Freshwater Invertebrate Assemblage Composition and Water Quality Assessment of an Urban Coastal Watershed in the Context of Land-Use Land-Cover and Reach-Scale Physical Habitat
Abstract
:1. Introduction
2. Materials and Methods
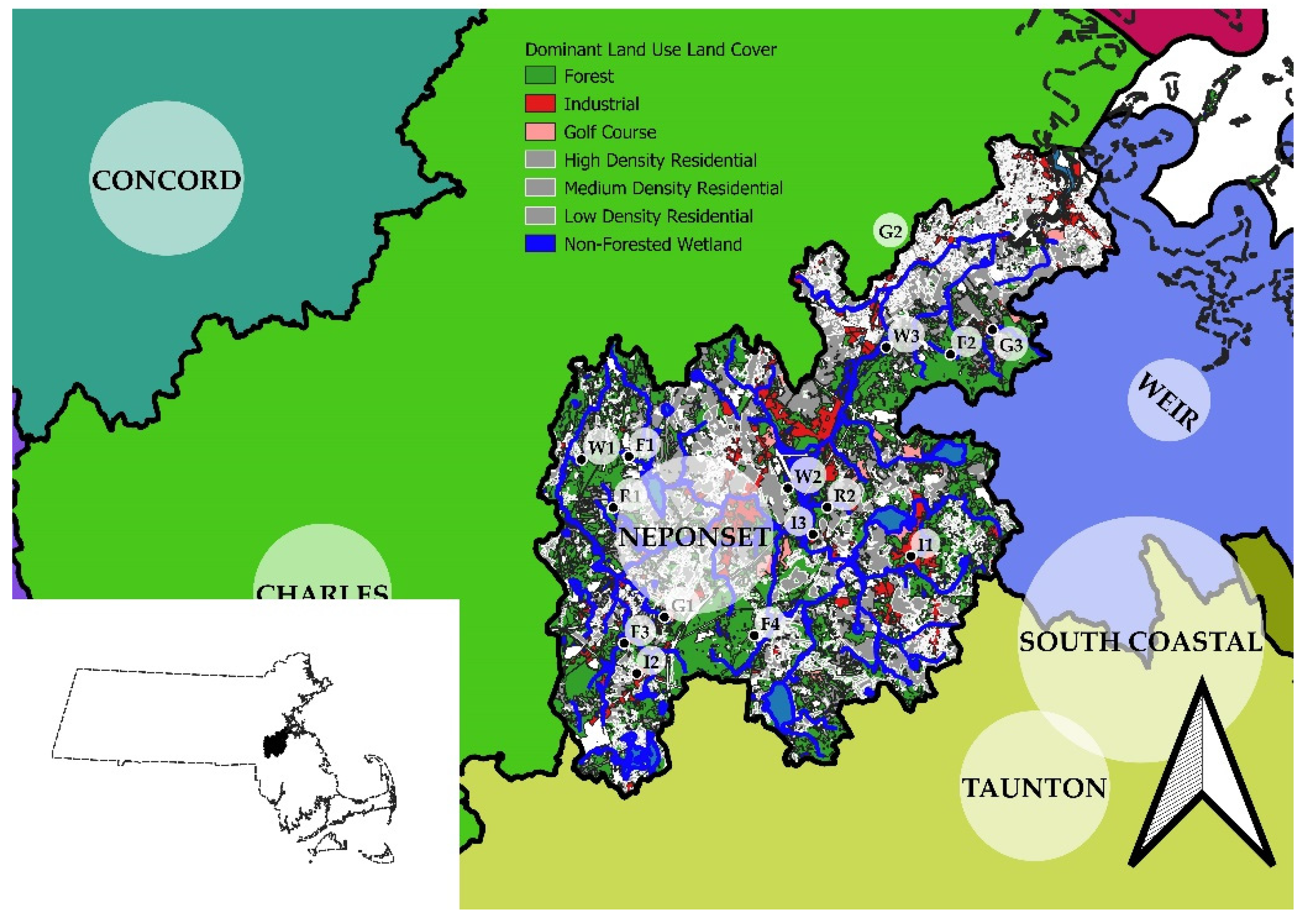
2.1. Study Area and Sampling Reaches
2.2. Physical Habitat Analyses: USEPA Rapid Bioassessment and Basin Area Stream Survey
2.3. FWI Assemblage Assessment
2.4. Statistical Analysis
3. Results
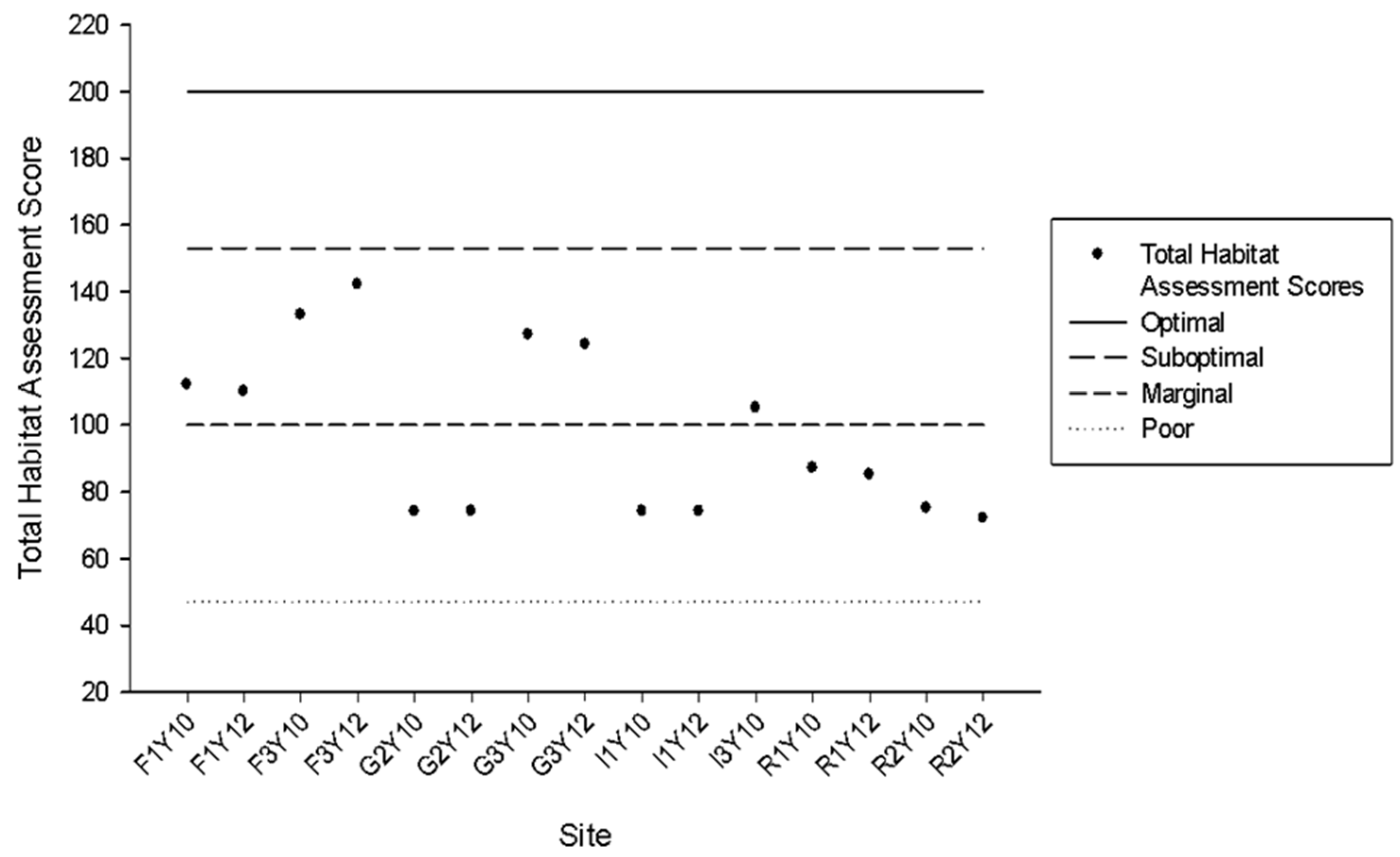
3.1. Habitat Assessment
3.2. Habitat Characterization
3.3. FWI Assemblage Diversity
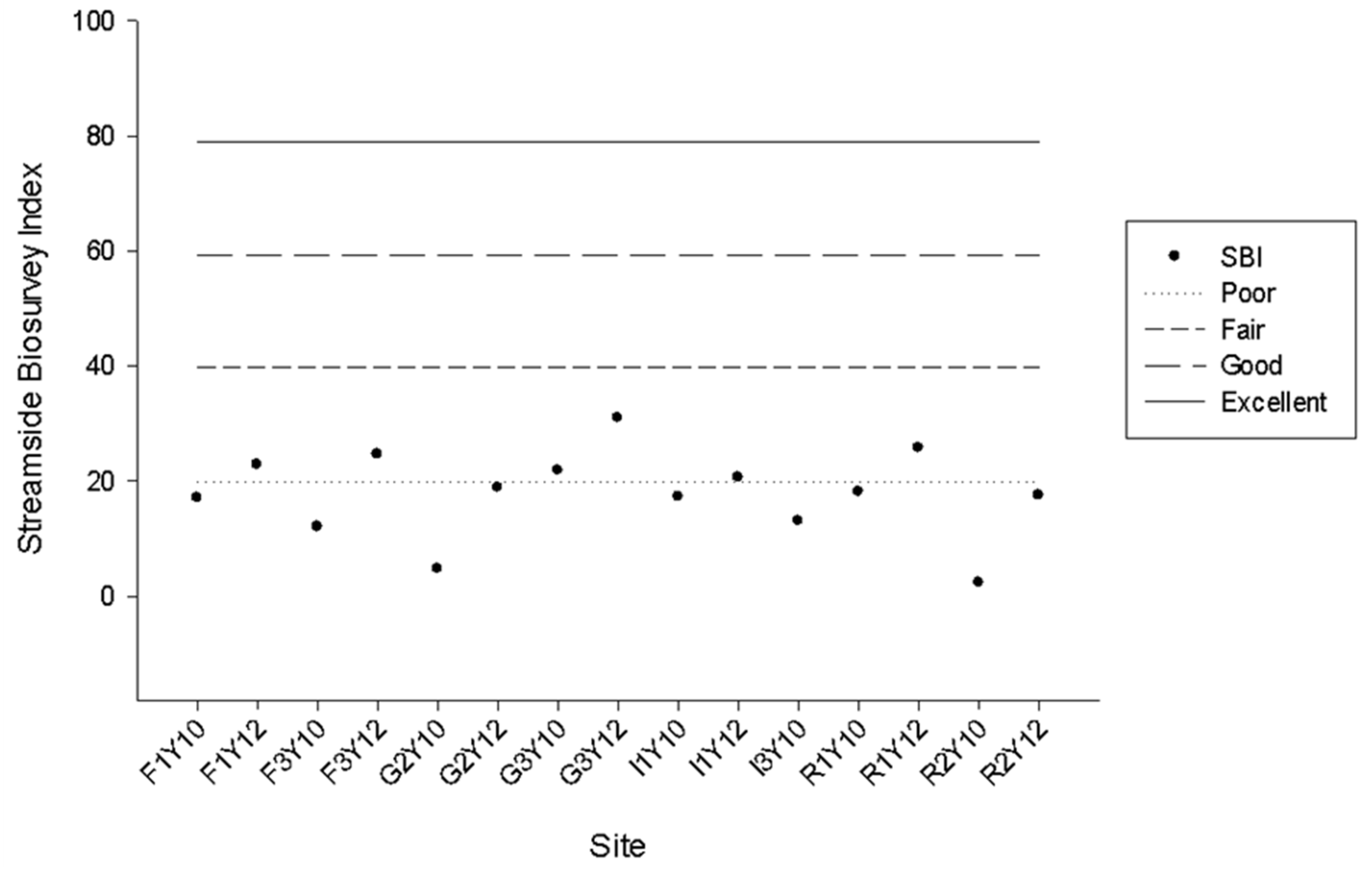
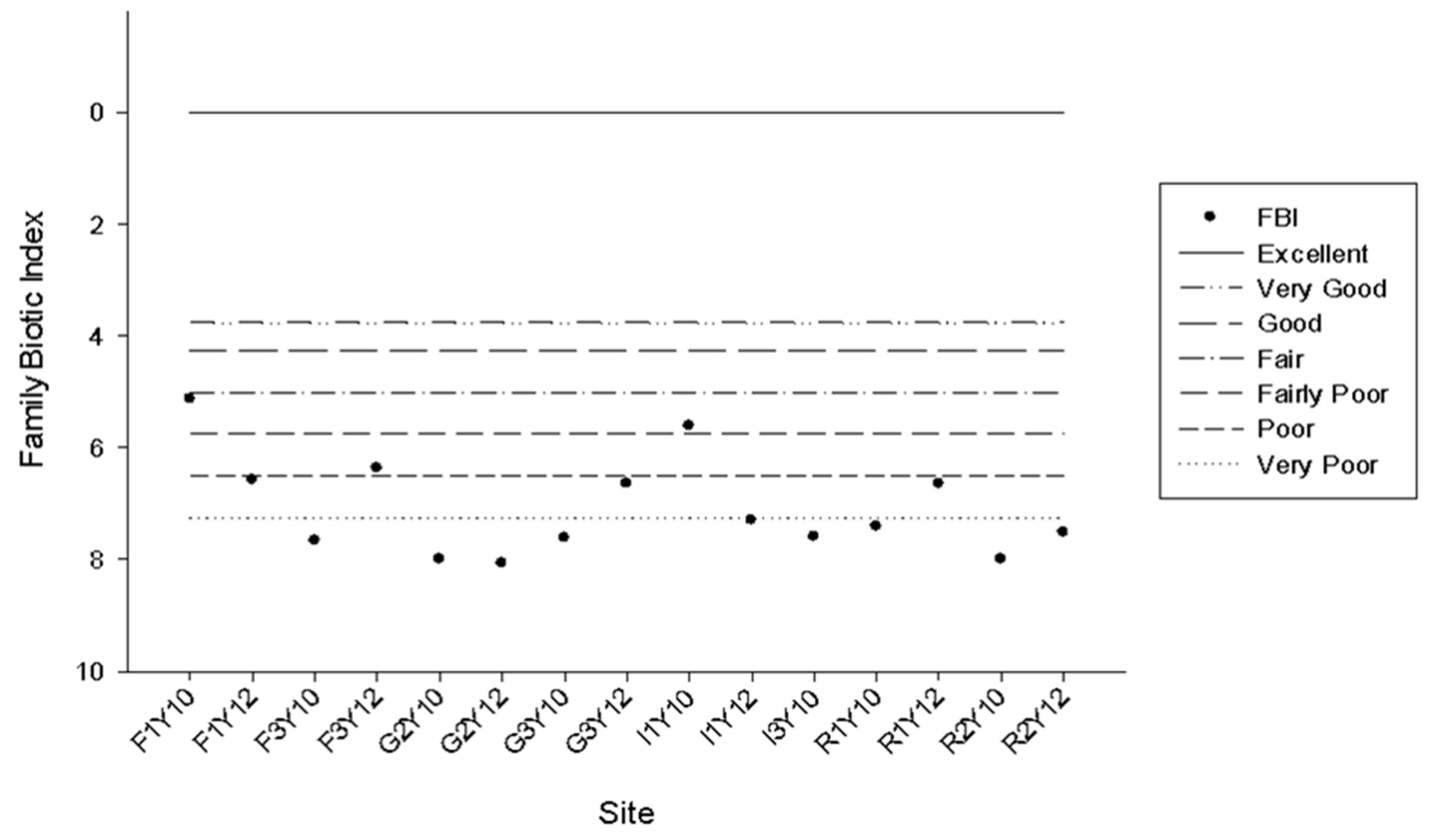
3.4. FWI Water Quality Assessment
3.5. Habitat Quality versus FWI Water Quality Scores
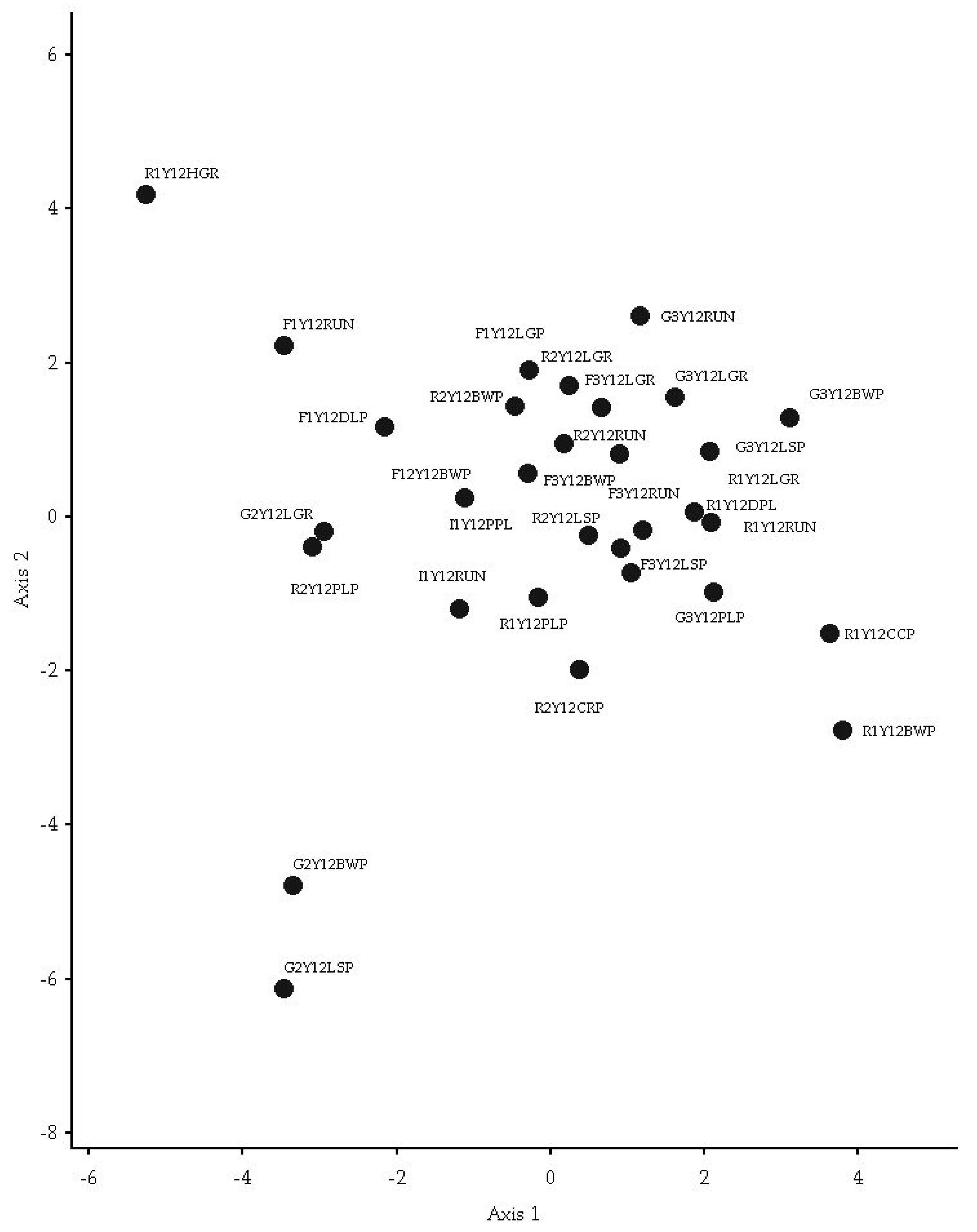
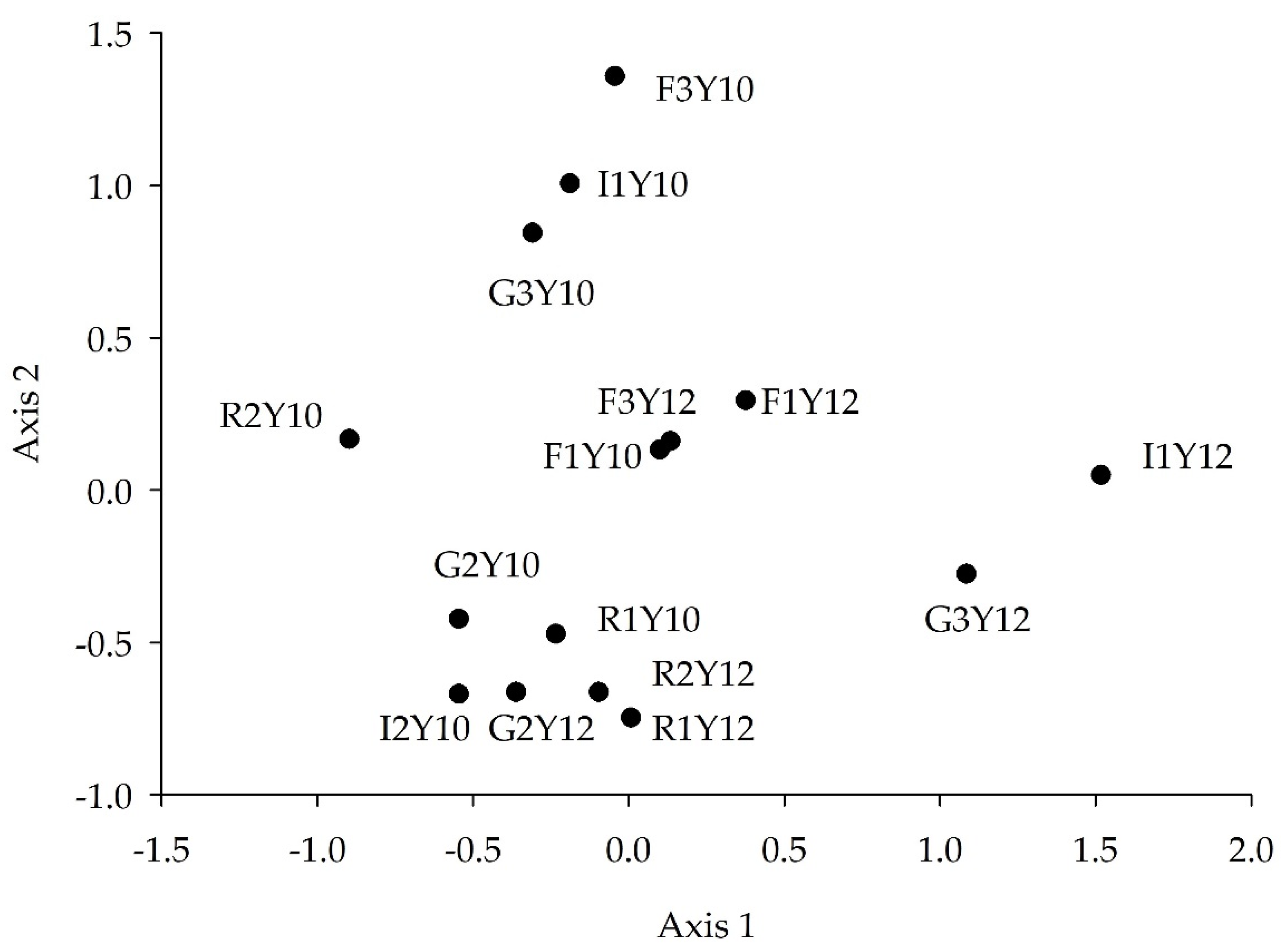
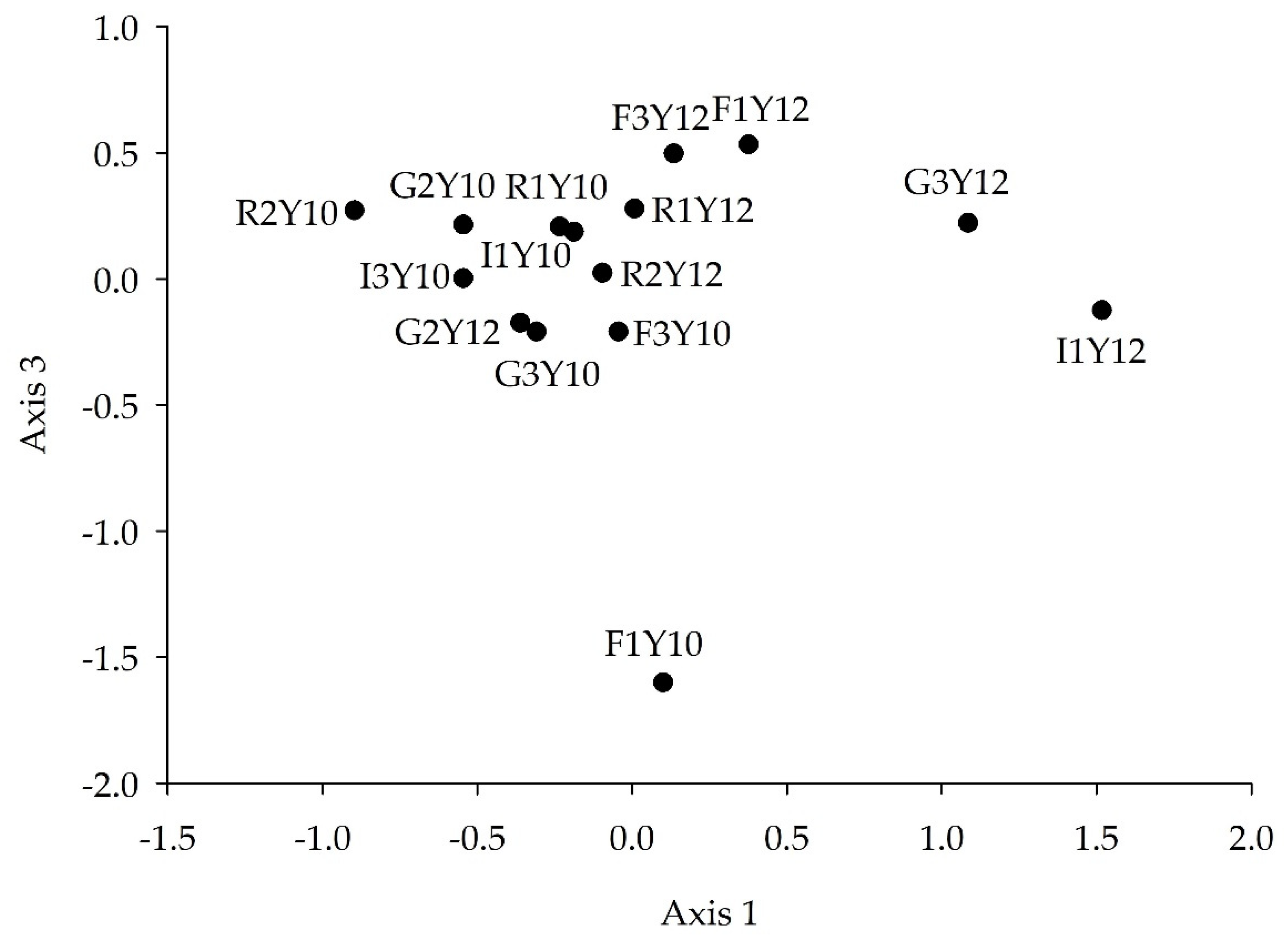
3.6. FWI Assemblage Structure Analysis
3.6.1. Axis 1 and 2
3.6.2. Axis 1 and 3
3.6.3. Axis 2 and 3
4. Discussion
4.1. Habitat Quality
4.2. Habitat Characterization
4.3. FWI Water Quality Assessment
4.4. Habitat Quality versus FWI Water Quality
4.5. FWI Assemblage Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Acronym | Term | Acronym | Term |
|---|---|---|---|
| LGR | Low gradient riffle | ICEMB | Instream-cover Embeddedness |
| HGR | High gradient riffle | ICUCB | Instream-cover undercut banks |
| BWP | Back-water pool | ICLWD | Instream-cover large woody debris |
| PLP | Plunge pool | ICSWD | Instream-cover small woody debris |
| LGP | Log lateral scour pool | ICWW | Instream-cover whitewater |
| DLP | Dammed pool | ICBO | Instream-cover boulder |
| RUN | Run | ICBRL | Instream-cover bedrock ledge |
| CCP | Channel confluence pool | ICCLN | Instream-cover clinging vegetation |
| CRP | Corner pool | ICRT | Instream-cover rooted vegetation |
| LSP | Lateral scour pool | LFTBK | Left bank angle |
| LEN | Macrohabitat length | LFTS | Left bank stability |
| BFW | Bankfull width | LFTVV | Left bank terrestrial vegetation |
| WAWID | Water width | MIDC | Mid-channel canopy |
| THAL | Thalweg depth | RGTBK | Right bank angle |
| DEPLS | Depth left bank | RGTS | Right bank stability |
| DEP25 | Depth ¼ width from left bank | RGTVV | Right bank terrestrial vegetation |
| DEP 50 | Depth ½ width from left bank | F | Forest |
| DEP 75 | Depth ¾ width from left bank | G | Golf |
| DEPRS | Depth right bank | I | Industrial |
| BTBo | % bottom boulders | R | Residential |
| BTMC | % bottom cobble | Y10 | 2010 |
| BTMG | % bottom gravel | Y12 | 2012 |
| BTMS | % bottom sand | ||
| BTMF | % bottom fines |
References
- Malmqvist, B.; Rundle, S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002, 29, 134–153. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Merriam-Webster, Incorporated. In Habitat; Merriam-Webster, Inc.: Springfield, MA, USA, 2022; Available online: https://www.merriam-webster.com/dictionary/habitat (accessed on 14 August 2022).
- Southwood, T.R.E. Tactics, Strategies and Templets. Oikos 1988, 52, 3–18. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Habitat, the Templet for Ecological Strategies? J. Anim. Ecol. 1977, 46, 337–365. [Google Scholar] [CrossRef]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Maddock, I.; Harby, A.; Kemp, P.; Wood, P.J. Ecohydraulics: An Integrated Approach; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Maddock, I. The importance of physical habitat assessment for evaluating river health. Freshw. Biol. 1999, 41, 373–391. [Google Scholar] [CrossRef]
- Raven, P.J.; Holmes, N.T.H.; Dawson, F.H.; Fox, P.J.A.; Everard, M.; Fozzard, I.R.; Rouen, K.J. River Habitat Quality: The Physical Character of Rivers and Streams in the UK and Isle of Man; Environment Agency: Bristol, UK, 1998. [Google Scholar]
- Nelson, G.C. Drivers of Ecosystem Change: Summary Chapter. In Ecosystems and Human Well-being: Current State and Trends; Island Press: Washington, DC, USA, 2005; Volume 1, pp. 73–76. [Google Scholar]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Karr, J.R. Biological Integrity: A Long-Neglected Aspect of Water Resource Management. Ecol. Appl. 1991, 1, 66–84. [Google Scholar] [CrossRef]
- DeShon, J.E. Development and Application of the Invertebrate Community Index (ICI). In Biological Assessment and Criteria: Tools for Risk-Based Decision Making and Planning; Davis, W.S., Simon, T., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 217–243. [Google Scholar]
- Roth, N.E.; Allan, J.D.; Erickson, D.L. Landscape Influences on Stream Biotic Integrity Assessed at Multiple Spatial Scales. Landsc. Ecol. 1996, 11, 141–156. [Google Scholar] [CrossRef]
- Allan, J.D.; Brenner, A.J.; Erazo, J.; Fernandez, L.; Flecker, A.S.; Karwan, D.L.; Segnini, S.; Taphorn, D.C. Land Use in Watersheds of the Venezuelan Andes: A Comparative Analysis. Conserv. Biol. 2002, 16, 527–538. [Google Scholar] [CrossRef]
- Maloney, K.O.; Weller, D. Anthropogenic disturbance and streams: Land use and land-use change affect stream ecosystems via multiple pathways. Freshw. Biol. 2010, 56, 611–626. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The Urban Stream Syn-drome: Current Knowledge and the Search for a Cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Roy, A.H.; Capps, K.A.; El-Sabaawi, R.W.; Jones, K.L.; Parr, T.B.; Ramírez, A.; Smith, R.F.; Walsh, C.J.; Wenger, S.J. Urbanization and stream ecology: Diverse mechanisms of change. Freshw. Sci. 2016, 35, 272–277. [Google Scholar] [CrossRef]
- Kilonzo, F.; Masese, F.O.; Van Griensven, A.; Bauwens, W.; Obando, J.; Lens, P.N. Spatial–temporal variability in water quality and macro-invertebrate assemblages in the Upper Mara River basin, Kenya. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 93–104. [Google Scholar] [CrossRef]
- Parr, L.; Mason, C. Long-term trends in water quality and their impact on macroinvertebrate assemblages in eutrophic lowland rivers. Water Res. 2003, 37, 2969–2979. [Google Scholar] [CrossRef]
- Sharma, K.K.; Chowdhary, S. Macroinvertebrate Assemblages as Biological Indicators of Pollution in a Central Himalayan River, Tawi (J&K). Int. J. Biodivers. Conserv. 2011, 3, 167–174. [Google Scholar]
- Brito, J.G.; Martins, R.T.; Oliveira, V.C.; Hamada, N.; Nessimian, J.; Hughes, R.M.; Ferraz, S.; de Paula, F.R. Biological indicators of diversity in tropical streams: Congruence in the similarity of invertebrate assemblages. Ecol. Indic. 2018, 85, 85–92. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish; EPA 841-B-99-002, 2nd ed.; U.S. Environmental Protection Agency: Office of Water: Washington, DC, USA, 1999; Volume EPA 841-B-99-002.
- Hilsenhoff, W.L. Using a Biotic Index to Evaluate Water Quality in Streams; Report; Wisconsin Department of Natural Resources: Madison, Wisconsin, 1982. [Google Scholar]
- Hilsenhoff, W.L. Rapid Field Assessment of Organic Pollution with a Family-Level Biotic Index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of Biological Integrity Using Fish Communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Rankin, E.T.; Yoder, C.O. Methods for Assessing Habitat in Flowing Waters Using the Qualitative Habitat Evaluation Index (QHEI); Report; Ecological Assessment Unit, Division of Surface Water, Ohio EPA: Columbus, OH, USA, 1999. [Google Scholar]
- United States Environmental Protection Agency. Volunteer Stream Monitoring: A Methods Manual; 4503F EPA 841-B-97-003; Office of Water, Environmental Protection Agency: Washington, DC, USA, 1997; p. 227.
- Huang, W.; Chen, R.F. Sources and transformations of chromophoric dissolved organic matter in the Neponset River Watershed. J. Geophys. Res. Earth Surf. 2009, 114. [Google Scholar] [CrossRef]
- McCain, M.E.; Fuller, D.; Decker, L.; Overton, K. Stream Habitat Classification and Inventory Procedures for Northern California. Fish Habitat Relatsh. Tech. Bull. 1990, 1, 1–15. [Google Scholar]
- Clingenpeel, J.A.; Cochran, B.G. Using Physical, Chemical, and Biological Indicators to Assess Water Quality on the Ouachita National Forest Utilizing Basin Area Stream Survey Methods. Proc. Ark. Acad. Sci. 1992, 46, 33–35. [Google Scholar]
- Merritt, R.W.; Cummins, K.S. An Introduction to the Aquatic Insects of North America, 3rd ed.; Kendall/Hunt Publishing Company: Dubuque, Iowa, 1996. [Google Scholar]
- Rogers, D.C.; Hill, M. Key to the Freshwater Malacostraca (Crustacea) of the Mid-Atlantic Region; US EPA, Office of Environmental Information: Washington, DC, USA, 2008; Above: The exotic rusty crayfish was the only crayfish species found in the main stem of Loyalsock Creek 2008. [Google Scholar]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates; Academic Press, Inc: San Diego, CA, USA, 1991. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America, 4th ed.; Kendall; Hunt Publishing Company: Dubuque, IA, USA, 2008. [Google Scholar]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD v. 6.255 Beta; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- Tran, C.P.; Bode, R.W.; Smith, A.J.; Kleppel, G.S. Land-use proximity as a basis for assessing stream water quality in New York State (USA). Ecol. Indic. 2010, 10, 727–733. [Google Scholar] [CrossRef]
- Allan, D.; Erickson, D.; Fay, J. The influence of catchment land use on stream integrity across multiple spatial scales. Freshw. Biol. 1997, 37, 149–161. [Google Scholar] [CrossRef]
- Strayer, D.L.; Beighley, R.E.; Thompson, L.C.; Brooks, S.; Nilsson, C.; Pinay, G.; Naiman, R.J. Effects of Land Cover on Stream Ecosystems: Roles of Empirical Models and Scaling Issues. Ecosystems 2003, 6, 407–423. [Google Scholar] [CrossRef]
- Gergel, S.E.; Turner, M.G.; Miller, J.R.; Melack, J.M.; Stanley, E.H. Landscape Indicators of Human Impacts to Riverine Systems. Aquat. Sci. 2002, 64, 118–128. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Iliopoulou-Georgudaki, J. Response of Biota to Land Use Changes and Water Quality Degra-dation in Two Medium-Sized River Basins in Southwestern Greece. Ecol. Indic. 2010, 10, 1231–1238. [Google Scholar] [CrossRef]
- Chakona, A.; Phiri, C.; Magadza, C.H.D.; Brendonck, L. The influence of habitat structure and flow permanence on macroinvertebrate assemblages in temporary rivers in northwestern Zimbabwe. Hydrobiologia 2008, 607, 199–209. [Google Scholar] [CrossRef]
- Heidkamp, L.C.; Christian, A.D. A Case Study Evaluating Water Quality and Reach-, Buffer-, and Water-shed-Scale Explanatory Variables of an Urban Coastal Watershed. Urban Sci. 2022, 6, 17. [Google Scholar] [CrossRef]
- Klauda, R.; Kazyak, P.; Stranko, S.; Southerland, M.; Roth, N.; Chaillou, J. Maryland Biological Stream Survey: A State Agency Program to Assess the Impact of Anthropogenic Stresses on Stream Habitat Quality and Biota. Environ. Monit. Assess. 1998, 51, 299–316. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P.; Gatti, R. Influences of Watershed Land Use on Habitat Quality and Biotic Integrity in Wisconsin Streams. Fisheries 1997, 22, 6–12. [Google Scholar] [CrossRef]
- Varnosfaderany, M.N.; Ebrahimi, E.; Mirghaffary, N.; Safyanian, A. Biological Assessment of the Zayandeh Rud River, Iran, Using Benthic Macroinvertebrates. Limnol. Ecol. Manag. Inland Waters 2010, 40, 226–232. [Google Scholar] [CrossRef]
- Roy, A.H.; Rosemond, A.D.; Leigh, D.S.; Paul, M.J.; Wallace, J.B. Habitat-specific responses of stream insects to land cover disturbance: Biological consequences and monitoring implications. J. N. Am. Benthol. Soc. 2003, 22, 292–307. [Google Scholar] [CrossRef]
- Nicklin, L.; Balas, M.T. Correlation between Unionid Mussel Density and EPA Habitat-assessment Parameters. Northeast. Nat. 2007, 14, 225–234. [Google Scholar] [CrossRef]
- Hannaford, M.J.; Resh, V.H. Variability in Macroinvertebrate Rapid-Bioassessment Surveys and Habitat Assess-ments in a Northern California Stream. J. N. Am. Benthol. Soc. 1995, 14, 430–439. [Google Scholar] [CrossRef]
- Stepenuck, K.F.; Crunkilton, R.L.; Wang, L. Impacts of urban landuse on macroinvertebrate communities in southeastern wisconsin streams. JAWRA J. Am. Water Resour. Assoc. 2002, 38, 1041–1051. [Google Scholar] [CrossRef]
- May, C.; Horner, R.R.; Karr, J.R.; Mar, B.W.; Welch, E.B. Effects of Urbanization on Small Streams in the Puget Sound Ecoregion. Watershed Prot. Tech. 1999, 2, 79. [Google Scholar]
- Walsh, C.J.; Sharpe, A.K.; Breen, P.F.; Sonneman, J.A. Effects of urbanization on streams of the Melbourne region, Victoria, Australia. I. Benthic macroinvertebrate communities. Freshw. Biol. 2001, 46, 535–551. [Google Scholar] [CrossRef]
- Walsh, C.J.; Waller, K.A.; Gehling, J.; MAC Nally, R. Riverine invertebrate assemblages are degraded more by catchment urbanisation than by riparian deforestation. Freshw. Biol. 2007, 52, 574–587. [Google Scholar] [CrossRef]
- Uriarte, M.; Yackulic, C.B.; Lim, Y.; Arce-Nazario, J.A. Influence of land use on water quality in a tropical landscape: A multi-scale analysis. Landsc. Ecol. 2011, 26, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Kernan, M.; Battarbee, R.W.; Moss, B.R. Climate Change Impacts on Freshwater Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hawley, R.J. Making Stream Restoration More Sustainable: A Geomorphically, Ecologically, and Socioeconomi-cally Principled Approach to Bridge the Practice with the Science. BioScience 2018, 68, 517–528. [Google Scholar] [CrossRef] [PubMed]

| Axis | Eigenvalue | % of Variance | Cum.% of Var. | Broken Stick Eigenvalue |
|---|---|---|---|---|
| 1 | 5.052 | 17.421 | 17.421 | 3.962 |
| 2 | 4.227 | 14.577 | 31.998 | 2.962 |
| 3 | 2.983 | 10.286 | 42.284 | 2.462 |
| 4 | 2.557 | 8.816 | 51.1 | 2.128 |
| 5 | 2.263 | 7.802 | 58.902 | 1.878 |
| 6 | 1.806 | 6.226 | 65.128 | 1.678 |
| 7 | 1.65 | 5.69 | 70.818 | 1.512 |
| 8 | 1.388 | 4.785 | 75.603 | 1.369 |
| 9 | 1.282 | 4.422 | 80.025 | 1.244 |
| 10 | 0.958 | 3.305 | 83.33 | 1.133 |
| Site | Mean | Std. Dev. | Sum | Richness | Evenness | Shannon’s Diversity | Simpson’s Diversity |
|---|---|---|---|---|---|---|---|
| F1Y2010 | 0.422 | 1.438 | 19 | 6 | 0.829 | 1.485 | 0.7258 |
| F1Y2012 | 0.222 | 0.636 | 10 | 6 | 0.946 | 1.696 | 0.8000 |
| F3Y2010 | 1.733 | 9.555 | 78 | 6 | 0.402 | 0.720 | 0.3176 |
| F3Y2012 | 0.822 | 2.092 | 37 | 11 | 0.855 | 2.051 | 0.8371 |
| G2Y2010 | 0.578 | 1.323 | 26 | 9 | 0.945 | 2.075 | 0.8639 |
| G2Y2012 | 3.022 | 18.169 | 136 | 7 | 0.252 | 0.490 | 0.1925 |
| G3Y2010 | 4.067 | 20.584 | 183 | 12 | 0.426 | 1.058 | 0.4211 |
| G3Y2012 | 0.6 | 3.201 | 27 | 3 | 0.573 | 0.630 | 0.3594 |
| I1Y2010 | 6.756 | 17.802 | 304 | 16 | 0.764 | 2.118 | 0.8269 |
| I1Y2012 | 6.044 | 22.983 | 272 | 11 | 0.659 | 1.581 | 0.6636 |
| I3Y2010 | 2.533 | 7.197 | 114 | 17 | 0.750 | 2.126 | 0.8024 |
| R1Y2010 | 2.956 | 9.568 | 133 | 13 | 0.660 | 1.693 | 0.7501 |
| R1Y2012 | 4.067 | 15.734 | 183 | 8 | 0.630 | 1.310 | 0.6525 |
| R2Y2010 | 13.311 | 34.651 | 599 | 19 | 0.719 | 2.116 | 0.8305 |
| R2Y2012 | 4.4 | 20.399 | 198 | 7 | 0.582 | 1.132 | 0.5108 |
| Sampling Event | EPA Habitat | SBI | FBI |
|---|---|---|---|
| F1Y2010 | 112 | 17.0 | 5.13 |
| F1Y2012 | 110 | 22.8 | 6.58 |
| F3Y2010 | 133 | 12.0 | 7.66 |
| F3Y2012 | 142 | 24.6 | 6.37 |
| G2Y2010 | 74 | 4.7 | 8.00 |
| G2Y2012 | 74 | 18.8 | 8.06 |
| G3Y2010 | 127 | 21.8 | 7.62 |
| G3Y2012 | 124 | 30.9 | 6.65 |
| I1Y2010 | 74 | 17.2 | 5.62 |
| I1Y2012 | 74 | 20.6 | 7.30 |
| I3Y2010 | 105 | 13.0 | 7.60 |
| R1Y2010 | 87 | 18.1 | 7.41 |
| R1Y2012 | 85 | 25.7 | 6.65 |
| R2Y2010 | 75 | 2.3 | 8.00 |
| R2Y2012 | 72 | 17.5 | 7.52 |
| F1Y10 | F3Y10 | G2Y10 | G3Y10 | I1Y10 | I3Y10 | R1Y10 | R2Y10 | R1Y12 | R2Y12 | F1Y12 | F3Y12 | G2Y12 | G3Y12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F3Y10 | 0.929 | |||||||||||||
| G2Y10 | 0.979 | 0.93 | ||||||||||||
| G3Y10 | 0.926 | 0.636 | 0.875 | |||||||||||
| I1Y10 | 0.956 | 0.543 | 0.903 | 0.541 | ||||||||||
| I3Y10 | 0.948 | 0.972 | 0.396 | 0.894 | 0.888 | |||||||||
| R1Y10 | 0.97 | 0.927 | 0.459 | 0.834 | 0.856 | 0.180 | ||||||||
| R2Y10 | 0.957 | 0.889 | 0.538 | 0.774 | 0.811 | 0.741 | 0.789 | |||||||
| R1Y12 | 0.963 | 0.981 | 0.601 | 0.917 | 0.933 | 0.517 | 0.494 | 0.849 | ||||||
| R2Y12 | 0.938 | 0.979 | 0.564 | 0.922 | 0.926 | 0.361 | 0.304 | 0.826 | 0.451 | |||||
| F1Y12 | 0.985 | 0.854 | 0.791 | 0.664 | 0.729 | 0.92 | 0.777 | 0.858 | 0.718 | 0.788 | ||||
| F3Y12 | 0.961 | 0.873 | 0.714 | 0.667 | 0.811 | 0.806 | 0.663 | 0.738 | 0.698 | 0.748 | 0.336 | |||
| G2Y12 | 0.911 | 0.969 | 0.454 | 0.927 | 0.914 | 0.384 | 0.468 | 0.8 | 0.507 | 0.367 | 0.865 | 0.829 | ||
| G3Y12 | 0.977 | 0.974 | 0.902 | 0.927 | 0.952 | 0.929 | 0.867 | 0.93 | 0.756 | 0.835 | 0.756 | 0.683 | 0.898 | |
| I1Y12 | 0.963 | 0.971 | 0.964 | 0.966 | 0.929 | 0.994 | 0.953 | 1 | 0.936 | 0.915 | 0.878 | 0.909 | 0.963 | 0.611 |
| Axis 1 | Axis 2 | Axis 3 |
|---|---|---|
| Asellidae (ASEL) | Calopterygidae (CALO) * | Ceratopogonidae (CERA) * |
| Caenidae (CAEN) | Chirinomidae (CHIR) * | Chironomidae (CHIR) * |
| Chirinomidae (CHIR) * | Elmidae (ELMI) * | Dytiscidae (DYTI) |
| Coenagionidae (COEN) | Gerridae (GERR)* | Gelastocoridae (GELA) |
| Dytiscidae (DYTI) | Halictidae (HALI) * | Hyalellidae (HYAL) |
| Elmidae (ELMI) * | Hirundinea (HIRU) * | Hydropsychidae (HYDR) * |
| Gammaridae (GAMM) | Hyalellidae (HYAL) | Odontoceridae (ODON) |
| Gomphidae (GOMP) | Hydropsychidae (HYDR) * | Polycentropodidae (POLY) |
| Halictidae (HALI) * | Oligochaeta (OLIG) * | Psephenidae (PSEP) |
| Libellulidae (LIBE) | Physidae (PHYS) * | |
| Nematoda (NEMA) * | Planoribidae (PLAN) * | |
| Olgiochaeta (OLIG) * | Psychomyiidae (PSYC) | |
| Ptychopteridae (PTYC) | Sphaeriidae (SPHA) * | |
| Sialidae (SIAL) | Tipulidae (TIPU) * | |
| Sphaeriidae (SPHA) * | ||
| Tabariidae (TABA) | ||
| Talitridae (TALI) | ||
| Turbellaria (TURB) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, N.D.; Christian, A.D. Freshwater Invertebrate Assemblage Composition and Water Quality Assessment of an Urban Coastal Watershed in the Context of Land-Use Land-Cover and Reach-Scale Physical Habitat. Ecologies 2022, 3, 376-394. https://doi.org/10.3390/ecologies3030028
Henderson ND, Christian AD. Freshwater Invertebrate Assemblage Composition and Water Quality Assessment of an Urban Coastal Watershed in the Context of Land-Use Land-Cover and Reach-Scale Physical Habitat. Ecologies. 2022; 3(3):376-394. https://doi.org/10.3390/ecologies3030028
Chicago/Turabian StyleHenderson, Nicole D., and Alan D. Christian. 2022. "Freshwater Invertebrate Assemblage Composition and Water Quality Assessment of an Urban Coastal Watershed in the Context of Land-Use Land-Cover and Reach-Scale Physical Habitat" Ecologies 3, no. 3: 376-394. https://doi.org/10.3390/ecologies3030028




