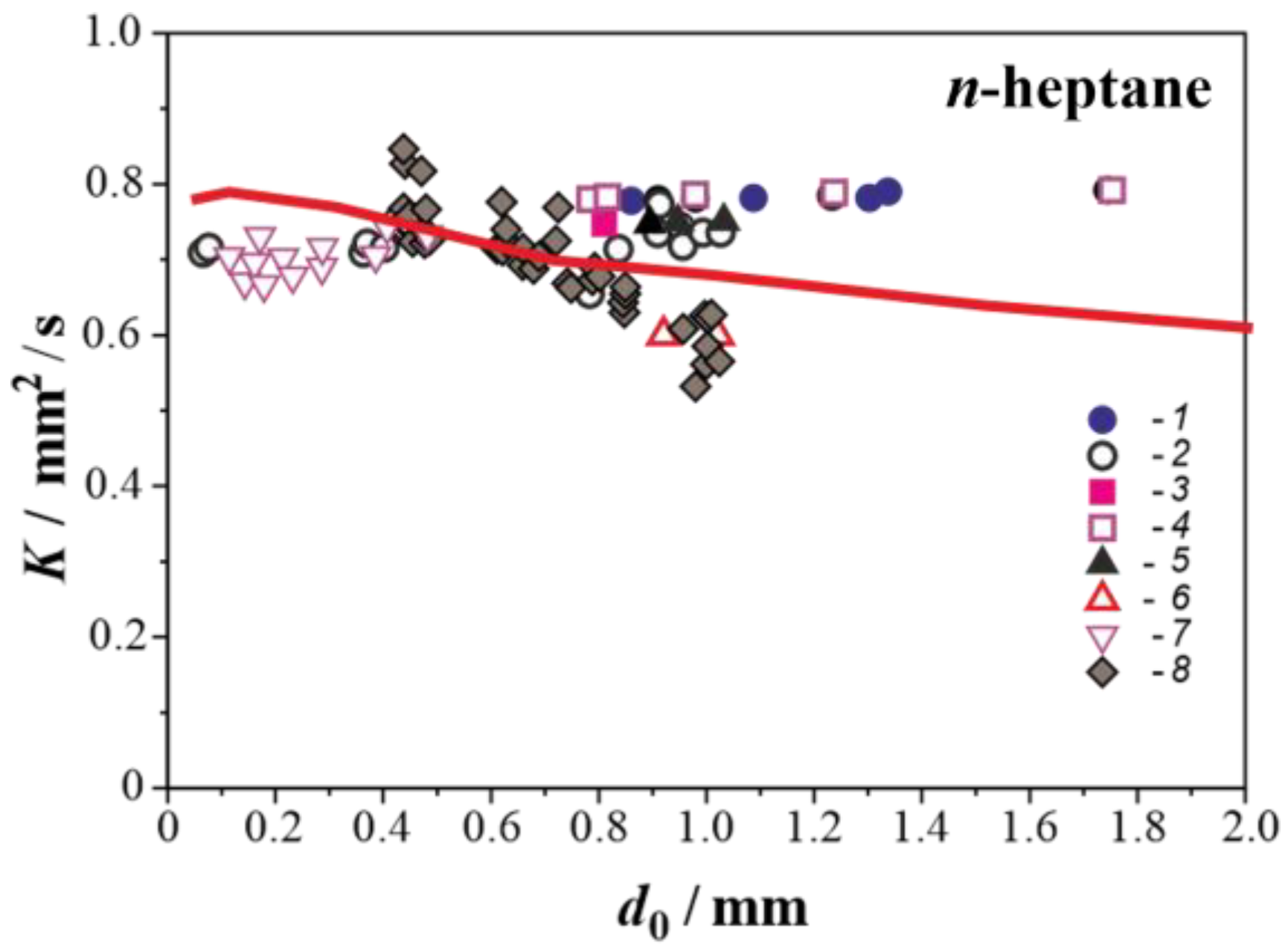
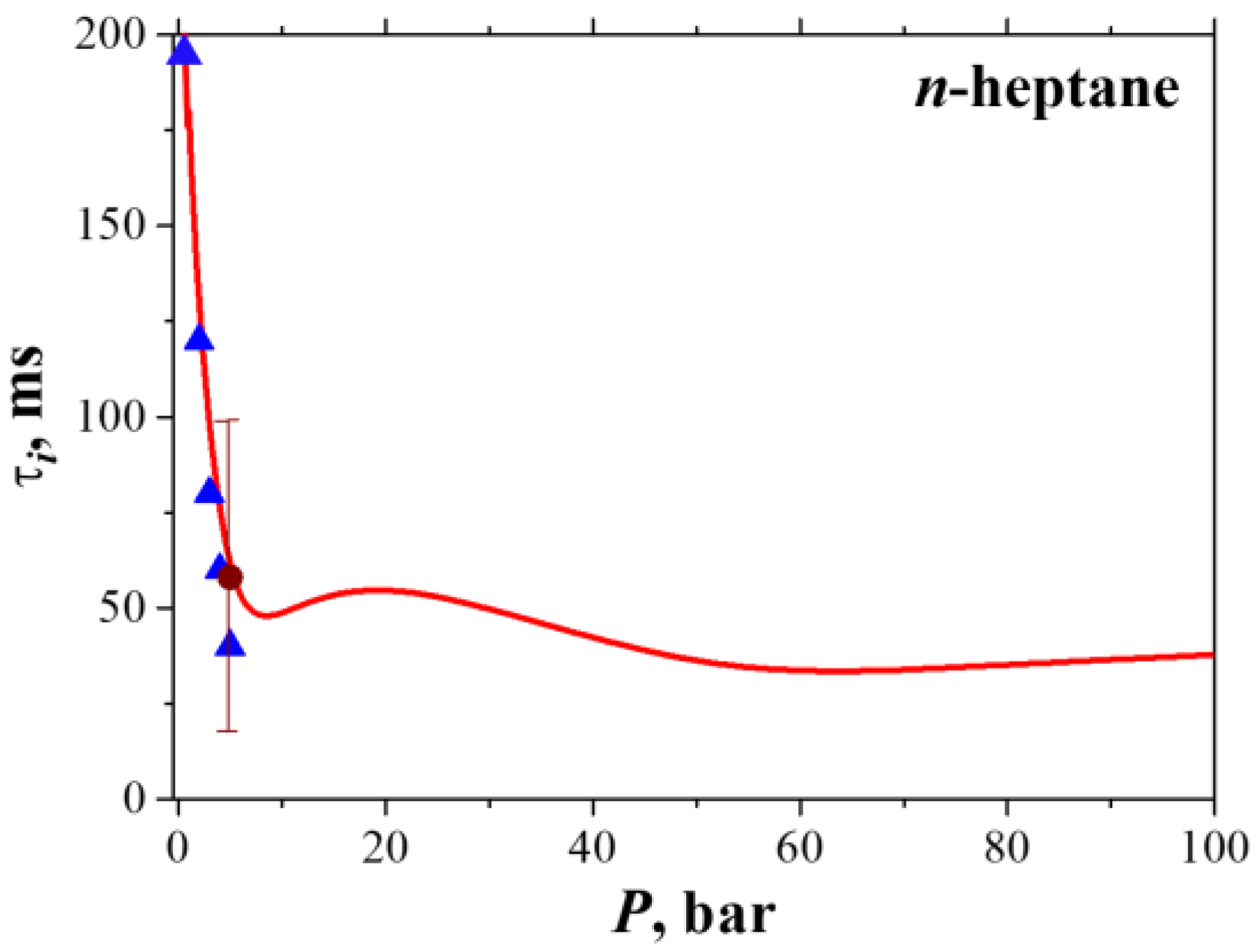
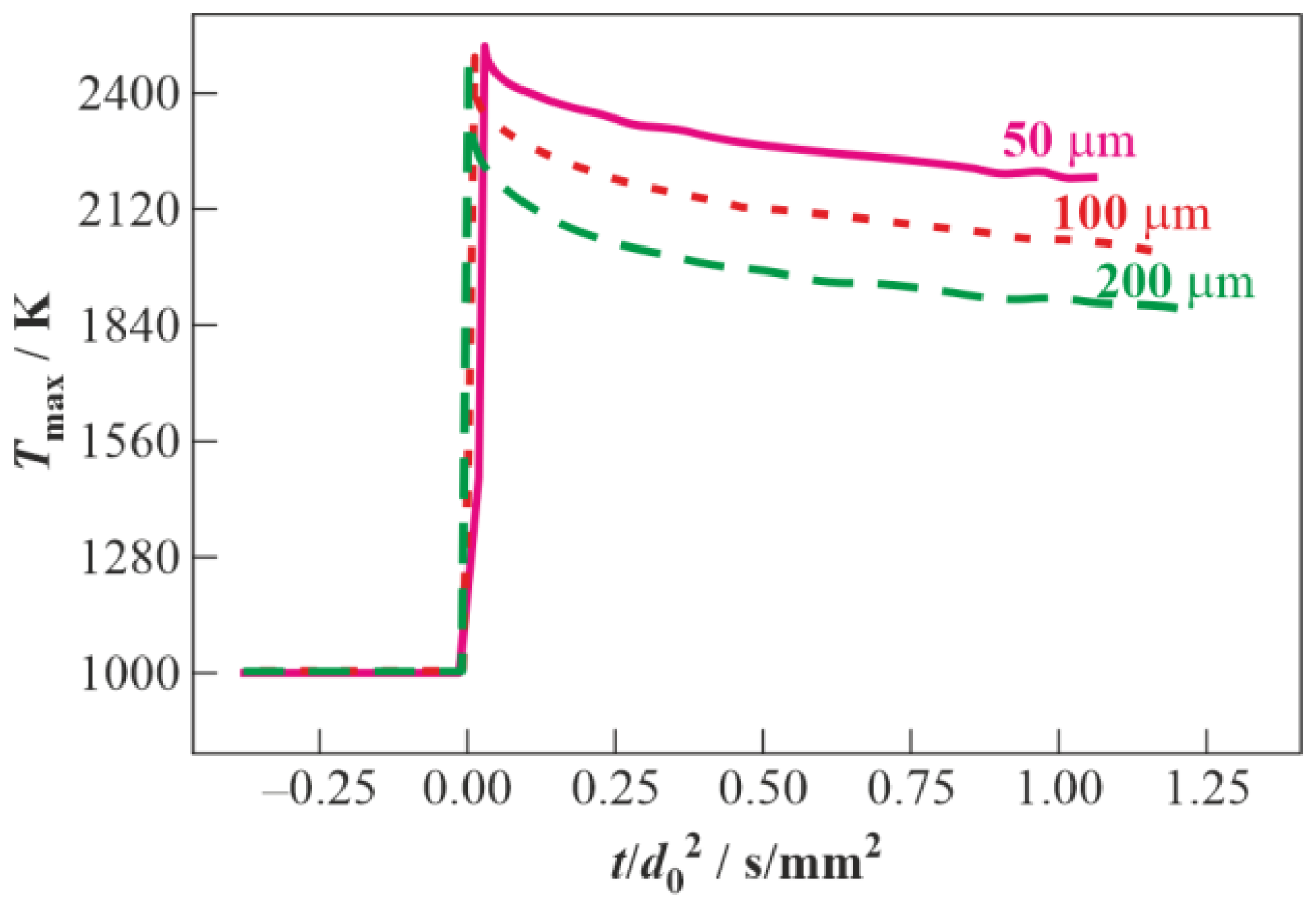
3.2. Droplet Autoignition
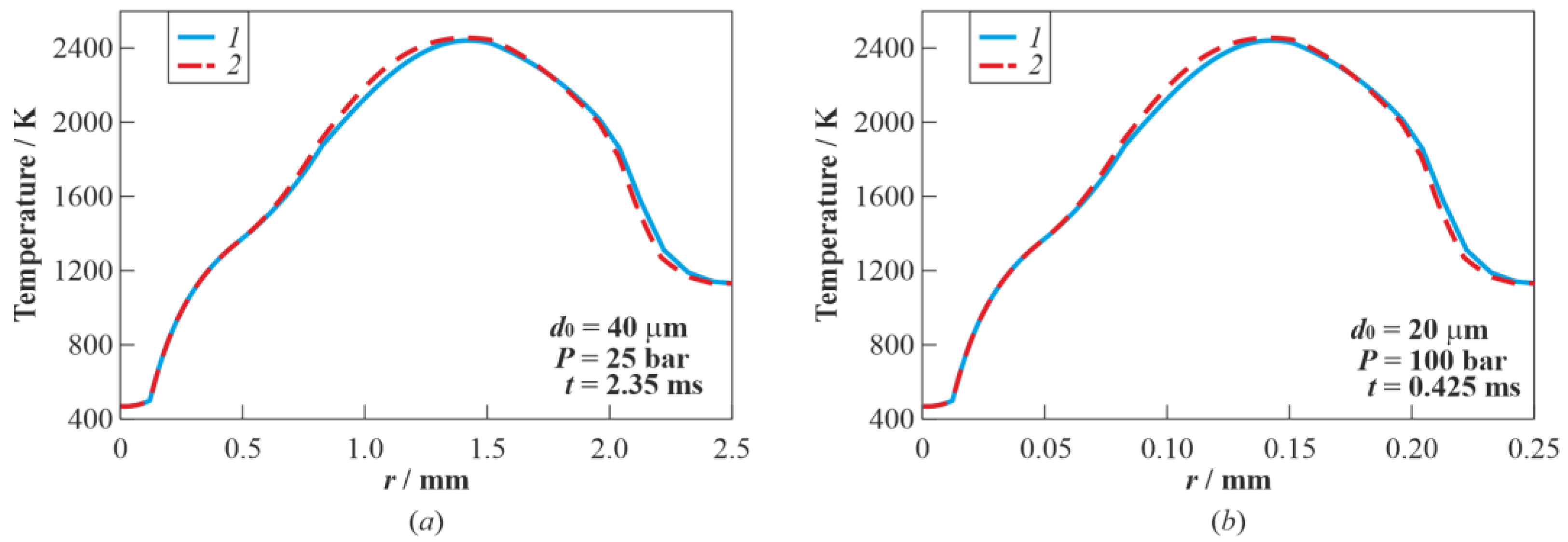
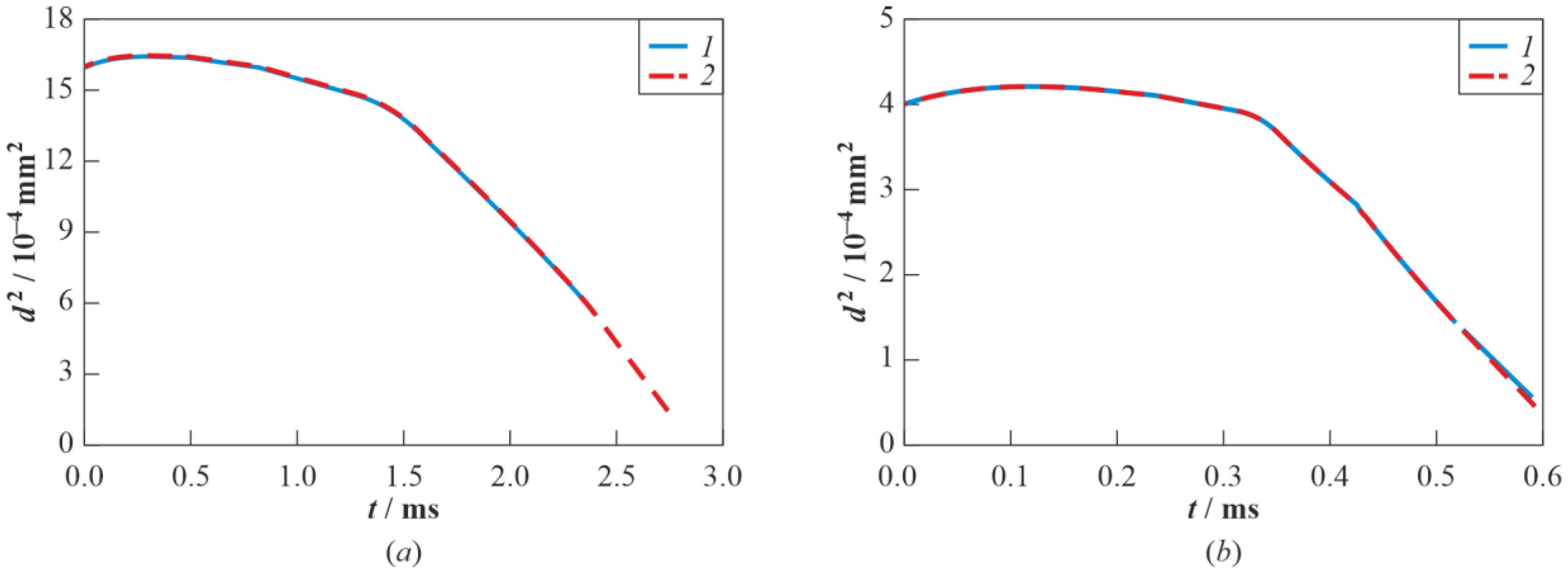
3.3. Real Gas Effects
4. Conclusions
- (1)
- reduces the maximum pressure and mass-averaged temperature in the combustion chamber by about 7 bar (6%) and 150 K (9%), respectively;
- (2)
- increases the autoignition delay time by a 1.6 crank angle degree;
- (3)
- increases the maximum heat release rate by 20%; and
- (4)
- reduces the yields of NO and soot by a factor of 2 and 4, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 0D | Zero-dimensional |
| 1D | One-dimensional |
| 3D | Three-dimensional |
| CAD | Crank angle degree |
| CFD | Computational fluid dynamics |
| CIE | Compression ignition engines |
| CV | Compensation volume |
| DKM | Detailed kinetic mechanism |
| EoS | Equation of state |
| OM | Overall mechanism |
| RANS | Reynolds-averaged Navier–Stokes |
| SIMPLE | Semi-implicit method for pressure linked equations |
| TDC | Top dead center |
| TVD | Total variation diminishing |
References
- Conway, G.; Joshi, A.; Leach, F.; García, A.; Senecal, P.K. A review of current and future powertrain technologies and trends in 2020. Transport. Eng. 2021, 5, 100080. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Kumar, D.; Sharma, N.; Sonawane, U.L. (Eds.) Engine Modeling and Simulation; Energy, Environment, and Sustainability Series; Springer Nature Singapore Pte Ltd.: Singapore, 2022. [Google Scholar] [CrossRef]
- Frolov, S.M.; Sergeev, S.S.; Basevich, V.Y.; Frolov, F.S.; Basara, B.; Priesching, P. Simulation of multistage autoignition in diesel engine based on the detailed reaction mechanism of fuel oxidation. In Advances in Engine and Powertrain Research and Technology. Mechanisms and Machine Science; Parikyan, T., Ed.; Springer: Cham, Switzerland, 2022; Volume 114, pp. 149–165. [Google Scholar] [CrossRef]
- Downs, D.; Street, J.C.; Wheeler, R.W. Cool flame formation in a motored engine. Fuel 1953, 32, 279–295. [Google Scholar]
- Sokolik, A.S. Autoignition, Flame, and Detonation in Gases; AN SSSR Publs.: Moscow, Russia, 1960. [Google Scholar]
- Basevich, V.Y.; Vedeneev, V.I. Kinetic interpretation of self-ignition of paraffin hydrocarbons. Khim. Fiz. 1998, 17, 73–80. [Google Scholar]
- Distaso, E.; Amirante, R.; Calò, G.; De Palma, P.; Tamburrano, P.; Reitz, R.D. Investigation of lubricant oil influence on ignition of gasoline-like fuels by a detailed reaction mechanism. Energy Procedia 2018, 148, 663–670. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Westbrook, C.K.; Mehl, M.; Pitz, W.J.; Togbe, C.; Dagaut, P. Comprehensive chemical kinetic modeling of the oxidation of 2-methylalkanes from C 7 to C 20. Combust. Flame 2011, 158, 2338–2357. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Reitz, R.D. Development of a reduced primary reference fuel mechanism for internal combustion engine combustion simulations. Energy Fuels 2013, 27, 7843–7853. [Google Scholar] [CrossRef]
- Distaso, E.; Amirante, R.; Calò, G.; De Palma, P.; Tamburrano, P.; Reitz, R.D. Predicting lubricant oil induced pre-ignition phenomena in modern gasoline engines: The reduced GasLube reaction mechanism. Fuel 2020, 281, 118709. [Google Scholar] [CrossRef]
- Hwang, W.; Dec, J.; Sjöberg, M. Spectroscopic and chemical-kinetic analysis of the phases of HCCI autoignition and combustion for single- and two-stage ignition fuels. Combust. Flame 2008, 154, 387–409. [Google Scholar] [CrossRef]
- Waqas, M.U.; Hoth, A.; Kolodziej, C.P.; Rockstroh, T.; Gonzalez, J.P.; Johansson, B. Detection of low temperature heat release (LTHR) in the standard cooperative fuel research (CFR) engine in both SI and HCCI combustion modes. Fuel 2019, 256, 115745. [Google Scholar] [CrossRef]
- Singh, E.; Sarathy, S.M. The role of intermediate-temperature heat release in octane sensitivity of fuels with matching research octane number. Energy Fuels 2021, 35, 4457–4477. [Google Scholar] [CrossRef]
- Yoo, K.H.; Voice, A.K.; Boehman, A.L. Influence of intermediate temperature heat release on autoignition reactivity of single-stage ignition fuels with varying octane sensitivity. Proc. Combust. Inst. 2021, 38, 5529–5538. [Google Scholar] [CrossRef]
- Peterson, J.; Mohammed, A.; Gorbatenko, I.; Singh, E.; Sarathy, S.M. The contribution of intermediate-temperature heat release to octane sensitivity. Fuel 2023, 352, 129077. [Google Scholar] [CrossRef]
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577. [Google Scholar] [CrossRef]
- Yu, L.; Qiu, Y.; Mao, Y.; Wang, S.; Ruan, C.; Tao, W.; Qian, Y.; Lu, X. A study on the low-to-intermediate temperature ignition delays of long chain branched paraffin: Iso-cetane. Proc. Combust. Inst. 2019, 37, 631–638. [Google Scholar] [CrossRef]
- Lebedev, A.B.; Sekundov, A.N.; Savel’ev, A.M.; Starik, A.M.; Titova, N.S. Numerical analysis of the influence of the operation mode of aviation gas turbine engine on the pollutant emission. In Nonequilibrium Physicochemical Processes in Gas Flows and New Principles of Combustion Organization; Starik, A.M., Ed.; Torus Press: Moscow, Russia, 2011; pp. 755–771. [Google Scholar]
- Titova, N.S.; Torokhov, S.A.; Starik, A.M. Specific features of ignition and combustion of heavy hydrocarbons and cool-flame phenomena. In Nonequilibrium Physicochemical Processes in Gas Flows and New Principles of Combustion Organization; Starik, A.M., Ed.; Torus Press: Moscow, Russia, 2011; pp. 88–110. [Google Scholar]
- Basevich, V.Y.; Belyaev, A.A.; Medvedev, S.N.; Posvyanskii, V.S.; Frolov, F.S.; Frolov, S.M. A detailed kinetic mechanism of multistage oxidation and combustion of isooctane. Rus. J. Phys. Chem. B 2016, 10, 801–809. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.H. Soot formation in laminar counterflow flames. Progr. Energy Combust. Sci. 2019, 74, 152–238. [Google Scholar] [CrossRef]
- Frenklach, M.; Mebel, A.M. On the mechanism of soot nucleation. Phys. Chem. Chem. Phys. 2020, 22, 5314–5331. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez–Fernández, J.; Sánchez-Valdepeñas, J. Soot reactivity analysis and implications on diesel filter regeneration. Progr. Energy Combust. Sci. 2020, 78, 100833. [Google Scholar] [CrossRef]
- Gleason, K.; Carbone, F.; Sumner, A.J.; Drollette, B.D.; Plata, D.L.; Gomez, A. Small aromatic hydrocarbons control the onset of soot nucleation. Combust. Flame 2021, 223, 398–406. [Google Scholar] [CrossRef]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot inception: Carbonaceous nanoparticle formation in flames. Progr. Energy Combust. Sci. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Avedisian, C.T. Recent advances in soot formation from spherical droplet flames at atmospheric pressure. J. Prop. Power 2000, 16, 628–635. [Google Scholar] [CrossRef]
- Abdul Rasid, A.F.; Zhang, Y. Comparison of the burning of a single diesel droplet with volume and surface contamination of soot particles. Proc. Combust. Inst. 2021, 38, 3159–3166. [Google Scholar] [CrossRef]
- Kumar, A.; Chen, H.-W.; Yang, S. Diffusion and its effects on soot production in the combustion of emulsified and nonemulsified fuel droplets. Energy 2023, 267, 126521. [Google Scholar] [CrossRef]
- Shaw, B.; Dryer, F.; Williams, F.; Haggard, J. Sooting and disruption in spherically symmetrical combustion of decane droplets in air. Acta Astronaut. 1988, 17, 1195–1202. [Google Scholar] [CrossRef]
- Nayagam, V.; Haggard, J.B.; Colantonio, R.O.; Marchese, A.J.; Dryer, F.L.; Zhang, B.L.; Williams, F.A. Microgravity n-heptane droplet combustion in oxygen-helium mixtures at atmospheric pressure. AIAA J. 1998, 36, 1369–1378. [Google Scholar] [CrossRef]
- Liu, Y.C.; Xu, Y.; Avedisian, C.T.; Hicks, M.C. The effect of support fibers on micro-convection in droplet combustion experiments. Proc. Combust. Inst. 2015, 35, 1709–1716. [Google Scholar] [CrossRef]
- Nayagam, V.; Dietrich, D.L.; Hicks, M.C.; Williams, F.A. Radiative extinction of large n-alkane droplets in oxygen-inert mixtures in microgravity. Combust. Flame 2018, 194, 107–114. [Google Scholar] [CrossRef]
- Frolov, S.M.; Basevich, V.Y.; Medvedev, S.N. Modeling of low-temperature oxidation and combustion of droplets. Dokl. Phys. Chem. 2016, 470, 150–153. [Google Scholar] [CrossRef]
- Frolov, S.M.; Basevich, V.Y. Simulation of low-temperature oxidation and combustion of n-dodecane droplets under microgravity conditions. Fire 2023, 6, 70. [Google Scholar] [CrossRef]
- Kaario, O.; Nuutinen, M.; Lehto, K.; Larmi, M. Real gas effects in high-pressure engine environment. SAE Intern. J. Engines 2010, 3, 546–555. Available online: https://www.jstor.org/stable/10.2307/26275498 (accessed on 22 August 2023). [CrossRef]
- Yue, Z.; Hessel, R.; Reitz, R.D. Investigation of real gas effects on combustion and emissions in internal combustion engines and implications for development of chemical kinetics mechanisms. Int. J. Engine Res. 2017, 19, 269–281. [Google Scholar] [CrossRef]
- Zheng, C.; Coombs, D.M.; Akih-Kumgeh, B. Real gas model parameters for high-density combustion from chemical kinetic model data. ACS Omega 2019, 4, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Oefelein, J.; Lacaze, G.; Dahms, R.; Ruiz, A.; Misdariis, A. Effects of real-fluid thermodynamics on high-pressure fuel injection processes. SAE Int. J. Engines 2014, 7, 1125–1136. [Google Scholar] [CrossRef]
- Perini, F.; Busch, S.; Reitz, R. An investigation of real-gas and multiphase effects on multicomponent diesel sprays. SAE Int. J. Adv. Curr. Prac. Mobil. 2020, 2, 1774–1785. [Google Scholar] [CrossRef]
- Ihme, M.; Ma, P.C.; Bravo, L. Large eddy simulations of diesel-fuel injection and auto-ignition at transcritical conditions. Intern. J. Engine Res. 2019, 20, 58–68. [Google Scholar] [CrossRef]
- Kogekar, G.; Karakaya, C.; Liskovich, G.J.; Oehlschlaeger, M.A.; DeCaluwe, S.C.; Kee, R.J. Impact of non-ideal behavior on ignition delay and chemical kinetics in high-pressure shock tube reactors. Combust. Flame 2018, 189, 1–11. [Google Scholar] [CrossRef]
- Span, R. Multiparameter Equations of State; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Detailed Kinetic Mechanism of Isooctane. Available online: http://ru.combex.org/lab1313.htm (accessed on 4 August 2023).
- Basevich, V.Y.; Belyaev, A.A.; Posvyanskii, V.S.; Frolov, S.M. Mechanisms of the oxidation and combustion of normal paraffin hydrocarbons: Transition from C1–C10 to C11–C16. Russ. J. Phys. Chem. B 2013, 7, 161–169. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Sherwood, T.K. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1977. [Google Scholar]
- Fieweger, K.; Blumenthal, R.; Adomeit, G. Shock-tube investigations on the self-ignition of hydrocarbon-air mixtures at high pressures. Proc. Combust. Symp. 1994, 25, 1579–1585. [Google Scholar] [CrossRef]
- Ciezki, H.K.; Adomeit, G. Shock-tube investigation of self-ignition of n-heptane-air mixtures under engine relevant conditions. Combust. Flame 1993, 93, 421–433. [Google Scholar] [CrossRef]
- Frolov, S.M.; Avdeev, K.A.; Ivanov, V.S.; Vlasov, P.A.; Frolov, F.S.; Semenov, I.V.; Belotserkovskaya, M.S. Evolution of the soot-particle size distribution function in the cylinder and exhaust system of piston engines: Simulation. Atmosphere 2023, 14, 13. [Google Scholar] [CrossRef]
- Massoli, P.; Lazzaro, M.; Beretta, F.; D’Alessio, A. Characterization of hydrocarbon droplets heating in a drop tube furnace. In Instituto Motori, C.N.R. Report on Research Activities and Facilities; Di Lorenzo, A., Ed.; Institute for Research on Engines: Napoli, Italy, 1993; pp. 36–37. [Google Scholar]
- Avedisian, C.T.; Yang, J.C.; Wang, C.H. On low-gravity droplet combustion. Proc. R. Soc. A 1988, 420, 183–200. [Google Scholar] [CrossRef]
- Okajima, S.; Kumagai, S. Further investigations of combustion of free droplets in a freely falling chamber including moving droplets. Proc. Combust. Inst. 1975, 15, 401–407. [Google Scholar] [CrossRef]
- Hara, H.; Kumagai, S. The effect of initial diameter of free droplet combustion with spherical flame. Proc. Combust. Inst. 1994, 25, 423–430. [Google Scholar] [CrossRef]
- Mikami, M.; Kato, H.; Sato, J.; Kono, M. Interactive combustion of two droplets in microgravity. Proc. Combust. Inst. 1994, 25, 431–438. [Google Scholar] [CrossRef]
- Kumagai, S.; Sakai, T.; Okajima, S. Combustion of free fuel droplets in a freely falling chamber. Proc. Combust. Inst. 1971, 13, 779–785. [Google Scholar] [CrossRef]
- Jackson, G.S.; Avedisian, C.T.; Yang, J.C. Observations of soot during droplet combustion at low gravity: Heptane and heptane/monochloroalkane mixtures. Int. J. Heat Mass Transf. 1992, 35, 2017–2033. [Google Scholar] [CrossRef]
- Monaghan, M.T.; Siddall, R.G.; Thring, M.W. The influence of initial diameter on the combustion of single drops of liquid fuel. Combust. Flame 1968, 12, 45–53. [Google Scholar] [CrossRef]
- Jackson, G.S.; Avedisian, C.T. The effect of initial diameter in spherically symmetric droplet combustion of sooting fuels. Proc. R. Soc. Lond. A 1994, 446, 255–276. [Google Scholar]
- Tanabe, M.; Bolik, T.; Eigenbrod, C.; Rath, H.J.; Sato, J.; Kono, M. Spontaneous ignition of liquid droplets from a view of non-homogeneous mixture formation and transient chemical reactions. Proc. Combust. Inst. 1996, 26, 1637–1643. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Moriue, O.; Coordes, T.; Eigenbrod, C.; Rath Zarm, H.J. Detailed numerical simulations of the multistage self-ignition process of n-heptane, isolated droplets and their verification by comparison with microgravity experiments. Proc. Combust. Inst. 2000, 28, 953–960. [Google Scholar] [CrossRef]
- Frolov, S.M.; Kuznetsov, N.M.; Krueger, C. Real-gas properties of n-alkanes, O2, N2, H2O, CO, CO2, and H2 for Diesel engine operation conditions. Rus. J. Phys. Chemi. B 2009, 3, 1191–1252. [Google Scholar] [CrossRef]
- Grigor’ev, B.A.; Rastorguev, Y.L.; Gerasimov, A.A. Thermodynamic Properties of Normal Hexane; Standard Publ.: Moscow, Russia, 1990. [Google Scholar]
- Sychev, V.V.; Vasserman, A.A.; Kozlov, A.D.; Spiridonov, G.A.; Tsymarnyi, V.A. Thermodynamic Properties of Oxygen; Standard Publ.: Moscow, Russia, 1981. [Google Scholar]
- Jacobsen, R.T.; Stewart, R.B.; Jahangiri, M. Thermodynamic properties of nitrogen from the freezing line to 2000 K at pressures to 1000 MPa. J. Phys. Chem. Ref. Data 1986, 15, 735. [Google Scholar] [CrossRef]
- Vukalovich, M.P.; Rivkin, S.L.; Alexandrov, A.A. Tables of Thermodynamic Properties of Water and Water Vapor; Standard Publ.: Moscow, Russia, 1969. [Google Scholar]
- Goodvin, R.D. Carbon monoxide thermophysical properties from 68 to 1000 K at pressures to 100 MPa. J. Phys. Chem. Ref. Data 1985, 14, 849. [Google Scholar] [CrossRef]
- Vukalovich, M.P.; Altunin, V.V. Thermophysical Properties of Carbon Dioxide; Atomizdat Publ.: Moscow, Russia, 1965. [Google Scholar]
- Vargaftik, N.B. Handbook on Thermophysical Properties of Gases and Liquids; Nauka Publ.: Moscow, Russia, 1972. [Google Scholar]
- CHEMKIN-PRO Release 15083 Tutorial Manual. Reaction Design, San Diego (CA). 2009. Available online: https://www.ansys.com/products/fluids/ansys-chemkin-pro (accessed on 22 August 2023).
- Frolov, S.M.; Ivanov, V.S.; Frolov, F.S.; Vlasov, P.A.; Axelbaum, R.; Irace, P.H.; Yablonsky, G.; Waddell, K. Soot formation in spherical diffusion flames. Mathematics 2023, 11, 261. [Google Scholar] [CrossRef]
- Agafonov, G.L.; Bilera, I.V.; Vlasov, P.A.; Kolbanovskii, Y.A.; Smirnov, V.N.; Teresa, A.M. Soot formation at pyrolysis and oxidation of acetylene and ethylene in shock tubes. Kinet. Catal. 2015, 56, 12–30. [Google Scholar] [CrossRef]
- AVL FIRE—Computational Fluid Dynamics for Conventional and Alternative Powertrain Development. Available online: https://www.avl.com/fire (accessed on 4 August 2023).
- Hanjalic, K.; Popovac, M.; Hadziabdic, M. A robust near-wall elliptic relaxation eddy-viscosity turbulence model for CFD. Int. J. Heat Fluid Flow 2004, 25, 897–901. [Google Scholar] [CrossRef]
- B’eard, P.; Colin, O.; Miche, M. Improved modelling of DI diesel engines using sub-grid descriptions of spray and combustion. In SAE Paper; No. 2003-01-0008; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Schiller, L.; Naumann, M. A drag coefficient correlation. VDI Zeitung 1935, 77, 318–320. [Google Scholar]
- Dukowicz, J.K. A particle–fluid numerical model for liquid sprays. J. Comput. Phys. 1980, 35, 229–253. [Google Scholar] [CrossRef]
- Liu, A.B.; Reitz, R.D. Modeling the effects of drop drag and breakup on fuel sprays. In SAE Paper; No. 930072; SAE International: Warrendale, PA, USA, 1993. [Google Scholar]
- Tao, F.; Liu, Y.; RempelEwert, B.A.; Foster, D.E.; Reitz, R.D.; Choi, D.; Miles, P.C. Modeling the effects of EGR and injection pressure on soot formation in a high-speed direct-injection (HSDI) diesel engine using a multi-step phenomenological soot model. In SAE Paper 2005-01-0121; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Zel’dovich, Y.B.; Sadovnikov, P.Y.; Frank-Kametentskii, D.A. Oxidation of Nitrogen under Combustion; Moscow–Leningrad: USSR Acad. Sci. Publ.: Moscow, Russia, 1947. [Google Scholar]
- Magnussen, B.F.; Hjertager, B.H. On mathematical modeling of turbulent combustion with special emphasis on soot formation and combustion. Proc. Combust. Ins. 1977, 16, 719–729. [Google Scholar] [CrossRef]
- Ferziger, J.H.; Peric, M. Computational Methods for Fluid Dynamics; Springer-Verlag: New York, NY, USA, 1996. [Google Scholar]
- Sweby, P.K. High resolution schemes using flux limiters for hyperbolic conservation laws. SIAMJ. Numer. Anal. 1984, 21, 995. [Google Scholar] [CrossRef]




| n-Hexane | ||||
| , K | , mol/dm3 | , MPa | , MPa [63] | , % |
| 530 | 2.526 | 4.010 | 4 | 0.25 |
| 550 | 1.619 | 4.032 | 4 | 0.79 |
| 600 | 1.106 | 4.008 | 4 | 0.19 |
| 630 | 0.9741 | 4.0025 | 4 | 0.06 |
| Oxygen | ||||
| , K | , kg/m3 | , MPa | , MPa [64] | , % |
| 500 | 45.6 | 6.0008 | 6 | 0.013 |
| 500 | 60.51 | 8.0033 | 8 | 0.041 |
| 500 | 75.25 | 10.007 | 10 | 0.07 |
| 500 | 111.31 | 15.028 | 15 | 0.19 |
| 500 | 146.15 | 20.076 | 20 | 0.38 |
| Nitrogen | ||||
| , K | , mol/dm3 | , MPa | , MPa [65] | , % |
| 500 | 0.94635 | 4.0007 | 4 | 0.018 |
| 500 | 1.4070 | 6.0011 | 6 | 0.018 |
| 500 | 1.8590 | 8.0018 | 8 | 0.023 |
| 500 | 2.3020 | 10.002 | 10 | 0.022 |
| 500 | 3.3700 | 15.004 | 15 | 0.027 |
| 500 | 4.3806 | 20.004 | 20 | 0.022 |
| Water | ||||
| , °C | , dm3/g | , MPa | , MPa [66] | , % |
| 300 | 5.885 | 4.0066 | 4 | 0.17 |
| 300 | 4.532 | 5.017 | 5 | 0.34 |
| 300 | 3.616 | 6.033 | 6 | 0.55 |
| 300 | 2.976 | 7.0052 | 7 | 0.07 |
| 300 | 2.425 | 8.090 | 8 | 1.1 |
| Carbon monoxide | ||||
| , K | , mol/dm3 | , MPa | , MPa [67] | , % |
| 500 | 0.94818 | 4.004 | 4 | 0.09 |
| 500 | 1.40962 | 6.002 | 6 | 0.04 |
| 500 | 1.86196 | 7.999 | 8 | 0.01 |
| 500 | 2.30518 | 9.994 | 10 | 0.06 |
| 500 | 2.73932 | 11.990 | 12 | 0.08 |
| 500 | 3.16448 | 13.988 | 14 | 0.09 |
| 500 | 3.58073 | 15.989 | 16 | 0.07 |
| 500 | 3.98813 | 17.994 | 18 | 0.03 |
| 500 | 4.38673 | 20.004 | 20 | 0.02 |
| Carbon dioxide | ||||
| , °C | , g/cm3 | , MPa | , MPa [68] | , % |
| 300 | 56.42 | 6.000 | 6 | 0.00 |
| 300 | 75.59 | 8.000 | 8 | 0.01 |
| 300 | 94.89 | 10.000 | 10 | 0.00 |
| 300 | 114.26 | 12.000 | 12 | 0.00 |
| 300 | 133.67 | 14.001 | 14 | 0.01 |
| 300 | 153.09 | 16.006 | 16 | 0.04 |
| 300 | 172.4 | 18.009 | 18 | 0.05 |
| 300 | 191.6 | 20.014 | 20 | 0.07 |
| Hydrogen | ||||
| , K | , mol/dm3 | , MPa | , MPa [69] | , % |
| 500 | 0.94818 | 3.998 | 4 | 0.05 |
| 500 | 1.40962 | 5.994 | 6 | 0.10 |
| 500 | 1.86196 | 7.990 | 8 | 0.13 |
| 500 | 2.30518 | 9.986 | 10 | 0.14 |
| 500 | 2.73932 | 14.990 | 15 | 0.07 |
| 500 | 3.16448 | 20.03 | 20 | 0.15 |
| Reaction | , (L, mol, s) | , K | |
|---|---|---|---|
| C2H2 + C2H2 = C + C + C2H4 | 2 × 1016 | 40,000 | 0 |
| C + CO2 = CO + CO | 1 × 1015 | 40,000 | 0 |
| C + H2O = H2 + CO | 1 × 1015 | 40,000 | 0 |
| C + OH = HCO | 1 × 1012 | 0 | 0 |
| Parameter | Value |
|---|---|
| Rotation speed, rpm | 2000 |
| Cylinder radius, mm | 42.5 |
| Compression ratio | 16 |
| Start of injection, CAD ** | 715.78 |
| End of injection, CAD | 730.06 |
| Injection angle, deg. | 150 |
| Mass of injected fuel, kg | 2.8 × 10−5 |
| Fuel temperature, K | 330.15 |
| Mass fraction of exhaust gases | 0.233 |
| Equivalence ratio in exhaust gases | 0.5606 |
| Flow swirl, 1/min | 5800 |
| Reaction Type | , K | , K | |
|---|---|---|---|
| No apparent reaction | 12.00 | <786 | <703 |
| Single cool flames | 13.00 | 808 | 703–838 |
| Double cool flames | 13.50 | 816 | 838–882 |
| Blue flames | 13.75 | 820 | 882–914 |
| Hot flames | 14.00 | >826 | >914 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basevich, V.Y.; Frolov, S.M.; Ivanov, V.S.; Frolov, F.S.; Semenov, I.V. The Effects of Multistage Fuel-Oxidation Chemistry, Soot Radiation, and Real Gas Properties on the Operation Process of Compression Ignition Engines. Eng 2023, 4, 2682-2710. https://doi.org/10.3390/eng4040153
Basevich VY, Frolov SM, Ivanov VS, Frolov FS, Semenov IV. The Effects of Multistage Fuel-Oxidation Chemistry, Soot Radiation, and Real Gas Properties on the Operation Process of Compression Ignition Engines. Eng. 2023; 4(4):2682-2710. https://doi.org/10.3390/eng4040153
Chicago/Turabian StyleBasevich, Valentin Y., Sergey M. Frolov, Vladislav S. Ivanov, Fedor S. Frolov, and Ilya V. Semenov. 2023. "The Effects of Multistage Fuel-Oxidation Chemistry, Soot Radiation, and Real Gas Properties on the Operation Process of Compression Ignition Engines" Eng 4, no. 4: 2682-2710. https://doi.org/10.3390/eng4040153





