Development of a High-Accuracy Statistical Model to Identify the Key Parameter for Methane Adsorption in Metal-Organic Frameworks
Abstract
:1. Introduction
1.1. Scheme Depicting the Main Content of This Work
1.2. Motivation and the Big Picture
1.3. Significance of Our Work and the Gap in the Literature
2. Relevant Literature Background, Specific Aims, and Key Contributions of This Work
2.1. Methane Detection Technologies
2.2. Comparison of MOFs vs. Inorganic Adsorbents and Suitability of MOFs in Terms of CH4
2.3. Studies Relevant to Our Hypothesis and Objective
2.4. Evidence of the Importance of Thermodynamic Factors in Storage Performance
2.5. Key Contributions of Important Research Works Involving Computational, Parameter Estimations, Thermodynamic Approaches, and Their Drawbacks
2.6. Key Contributions of This Work
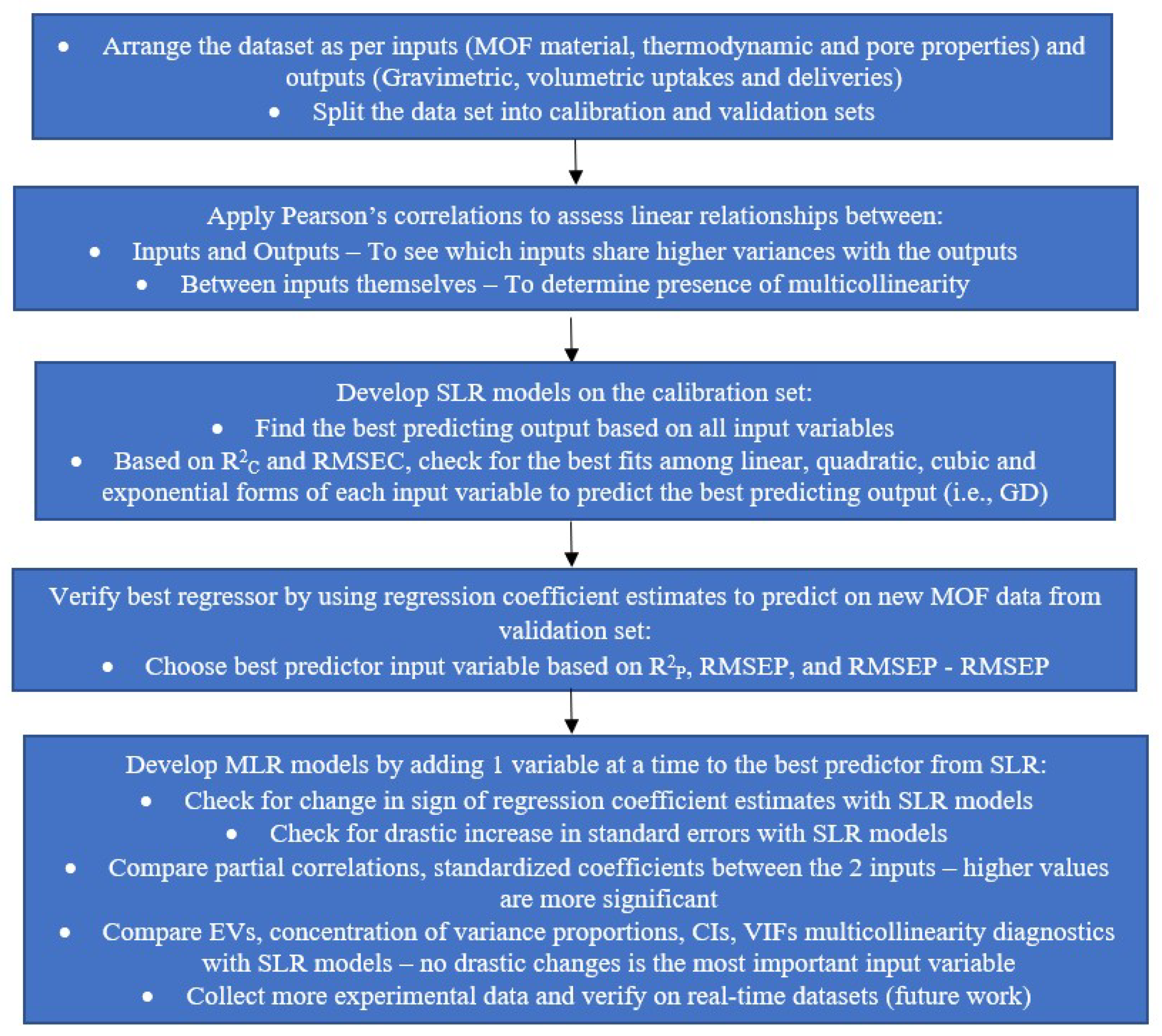
3. Methods and the Significance/Importance of Each Step to Our Objective
3.1. Data Sets and Splitting
3.2. Software Tools Used
3.3. Steps in the Statistical Approach Followed in This Work
3.3.1. Pearson’s Correlation and Its Significance
3.3.2. SLR and MLR Models—Assumptions, Regression Coefficient Estimates, and Standard Errors
3.3.3. Model Performance Evaluations and the Significance of the Overall Model
3.3.4. Statistical Analysis Procedure: Step-by-Step
- (i)
- Changes in signs or a drastic decrease in the regression coefficient estimates from the SLR to the MLR model for an input variable make it unstable in estimating the output;
- (ii)
- Increase in standard errors of the regression coefficient estimate of that variable indicates that it is rendered insignificant, compared to the other input variable;
- (iii)
- If the standardized coefficients of one variable in the MLR model are higher than that of the other variable, it signifies that the variable with a higher standardized coefficient can explain the variance in the output better and will have a higher prediction accuracy, independent of the less significant variable;
- (iv)
- Partial correlations of the inputs with the output indicate the influence of 1 variable on the output, in the presence of the other variables. The input variable with a higher partial correlation will be the more significant variable;
- (v)
- Variance inflation factors (VIFs) are defined by , where is the coefficient of determination when the variable is regressed on , which represents the set of all other explanatory variables in the model. VIF would be 1 for simple linear regression, while a higher value in the MLR model will indicate that multicollinearity is more prevalent in that particular variable, making the other variable more significant. As with Pearson’s correlation coefficient, there is the risk of a false diagnosis of multicollinearity with VIF as well, since there is no consensus on the threshold value [60]. Kutner et al. [61] suggest a minimum value of 10, while Vatcheva et al. [62] demonstrated that even a value of < 5 could be problematic. More than the absolute value, a change in VIF magnitude toward the higher side could provide evidence supporting multicollinearity, which is what is pursued in this study by comparing multiple regression models with the simple regression counterparts, as detailed in further sections in the manuscript. In addition, VIF can also be compared with to establish whether the correlation between the regressors is stronger than the overall regression model [56].
- (vi)
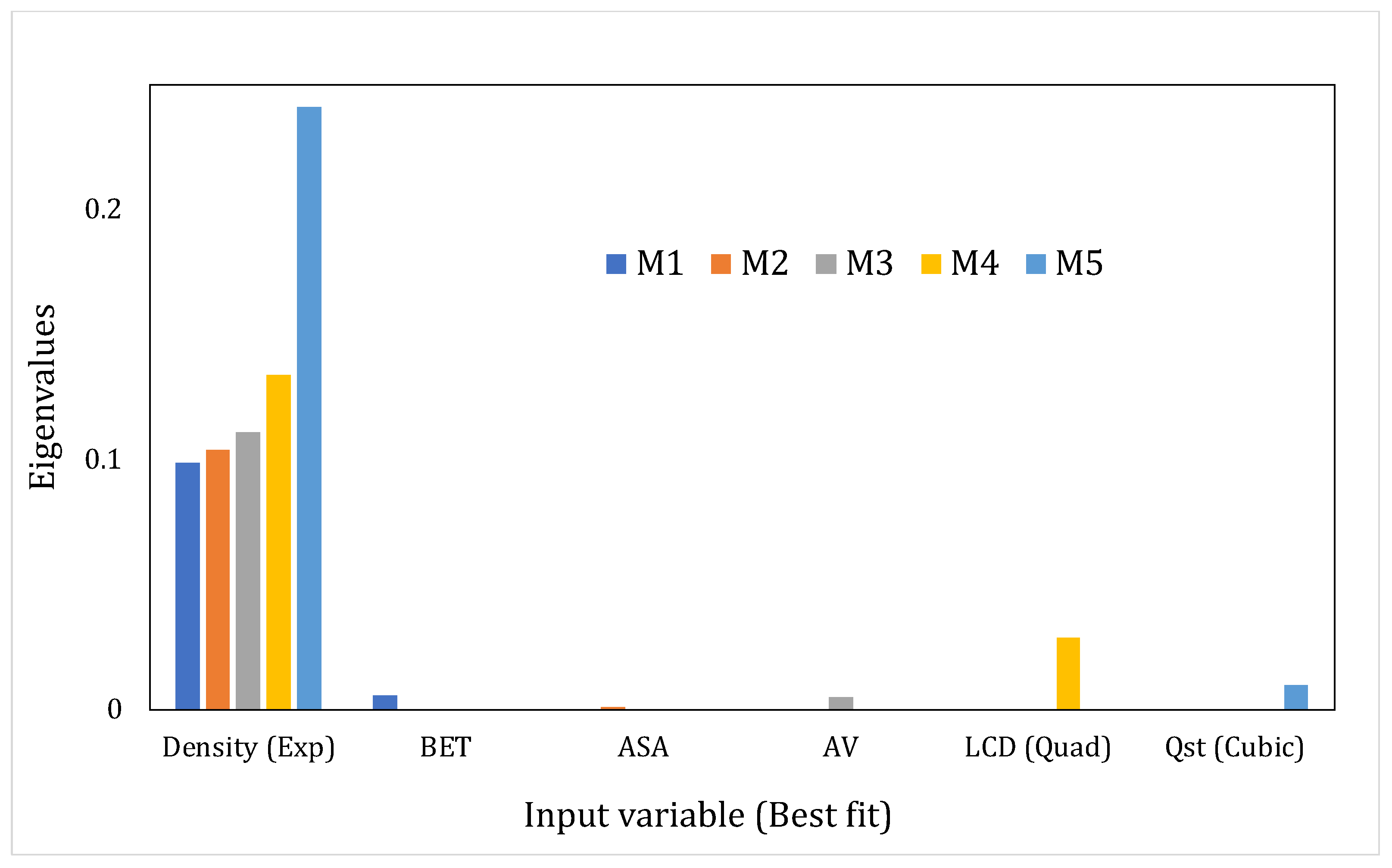
- Eigenvalues (EV) and Condition Index (CI)The sum of the eigenvalues of the correlation matrix (obtained through eigenvalue decomposition) will equal the number of explanatory variables in the system but the distribution of the eigenvalues across the dimensions of the matrix would point toward the presence or absence of linear dependencies [57]. If the variables are linearly independent, all eigenvalues will equal unity; in the case of correlated variables, certain dimensions would show eigenvalues that are close to 0. The latter situation indicates that the regression parameter estimates, when regressed using these input variables, would be very sensitive to changes in the data. The condition index (CI) helps in amplifying the unequal distribution of the eigenvalues and is given in Equation (1) as:where and are the maximum and the th eigenvalue, respectively.According to Midi et al., [58] if the falls below 15, then multicollinearity is not a serious concern. Johnston [63] proclaimed inconsequential collinearity until < 20. Furthermore, the detection process will also be assisted by the variance decomposition proportions for each predictor, i.e., the proportion of variance for the regression coefficient estimates of each input variable that belongs to every dimension. Significantly correlated variables would have higher variance proportions, concentrated on the same eigenvalue dimension. We have considered this aspect in our study as well. Another diagnostic that has been reported in the literature but that has been used on fewer occasions is the determinant of the correlation matrix, where a lower value indicates multicollinearity. However, this diagnostic is beyond the scope of our study.
4. Results and Discussion
4.1. Bivariate Pearson’s Correlations
4.1.1. Input-Output Correlations
4.1.2. Input-Input Correlations
4.2. Ascertaining the Prediction Accuracy and Best Predictor for GD from SLR Models
4.2.1. SLR Models Applied to the Calibration Set
4.2.2. SLR Models Applied to the Validation Set
4.3. Assessing the Interdependencies between the Input Variables Using the MLR Models
5. Future Extensions of Our Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derwent, R.G. Global Warming Potential (GWP) for Methane: Monte Carlo Analysis of the Uncertainties in Global Tropospheric Model Predictions. Atmosphere 2020, 11, 486. [Google Scholar] [CrossRef]
- Whittenberg, L. Methane: A Crucial Opportunity in the Climate Fight. Available online: https://www.edf.org/climate/methane-crucial-opportunity-climate-fight (accessed on 30 September 2021).
- McMillan, D. Canada Has Pledged to Reduce Methane Emissions—Here Are Some Ways to Get There. Available online: https://www.cbc.ca/news/methane-reduction-canada-1.6228361 (accessed on 21 May 2022).
- Alemu, A.W.; Pekrul, L.K.D.; Shreck, A.L.; Booker, C.W.; McGinn, S.M.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol Decreased Enteric Methane Production from Growing Beef Cattle in a Commercial Feedlot: Implications for Sustainable Beef Cattle Production. Front. Anim. Sci. 2021, 2, 641590. [Google Scholar] [CrossRef]
- McGee, M. Major Studies Reveal 60% More Methane Emissions. Available online: https://www.edf.org/climate/methane-studies (accessed on 20 June 2022).
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Leitzell, K. Climate Change Widespread, Rapid, and Intensifying. Available online: https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/ (accessed on 21 June 2022).
- Keohane, N. Carbon Removal Technologies to Help Tackle Climate Change? Here’s What It’ll Take. Available online: https://www.edf.org/blog/2019/02/12/carbon-removal-technologies-help-tackle-climate-change-heres-what-itll-take (accessed on 30 September 2021).
- Saleman, T.L.; Li, G.K.; Rufford, T.E.; Stanwix, P.L.; Chan, K.I.; Huang, S.H.; May, E.F. Capture of Low Grade Methane from Nitrogen Gas Using Dual-Reflux Pressure Swing Adsorption. Chem. Eng. J. 2015, 281, 739–748. [Google Scholar] [CrossRef]
- Fakhraei Ghazvini, M.; Vahedi, M.; Najafi Nobar, S.; Sabouri, F. Investigation of the MOF Adsorbents and the Gas Adsorptive Separation Mechanisms. J. Environ. Chem. Eng. 2021, 9, 104790. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Alcaniz-Monge, J.; De la Casa-Lillo, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Advances in the Study of Methane Storage in Porous Carbonaceous Materials. Fuel 2002, 81, 1777–1803. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Yuan, C.; Tao, Q.; Zhao, Y.; Zhang, G.; Liu, J. Influence of Temperature on Adsorption Selectivity: Coal-Based Activated Carbon for CH4 Enrichment from Coal Mine Methane. Powder Technol. 2019, 347, 42–49. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Cansado, I.P.P.; Carrott, M.M.L.R. Carbon Molecular Sieves from PET for Separations Involving CH4, CO2, O2 and N2. Appl. Surf. Sci. 2006, 252, 5948–5952. [Google Scholar] [CrossRef]
- Sridhar, P.; Kaisare, N.S. A Critical Analysis of Transport Models for Refueling of MOF-5 Based Hydrogen Adsorption System. J. Ind. Eng. Chem. 2020, 85, 170–180. [Google Scholar] [CrossRef]
- Zhou, W. Methane Storage in Porous Metal−organic Frameworks: Current Records and Future Perspectives. Chem. Rec. 2010, 10, 200–204. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane Storage in Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef]
- Okoro, H.K.; Ayika, S.O.; Ngila, J.C.; Tella, A.C. Rising Profile on the Use of Metal–Organic Frameworks (MOFs) for the Removal of Heavy Metals from the Environment: An Overview. Appl. Water Sci. 2018, 8, 169. [Google Scholar] [CrossRef]
- Ursueguía, D.; Díaz, E.; Ordóñez, S. Metal-Organic Frameworks (MOFs) as Methane Adsorbents: From Storage to Diluted Coal Mining Streams Concentration. Sci. Total Environ. 2021, 790, 148211. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E. Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks. Crystals 2020, 10, 700. [Google Scholar] [CrossRef]
- Hmiel, B.; Petrenko, V.V.; Dyonisius, N.M.; Buizert, C.; Smith, A.M.; Place, P.F.; Harth, C.; Beaudette, R.; Hua, Q.; Yang, B.; et al. Preindustrial 14CH4 Indicates Greater Anthropogenic Fossil CH4 Emissions. Nature 2020, 578, 409–412. [Google Scholar] [CrossRef]
- Coifman, J. How MethaneSAT Is Different from Other Satellites. Available online: https://www.edf.org/climate/how-methanesat-is-different (accessed on 21 June 2022).
- Menon, V.C.; Komarneni, S. Porous Adsorbents for Vehicular Natural Gas Storage: A Review. J. Porous Mater. 1998, 5, 43–58. [Google Scholar] [CrossRef]
- Gholipour, F.; Mofarahi, M. Adsorption Equilibrium of Methane and Carbon Dioxide on Zeolite 13X: Experimental and Thermodynamic Modeling. J. Supercrit. Fluids 2016, 111, 47–54. [Google Scholar] [CrossRef]
- Kim, J.; Maiti, A.; Lin, L.-C.; Stolaroff, J.K.; Smit, B.; Aines, R.D. New Materials for Methane Capture from Dilute and Medium-Concentration Sources. Nat. Commun. 2013, 4, 1694. [Google Scholar] [CrossRef]
- Kondo, M.; Yoshitomi, T.; Seki, K.; Matsuzaka, H.; Kitagawa, S. Three-Dimensional Framework with Channeling Cavities for Small Molecules. Angew. Chem. Int. Ed. 1997, 36, 1725. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Yang, L.; Su, C.; Wang, K.; Jiang, K. Preparation of Hybrid CaCO3–Pepsin Hemisphere with Ordered Hierarchical Structure and the Application for Removal of Heavy Metal Ions. J. Cryst. Growth 2012, 338, 272–279. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. High Pressure Methane Adsorption in the Metal-Organic Frameworks Cu3(Btc)2, Zn2(Bdc)2dabco, and Cr3F(H2O)2O(Bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, D.; Simmons, J.M.; Collier, C.D.; Yuan, D.; Zhou, H.-C. Metal-Organic Framework from an Anthracene Derivative Containing Nanoscopic Cages Exhibiting High Methane Uptake. J. Am. Chem. Soc. 2008, 130, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, H.; Hartman, M.R.; Yildirim, T. Hydrogen and Methane Adsorption in Metal−Organic Frameworks: A High-Pressure Volumetric Study. J. Phys. Chem. C 2007, 111, 16131–16137. [Google Scholar] [CrossRef]
- Düren, T.; Sarkisov, L.; Yaghi, O.M.; Snurr, R.Q. Design of New Materials for Methane Storage. Langmuir 2004, 20, 2683–2689. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. High-Capacity Methane Storage in Metal−Organic Frameworks M 2 (Dhtp): The Important Role of Open Metal Sites. J. Am. Chem. Soc. 2009, 131, 4995–5000. [Google Scholar] [CrossRef]
- Wu, H.; Simmons, J.M.; Liu, Y.; Brown, C.M.; Wang, X.-S.; Ma, S.; Peterson, V.K.; Southon, P.D.; Kepert, C.J.; Zhou, H.-C.; et al. Metal-Organic Frameworks with Exceptionally High Methane Uptake: Where and How Is Methane Stored? Chem. A Eur. J. 2010, 16, 5205–5214. [Google Scholar] [CrossRef]
- Mahmoud, E. Mitigating Global Methane Emissions Using Metal-Organic Framework Adsorbents. Appl. Sci. 2020, 10, 7733. [Google Scholar] [CrossRef]
- Sezginel, K.B.; Uzun, A.; Keskin, S. Multivariable Linear Models of Structural Parameters to Predict Methane Uptake in Metal–Organic Frameworks. Chem. Eng. Sci. 2015, 124, 125–134. [Google Scholar] [CrossRef]
- Hechinger, M.; Leonhard, K.; Marquardt, W. What Is Wrong with Quantitative Structure–Property Relations Models Based on Three-Dimensional Descriptors? J. Chem. Inf. Model. 2012, 52, 1984–1993. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal–Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Zhou, W.; Xu, J.Q.; Chen, B. Porous Metal-Organic Frameworks: Promising Materials for Methane Storage. Chem 2016, 1, 557–580. [Google Scholar] [CrossRef]
- Knyazeva, M.K.; Tsivadze, A.Y.; Solovtsova, O.V.; Fomkin, A.A.; Pribylov, A.A.; Shkolin, A.V.; Pulin, A.L.; Men’shchikov, I.E. Methane Adsorption on the Metal–Organic Framework Structure Al-BTC. Prot. Met. Phys. Chem. Surfaces 2019, 55, 9–14. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, Ł.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.-H.; et al. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based Soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.-S.; Hong, D.-Y.; Kyu Hwang, Y.; et al. High Uptakes of CO2 and CH4 in Mesoporous Metal—Organic Frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef]
- Wiersum, A.D.; Chang, J.-S.; Serre, C.; Llewellyn, P.L. An Adsorbent Performance Indicator as a First Step Evaluation of Novel Sorbents for Gas Separations: Application to Metal–Organic Frameworks. Langmuir 2013, 29, 3301–3309. [Google Scholar] [CrossRef]
- Vandenbrande, S.; Verstraelen, T.; Gutiérrez-Sevillano, J.J.; Waroquier, M.; Van Speybroeck, V. Methane Adsorption in Zr-Based MOFs: Comparison and Critical Evaluation of Force Fields. J. Phys. Chem. C 2017, 121, 25309–25322. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, A.; Font, X. Critical Review of Existing Nanomaterial Adsorbents to Capture Carbon Dioxide and Methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M.; et al. Methane Storage in Flexible Metal–Organic Frameworks with Intrinsic Thermal Management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef]
- Tahmooresi, M.; Sabzi, F. Sorption of Methane in a Series of Zn-Based MOFs Studied by PHSC Equation of State. Fluid Phase Equilib. 2014, 381, 83–89. [Google Scholar] [CrossRef]
- Becker, T.M.; Heinen, J.; Dubbeldam, D.; Lin, L.-C.; Vlugt, T.J.H. Polarizable Force Fields for CO 2 and CH 4 Adsorption in M-MOF-74. J. Phys. Chem. C 2017, 121, 4659–4673. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Gomez-Gualdron, D.A.; Gutov, O.V.; Krungleviciute, V.; Borah, B.; Mondloch, J.E.; Hupp, J.T.; Yildirim, T.; Farha, O.K.; Snurr, R.Q. Computational Design of Metal–Organic Frameworks Based on Stable Zirconium Building Units for Storage and Delivery of Methane. Chem. Mater. 2014, 26, 5632–5639. [Google Scholar] [CrossRef]
- Zaiontz, C. Pearson’s Correlation Table. Available online: http://www.real-statistics.com/statistics-tables/pearsons-correlation-table/ (accessed on 7 May 2018).
- Kowalski, B.R. Chemometrics; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Thode, H.C. Testing for Normality; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Adeboye, N.O.; Fagoyinbo, I.S.; Olatayo, T.O. Estimation of the Effect of Multicollinearity on the Standard Error for Regression Coefficients. J. Math. 2014, 10, 16–20. [Google Scholar] [CrossRef]
- Daoud, J.I. Multicollinearity and Regression Analysis. J. Phys. Conf. Ser. 2017, 949, 012009. [Google Scholar] [CrossRef]
- Freund, R.J.; Wilson, W.J. Regression Analysis: Statistical Modeling of a Response Variable; Academic Press: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]
- Midi, H.; Sarkar, S.K.; Rana, S. Collinearity Diagnostics of Binary Logistic Regression Model. J. Interdiscip. Math. 2010, 13, 253–267. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Sivaramakrishnan, K.; Wu, Y.; de Klerk, A.; Nazemifard, N. A Statistical Approach Dealing with Multicollinearity among Predictors in Microfluidic Reactor Operation to Control Liquid-Phase Oxidation Selectivity. React. Chem. Eng. 2018, 3, 972–990. [Google Scholar] [CrossRef]
- O’Brien, R.M. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Kutner, M.; Nachtsheim, C.; Neter, J. Applied Linear Statistical Models; McGraw-Hill: Irwin, CA, USA, 2004. [Google Scholar]
- Vatcheva, K.P.; MinJae, L.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology 2016, 6, 227. [Google Scholar] [CrossRef]
- Johnston, J. Econometric Methods, 3rd ed.; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Nematollahzadeh, A.; Abdekhodaie, M.J.; Shojaei, A. Submicron Nanoporous Polyacrylamide Beads with Tunable Size for Verapamil Imprinting. J. Appl. Polym. Sci. 2012, 125, 189–199. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of Tetracycline by NaOH-Activated Carbon Produced from Macadamia Nut Shells: Kinetic and Equilibrium Studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Haldoupis, E.; Nair, S.; Sholl, D.S. Pore Size Analysis of >250 000 Hypothetical Zeolites. Phys. Chem. Chem. Phys. 2011, 13, 5053. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-H.; Lyu, Q.; Lin, L.-C.; Kang, D.-Y. Transport-Relevant Pore Limiting Diameter for Molecular Separations in Metal–Organic Framework Membranes. J. Phys. Chem. C 2021, 125, 20416–20425. [Google Scholar] [CrossRef]
- Miklitz, M.; Jelfs, K.E. Pywindow: Automated Structural Analysis of Molecular Pores. J. Chem. Inf. Model. 2018, 58, 2387–2391. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; Pascal, T.A.; Goddard, W.A. Design of Covalent Organic Frameworks for Methane Storage. J. Phys. Chem. 2011, 115, 13852–13857. [Google Scholar] [CrossRef] [PubMed] [Green Version]



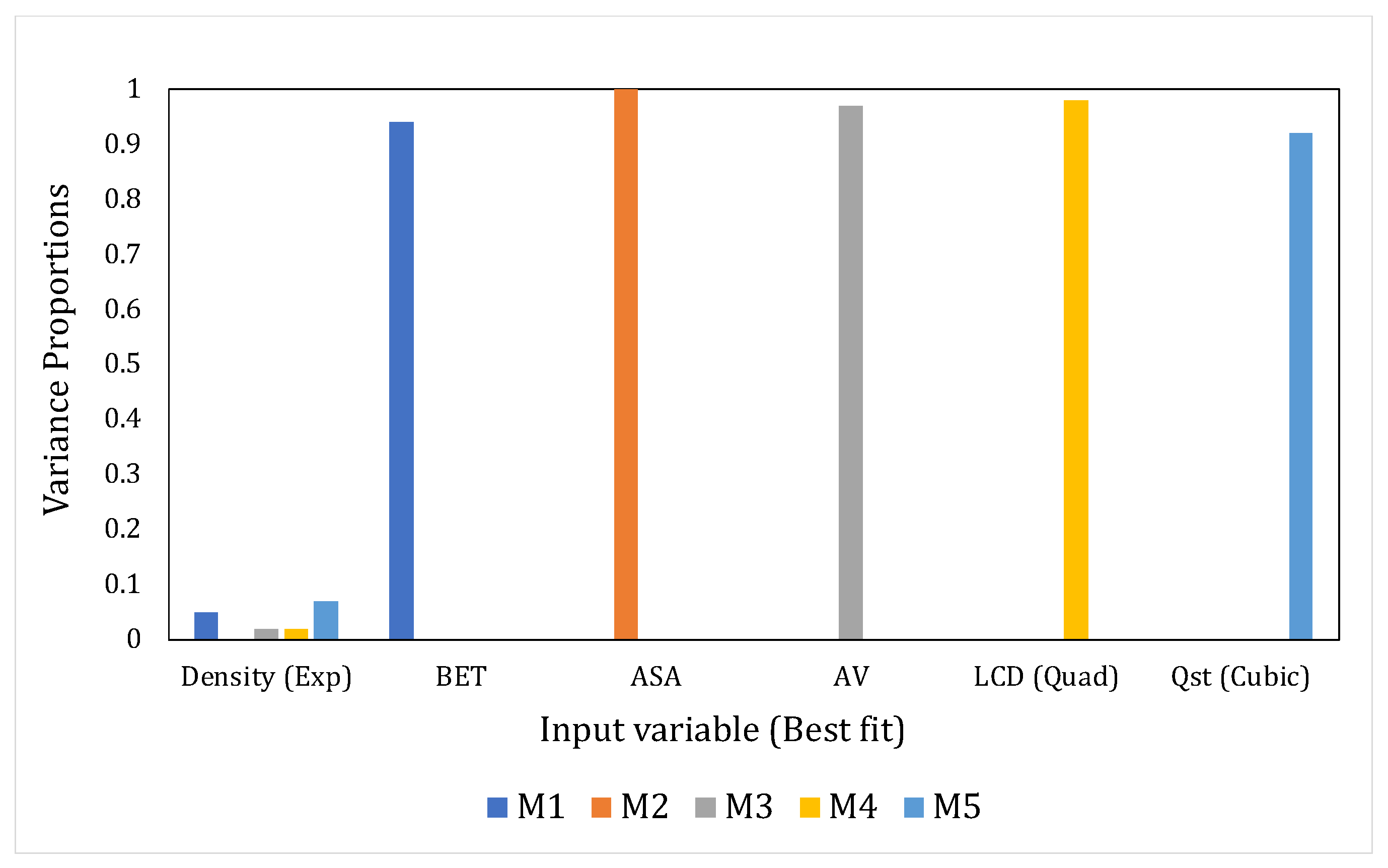
| Authors | Description/Agenda | Parameters Considered | Drawbacks/Motivation for Our Work |
|---|---|---|---|
| Wu et al. [33] | Recommended minimization of larger pores and an increase in the percentage of small cages and open metal sites | Pore volume, accessible surface area, density | Purely experimental study and takes longer. Correlates with our results that density can be a crucial parameter |
| Wu et al. [34] Sezginel et al. [36] | Molecular simulations to visualize the site structures | Assumptions on pore size, volume, density to get adsorption energies | Does not account for dynamic changes in structure during experiments |
| Hechinger et al. [37] | DFT simulations provided 3-D structural descriptors that were taken as inputs to QSPR models | Pore size, pore volume, surface area | Assumption of most stable conformation can lead to erroneous energies |
| Peng et al. [38] | Methane uptakes of 6 promising MOFs were determined to meet the DOE 2012 target and also seen to vary linearly with their pore surface area and pore volume but inversely proportional to density | Pore surface area, pore volume, packing density | The analysis was not comprehensive enough to find out the best descriptor of the MOF that can predict the uptakes with the highest accuracy |
| Li et al. [39] (2016) | The computational screening of MOFs -> revealed physical limitation to storage | Structural descriptors | Thermodynamic descriptors not considered Molecular simulations consume a great deal of time |
| Becker et al. [48] (2017) | Compared polarizable force fields vs. orbital interaction energies DFT -> adsorption isotherms -> adsorption energies Calculated for CO2 and CH4 on MOFs with 10 different metal ions | , assumptions of structural descriptors such as pore size, volume, density | Computational tools involving quantum mechanics consume lots of time and computer power and memory |
| Vandenbrande et al. [44] (2017) | Computed force fields for Zr-based MOFs adsorbing CH4 | General stable form of MOF assumed | Significant quantitative differences in methane uptakes, predictions are highly sensitive to the computed PES |
| Wiersum et al. [43] (2013) | Developed API considering weighting factors for performance sensitivity towards temperature and pressure | Adsorption energies, working capacities as inputs | Other material and pore properties of the MOF not considered whereas our model considers 8 input parameters |
| Tahmooresi and Sabzi [47] (2014) | Methane sorption in five different MOFs was modeled from experimental data PHSC) equation of state used—thermodynamic approach | Molecular dimensions and interaction energies used as inputs | Only 5 MOFs were considered, whereas our model considers 83 MOFs Other material and pore properties of the MOF were not considered Molecular parameters were estimated using a group contribution method—may not represent a real system |
| Gomez-Gualdron et al. [50] | Identified high-performance MOFs with Zr as central metal and OH and CO2 as ligands from a computational screening of 204 MOFs | Requires exact structures of MOFs as inputs—very difficult and time-consuming in the real system The MOFs used in the screening were hypothetical |
| Input or Output | GD 8 | GU 9 | VD 10 | VU 11 |
|---|---|---|---|---|
| ASA 1 | 0.96 (<0.001) | 0.947 (<0.001) | 0.399 (0.059) | −0.592 (<0.001) |
| Density | −0.941 (<0.001) | −0.908 (<0.001) | −0.406 (0.003) | 0.597 (<0.001) |
| BET SA 2 | 0.924 (<0.001) | 0.889 (<0.001) | 0.298 (0.049) | −0.491 (<0.001) |
| VP 3 | 0.925 (<0.001) | 0.883 (<0.001) | 0.249 (0.075) | −0.558 (<0.001) |
| AV 4 | 0.918 (<0.001) | 0.885 (<0.001) | 0.206 (0.346) | −0.613 (<0.001) |
| LCD 5 | 0.819 (<0.001) | 0.778 (<0.001) | 0.090 (0.684) | −0.501 (0.009) |
| PLD 6 | −0.687 (<0.001) | −0.642 (<0.001) | −0.731 (<0.001) | 0.246 (0.226) |
| Qst 7 | −0.68 (<0.001) | −0.374 (0.014) | −0.185 (0.24) | 0.727 (<0.001) |
| Input Variables | ASA | Density | BET SA | VP | AV | LCD | PLD | Qst |
|---|---|---|---|---|---|---|---|---|
| ASA | 1 | −0.99 (<0.001) | 0.944 (<0.001) | 0.952 (<0.001) | 0.956 (<0.001) | 0.804 (<0.001) | −0.566 (0.003) | −0.881 (<0.001) |
| Density | −0.99 (<0.001) | 1 | −0.89 (<0.001) | −0.898 (<0.001) | −0.917 (<0.001) | −0.745 (<0.001) | 0.653 (<0.001) | 0.572 (<0.001) |
| BET SA | 0.944 (<0.001) | −0.89 (<0.001) | 1 | 0.979 (<0.001) | 0.982 (<0.001) | 0.871 (<0.001) | −0.252 (0.284) | −0.49 (0.002) |
| VP | 0.952 (<0.001) | −0.898 (<0.001) | 0.979 (<0.001) | 1 | 0.989 (<0.001) | 0.863 (<0.001) | −0.345 (0.084) | −0.484 (<0.001) |
| AV | 0.956 (<0.001) | −0.917 (<0.001) | 0.982 (<0.001) | 0.989 (<0.001) | 1 | 0.902 (<0.001) | −0.346 (0.084) | −0.781 (<0.001) |
| LCD | 0.804 (<0.001) | −0.745 (<0.001) | 0.871 (<0.001) | 0.863 (<0.001) | 0.902 (<0.001) | 1 | 0.653 (<0.001) | −0.563 (0.01) |
| PLD | −0.566 (0.003) | 0.653 (<0.001) | −0.252 (0.284) | −0.345 (0.084) | −0.346 (0.084) | −0.237 (0.244) | 1 | 0.644 (0.002) |
| Qst | −0.881 (<0.001) | 0.572 (<0.001) | −0.49 (0.002) | −0.484 (<0.001) | −0.781 (<0.001) | −0.563 (0.01) | 0.644 (0.002) | 1 |
| Output | Input | Top 2 Best Performing Fits | F-Statistic | p-Value for bo * | p-Value for b1 ** | Best Fit according to the Calibration Set | Sign of the Correlation | |
|---|---|---|---|---|---|---|---|---|
| GD | ASA | Linear | 0.92 | 243 | 0.047 | <0.001 | Linear | Positive |
| Quadratic | 0.88 | 151 | <0.001 | <0.001 | ||||
| GD | Density | Exponential | 0.91 | 490 | <0.001 | <0.001 | Exponential | Negative |
| Linear | 0.89 | 381 | <0.001 | <0.001 | ||||
| GD | BET SA | Linear | 0.85 | 240.5 | <0.001 | <0.001 | Linear | Positive |
| Quadratic | 0.74 | 116 | <0.001 | <0.001 | ||||
| GD | AV | Linear | 0.84 | 113 | 0.011 | <0.001 | Linear | Positive |
| Exponential | 0.74 | 60 | <0.001 | <0.001 | ||||
| GD | LCD | Linear | 0.67 | 42 | 0.184 | <0.001 | Quadratic | Positive |
| Quadratic | 0.66 | 41 | <0.001 | <0.001 | ||||
| GD | Qst | Cubic | 0.54 | 46 | <0.001 | <0.001 | Cubic | Negative |
| Exponential | 0.52 | 42 | <0.001 | <0.001 |
| Best-Fit of the Input | RMSEP | RMSEC | abs (RMSEP—RMSEC) | ||
|---|---|---|---|---|---|
| Density (Exponential) | 0.88 | 0.015 | 0.91 | 0.011 | 0.004 |
| BET (Linear) | 0.85 | 0.02 | 0.85 | 0.015 | 0.012 |
| ASA (Linear) | 0.76 | 0.021 | 0.92 | 0.013 | 0.008 |
| AV (Linear) | 0.75 | 0.031 | 0.84 | 0.018 | 0.013 |
| LCD (Quadratic) | 0.36 | 0.053 | 0.66 | 0.027 | 0.026 |
| Qst (Cubic) | 0.26 | 0.046 | 0.54 | 0.027 | 0.019 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Input Variable | Coefficient Estimates for SLR Models | Coefficient Estimates for MLR Models | ||||
| M1 | M2 | M3 | M4 | M5 | ||
| Density_exp 1 | −1.49 | −0.32 | −0.31 | −0.44 | −0.34 | −0.38 |
| BET SA_linear 2 | ||||||
| ASA_linear 3 | ||||||
| AV_linear 4 | 0.092 | −0.02 | ||||
| LCD_quad 5 | ||||||
| Qst_cubic 6 | ||||||
| (b) | ||||||
| Input Variable | SEs for SLR Models | Standard Errors (SEs) of Regression Coefficient for MLR Models | ||||
| M1 | M2 | M3 | M4 | M5 | ||
| Density_exp 1 | 0.016 | 0.058 | 0.018 | 0.085 | 0.038 | 0.027 |
| BET SA_linear 2 | ||||||
| ASA_linear 3 | ||||||
| AV_linear 4 | 0.009 | 0.023 | ||||
| LCD_quad 5 | ||||||
| Qst_cubic 6 | ||||||
| (c) | ||||||
| Input Variable | Standardized Coefficients for SLR Models | Standardized Coefficients for MLR Models | ||||
| M1 | M2 | M3 | M4 | M5 | ||
| Density_exp 1 | 0.94 | 0.84 | 0.82 | 1.19 | 0.92 | 1.03 |
| BET SA_linear 2 | 0.92 | 0.12 | ||||
| ASA_linear 3 | 0.96 | 0.15 | ||||
| AV_linear 4 | 0.92 | 0.23 | ||||
| LCD_quad 5 | 0.81 | 0.06 | ||||
| Qst_cubic 6 | 0.73 | 0.08 | ||||
| (a) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Input Variable | t-Statistics for SLR Models | t-Statistic Values for MLR Models | |||||||||||
| M1 | M2 | M3 | M4 | M5 | |||||||||
| Density_exp 1 | −23 | −5.6 | −1.7 | −5.2 | −8.9 | −14.3 | |||||||
| BET SA_linear 2 | 15.5 | 0.8 | |||||||||||
| ASA_linear 3 | 15.6 | 0.3 | |||||||||||
| AV_linear 4 | 10.6 | −1 | |||||||||||
| LCD_quad 5 | 6.4 | 0.5 | |||||||||||
| Qst_cubic 6 | −6.6 | 1.1 | |||||||||||
| (b) | |||||||||||||
| Input Variable | Confidence Intervals for SLR Models | Confidence Intervals for MLR Models | |||||||||||
| Lower Bound (LB) | Upper Bound (UB) | M1 | M2 | M3 | M4 | M5 | |||||||
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | ||||
| Density_exp 1 | −3 | −0.39 | −2.1 | −0.44 | −3.1 | −0.7 | −2.6 | −0.6 | −2.1 | −0.5 | −3.3 | −0.4 | |
| BET SA_linear 2 | |||||||||||||
| ASA_linear 3 | |||||||||||||
| AV_linear 4 | 0.074 | 0.11 | −0.07 | 0.02 | |||||||||
| LCD_quad 5 | |||||||||||||
| Qst_cubic 6 | |||||||||||||
| (c) | |||||||||||||
| Input Variable | Partial Correlations for SLR Models | Partial Correlations for MLR Models | |||||||||||
| M1 | M2 | M3 | M4 | M5 | |||||||||
| Density_exp 1 | −0.94 | −0.66 | −0.45 | −0.76 | −0.9 | −0.92 | |||||||
| BET SA_linear 2 | 0.92 | 0.13 | |||||||||||
| ASA_linear 3 | 0.96 | 0.07 | |||||||||||
| AV_linear 4 | 0.92 | −0.22 | |||||||||||
| LCD_quad 5 | 0.81 | 0.13 | |||||||||||
| Qst_cubic 6 | −0.73 | 0.18 | |||||||||||
| (d) | |||||||||||||
| Input Variable | F-Statistics for SLR Models | F-Statistic Values for MLR Models | |||||||||||
| M1 | M2 | M3 | M4 | M5 | |||||||||
| Density_exp 1 | 490 | −0.66 | −0.45 | −0.76 | −0.9 | −0.92 | |||||||
| BET SA_linear 2 | 241 | 0.13 | |||||||||||
| ASA_linear 3 | 243 | 0.07 | |||||||||||
| AV_linear 4 | 0.92 | −0.22 | |||||||||||
| LCD_quad 5 | 0.81 | 0.13 | |||||||||||
| Qst_cubic 6 | −0.73 | 0.18 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaramakrishnan, K.; Mahmoud, E. Development of a High-Accuracy Statistical Model to Identify the Key Parameter for Methane Adsorption in Metal-Organic Frameworks. Analytica 2022, 3, 335-370. https://doi.org/10.3390/analytica3030024
Sivaramakrishnan K, Mahmoud E. Development of a High-Accuracy Statistical Model to Identify the Key Parameter for Methane Adsorption in Metal-Organic Frameworks. Analytica. 2022; 3(3):335-370. https://doi.org/10.3390/analytica3030024
Chicago/Turabian StyleSivaramakrishnan, Kaushik, and Eyas Mahmoud. 2022. "Development of a High-Accuracy Statistical Model to Identify the Key Parameter for Methane Adsorption in Metal-Organic Frameworks" Analytica 3, no. 3: 335-370. https://doi.org/10.3390/analytica3030024








