Abstract
Phthalocyanines are a group of porphyrin synthetic molecules. These compounds show outstanding photodynamic properties. This is due to their high ability to photogenerate free radicals, such as 1O2 and O.−2. However, the presence of different metals in the central core of phthalocyanines are able to modulate these characteristics. This study demonstrated that the photochemical activity of Zn, Cu, Ni, Co, Fe and Mn unsubstituted phthalocyanines differs, depending on the size and electronic configuration of the central metal. In addition, phthalocyanines showed different levels of photodamage against plasmid pBR322. Outcoming computational calculations will aim to clarify the photodynamic mechanisms of phthalocyanines.
1. Introduction
Phthalocyanines are organic compounds belonging to the porphyrin group. They are aromatic molecules containing four isoindole units. In their central region, they can bind to a wide variety of metallic and non-metallic elements, giving the phthalocyanine molecule different photochemical and photobiological properties. In general, phthalocyanines are planar compounds with highly symmetrical (D4h) point groups, although the latter can be distorted depending on the metal bonded [1,2].
The synthesis of phthalocyanines can be achieved by various routes. The most common starting materials are phthalimide, phthalic anhydride and phthalonitrile in the presence of high-boiling solvents such as nitrobenzene [3]. In addition, a strong base such as DBU or a nitrogen source such as urea and a hydrated metal salt (chlorides, acetates, etc.) are required. These reactions can be carried out using microwave irradiation, which is a more effective, cleaner and higher yielding process [4].
Phthalocyanines have π-π* transitions responsible for their absorption spectra, which consist of two bands: (1) the Sohret band, of lower intensity (300–400 nm), and (2) the Q band, of higher intensity (650–700 nm). In addition, the positions of the Q-bands are sensitive to the type of central metal. In general, the insertion of a metal with more electrons shifts the Q-band towards the red regions due to the destabilization of the HOMO orbitals. In addition, phthalocyanines show fluorescent emission bands between 650 and 700 nm [2,5].
Research into phthalocyanines is very broad and important, with a wide range of applications from medicine to technology [6]. In the field of biomedicine, phthalocyanines are good candidates for use as photosensitizers in antibacterial photodynamic therapy. This involves the initiation of phototoxic processes that generate reactive oxygen species (ROS) such as 1O2, OH and H2O2 via type II and type I mechanisms, respectively. These species are highly cytotoxic and are responsible for killing bacterial cells [7,8].
2. Materials and Methods
2.1. Materials and Equipment
Phthalic anhydride, urea, metal salts (Zn, Cu, Ni, Co, Fe and Mn acetates and chlorides), ammonium molybdate, dimethyl formamide (DMF), dimethyl sulphoxide (DMSO), PBS buffer, nitroblue tetrazolium (NBT), diphenyl anthracene (DPA), lyophilized pBR322, agarose gel, ethidium bromide (EtBr), luminol, Luzchem L2C-4V, Perkin Elmer Lambda 35 UV-Vis Spectrophotometer, Perkin Elmer LS 45 Fluorescence Spectrometer, Mars 6 230/60 CEM Microwave Reactor, Luminoskan Ascent 392 and UPLAND UV Transilluminator—most of these materials and equipment were purchased from Sigma Aldrich (St. Louis, MO, USA) and Perkin Elmer (Waltham, MA, USA).
2.2. Methods
2.2.1. Synthesis of Unsubstituted Phthalocyanines
The method in [9] was carried out for the synthesis of unsubstituted phthalocyanines: 1.48 g of phthalic anhydride, 3 g of urea, 0.012 g of ammonium molybdate and 2.6 mmol of the metal salt. These starting products were homogenized in a mortar, then placed in a beaker and subjected to microwave irradiation for 10 min. For this, a Mars 6 320/60 microwave reactor with a power of 2455 MHz was used. The resulting product was washed and filtered with many solvents.
2.2.2. UV-Vis Spectra and ΦF Values
The UV-Vis spectra of the synthesized phthalocyanines were taken in DMF and DMSO, using a Lambda 35 spectrophotometer. The spectra were taken in a 5 × 10−6 M solution. On the other hand, for the determination of the fluorescence quantum yields (ΦF) in DMF, an LS 45 spectrofluorometer was used to obtain the fluorescence spectra of the phthalocyanines. For this purpose, the Q-band values of each of the phthalocyanines were used as the excitation wavelength (660–670 nm). Calculations of ΦF were obtained with the comparative method, using tetraphenylporphyrin (TPP, ΦF = 0.110) as the standard [10]. Fluorescence spectra were taken at a concentration of 7 × 10−6 M.
2.2.3. Singlet Oxygen and Free-Radical Photogeneration
For the evaluation of the singlet oxygen generation of the different phthalocyanines, an indirect methodology was used [10,11]. For this, a standard molecule with 1O2 trapping capacity, diphenyl anthracene (DPA), which absorbs at 373–375 nm and forms a derivative oxidized by 1O2, was used. The disappearance kinetics was followed by UV-Vis spectrophotometry. An LED lamp was used as an irradiation source (irradiance 14.65 LUX, visible light) and a continuous flow of oxygen was put into the solution composed of phthalocyanine (5 × 10−6 M) and DPA. The gradual decrease in absorbance was evaluated at 373 nm by successive measurements every 15 min.
For the determination of superoxide anion (O.−2), the nitroblue tetrazolium (NBT) assay was carried out, where the conversion of NBT to diformazan (DF) at 560 nm is measured [12]. For this, a solution of NBT in DMF at 2 × 10−3 M was prepared and 50 μL of phthalocyanine solution in DMF at 5 × 10−5 M was added. Irradiation was carried out in a Luzchem L2C-4V type reactor (equipped with white lamps, 38.40 LUX) for 80 min.
On the other hand, the H2O2 photogeneration of phthalocyanines was studied via the chemiluminescence reaction [13]. For this purpose, 15 μL of phthalocyanine (5 × 10−5 M) and 10 μL of luminol (250 μM, prepared in 2 M NaOH and diluted with PBS) were added to a microtiter plate. Chemiluminescence signals were measured on a Luminoskan Ascent 392 instrument in the presence of NADH. The same tests were repeated in the presence of superoxide dismutase (SOD) to further investigate the phototoxic mechanisms of phthalocyanines.
2.2.4. Photobiological Activity: Electrophoresis and Photo Hemolysis
Electrophoresis was performed to determine the photoactivity of phthalocyanines against pBR322 plasmid [14]. For this purpose, 4 μL of 2 × 10−5 M phthalocyanine solutions and 20 μL of pBR322 were mixed and incubated for 20 min. After that, solutions were irradiated for 30 min in a Luzchem L2C-4V type reactor (equipped with white lamps, 38.40 LUX). Electrophoretic experiments were made in 1% agarose gel with 5 μL of ethidium bromide (EtBr, 0.5 mg.L−1). Voltage gradients of 40–45 V.cm−1 and a current of 60 mA were applied. Results were obtained after a runtime of 1 h. Electrophoretic bands were observed by using an UPLAND UV Transilluminator.
For photo hemolysis assays, solutions of human erythrocytes were prepared in a PBS buffer after the centrifugation of human blood. Irradiation of 4 mL of human erythrocytes was performed in the presence of 50 μL of a phthalocyanine solution (2 × 10−5 M) for 40 min in a Luzchem L2C-4V type reactor. The addition of chemical additives, such superoxide dismutase (SOD), glutathione (GSH) and sodium azide (NaN3), allowed us to elucidate the phototoxic mechanisms of phthalocyanines against human erythrocytes. Measurements were made at 545 nm [15].
3. Discussion and Results
3.1. UV-Vis Spectra and ΦF Values
All phthalocyanines showed the typical UV-Vis bands in DMF and DMSO. The Q band appeared at about 650–670 nm, and the Soret band appeared at 300–350 nm. Both bands are π-π* transitions in nature. The positions of the bands depend on the central metal (Figure 1). For zinc phthalocyanine (ZnPc), Q and Soret bands appeared at 672 nm and 343 nm, respectively (see Figure 1a). However, manganese phthalocyanine (MnPc) showed different Q and Soret bands at 712 nm and 347 nm (see Figure 1b). These results can be ascribed by the fact that Mn, as well as other metals with empty d-orbitals, are more prone to suffer charge-transfer bands [2,6].
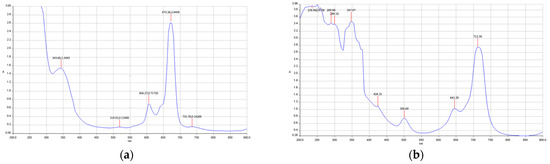
Figure 1.
Typical UV-Vis spectra of phthalocyanines: (a) PcZn UV-Vis Spectrum, showing Q band (672 nm) and Soret band (343 nm); (b) MnPc UV-Vis spectrum, showing Q band (712 nm) and Soret band (347 nm).
Table 1 shows the different bands of phthalocyanines in DMF and DMSO. Q bands are very sensitive to the central metal and solvent. Q bands are red-shifted as the size of the central metal increases. In addition, the Q bands in DMSO are different from the Q bands in DMF. This is due to the different values of the refractive indices of the solvents [5]. It should be noted that Q bands are also sensitive to the oxidation states of the central metals and the possibility of charge-transfer processes [2,6].

Table 1.
Q bands and quantum yield values of phthalocyanines.
The (ΦF) values of phthalocyanines were measured in DMF with the comparative method. These values were calculated using the Q-band value of the phthalocyanines as the excitation wavelength. Different ΦF values were observed due to the different central metals (Table 1). It is well known that metals with empty d-orbitals tend to deactivate the excited states. Therefore, the fluorescence tends to be weaker. However, NiPc showed the highest fluorescence intensity at its Q-band value. The diamagnetic nature and the production of dimeric species of NiPc may account for this result [6].
3.2. Singlet Oxygen and Free Radical Photogeneration
The electronic configurations of metals with unpaired electrons are assumed to inhibit the π-π* transitions of phthalocyanines. This translates into a decrease in the triplet excited states and, therefore, in a decrease in the capabilities to generate singlet oxygen. However, NiPc appears to be an exception for this rule (Table 1). This result can be ascribed by the fact that NiPc is more prone to create aggregates. The aggregation produces a transfer of electronic energy among aggregates, which in turn, will be used for the energy transfer reaction [6]. On the other hand, NiPc and CoPc showed the highest photogeneration of free radicals. These results show that these phthalocyanines are more prone to have photoactivity through the type I mechanism, which can be very harmful for tumoral and bacterial cells.
3.3. Photobiological Activity: Electrophoresis and Photo Hemolysis
All phthalocyanines showed photoactivity against pBR322 plasmid in gel agarose electrophoresis, except for MnPc (Figure 2a). These results can be described on the basis of free radical photogeneration, which are able to produce DNA damage. These first results showed that phthalocyanines may harm pBR322 plasmid due to its transformation of the supercoiled form into linear and open circular forms [6,8]. On the other hand, ZnPc showed the highest phototoxic activity against human erythrocytes (Figure 2b). These damages have contributions from both phototoxic mechanisms (singlet oxygen and other free radicals). However, it is expected that the level of damage also depends on how the phthalocyanine molecules interact with the human erythrocyte membrane [6,15].

Figure 2.
(a) Photoactivity of phthalocyanines on pBR322; (b) photoactivity of phthalocyanines on human erythrocytes.
4. Conclusions
In general, phthalocyanines are good candidates for use in antibacterial photodynamic therapy. These compounds can generate photoinduced free radicals, which are highly cytotoxic species. The extent to which these free radicals are generated depends on the electronic properties of the central metallic element, since in some cases they can promote the deactivation of the excited states and thus the generation of type I or type II phototoxic mechanisms. In the future, we intend to perform some computational calculations that will allow us to further investigate how central metals can modulate the photochemical and photobiological properties of phthalocyanines.
Author Contributions
Conceptualization, F.V. and M.L.; methodology, F.V. and M.L.; software, S.D. and A.M.; validation, F.V.; formal analysis, F.V. and M.L.; investigation, M.L.; resources, Á.Á.; writing—original draft preparation, M.L.; writing—review and editing, M.L.; supervision, M.L.; funding acquisition, F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Centro de Estudios Avanzados (CEA) of the Instituto Venezolano de Investigaciones Científicas (IVIC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are contained within the article.
Acknowledgments
We thank the head of the Organic Physicochemistry Laboratory, Álvaro Álvarez, for his permission and assistance with the computational calculations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitra, K.; Hartman, M.C. Silicon phthalocyanines: Synthesis and resurgent applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K. Functional singlet oxygen generators based on phthalocyanines. Coord. Chem. Rev. 2012, 256, 1556–1558. [Google Scholar] [CrossRef]
- Kong, X.; Wang, H.; Zhang, J. A new method for synthesis of iron phthalocyanine. Adv. Mater. Res. 2014, 1033–1034, 3–6. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Srinivas, K.; Srivastava, P.; Sahoo, B. Solvent-free synthesis of phthalocyanines. J. Porphyr. Phthalocyanines 2003, 7, 548–550. [Google Scholar] [CrossRef]
- Chidawanyika, W.; Ogunsipe, A.; Nyokong, T. Syntheses and photophysics of new phthalocyanine derivatives of zinc, cadmium and mercury. New J. Chem. 2007, 31, 377–384. [Google Scholar] [CrossRef]
- Pereira, G.F.M.; Tasso, T.T. From cuvette to cells: How the central metal ion modulates the properties of phthalocyanines and porphyrazines as photosensitizers. Inorganica Chim. Acta 2021, 519, 120271. [Google Scholar] [CrossRef]
- Barut, B.; Sofuoğlu, A.; Biyiklioglu, Z.; Özela, A. The water soluble peripherally tetra-substituted zinc(II), manganese(III) and copper(II) phthalocyanines as new potential anticancer agents. Dalton Trans. 2016, 45, 14301–14310. [Google Scholar] [CrossRef] [PubMed]
- Ağırtaş, M.S.; Cabir, B.; Yıldıko, Ü.; Özdemir, S.; Gonca, S. Synthesis, antioxidant, DNA cleavage and antimicrobial properties of phthalocyanine complexes bearing the poly-hydroxyl groups. Chem. Pap. 2021, 75, 1749–1760. [Google Scholar] [CrossRef]
- Staicu, A.; Nuta, A.; Pascu, A.; Sorescu, A.A. Studies about phthalocyanine photosensitizers to be used in photodynamic therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. Available online: https://rrp.nipne.ro/2013_65_3/A38.pdf (accessed on 22 January 2023).
- .Zoltan, T.; Vargas, F.; Rivas, C.; López, V.; Perez, J.; Biasutto, A. Synthesis, photochemical and photoinduced antibacterial activity studies of meso-tetra(pyren-1-yl)porphyrin and its Ni, Cu and Zn complexes. Sci. Pharm. 2010, 78, 767–789. [Google Scholar] [CrossRef] [PubMed]
- Burguete, M.; Galindo, F.; Gavara, R.; Lafuente, S.; Moreno, M.; Thomas, P.; Russell, D. Singlet oxygen generation using a porous monolithic polymer supported photosensitizer: Potential application to the photodynamic destruction of melanoma cells. Photochem. Photobiol. Sci. 2009, 8, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Méndez, H.; Fuentes, A.; Sequera, J.; Fraile, G.; Velásquez, M.; Cuello, K. Photosensitizing activity of thiocolchicoside: Photochemical and in vitro phototoxicity studies. Pharmazie 2001, 56, 83–88. Available online: https://pubmed.ncbi.nlm.nih.gov/11210677/ (accessed on 22 January 2023). [PubMed]
- Vargas, F.; Zoltan, T.; Pujol, F.; Rangel, H. Comparative antiviral (HIV) photoactivity of metalized meso-tetraphenylsulfonated porphyrins. Med. Chem. 2008, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; An, J.-Y.; Jiang, L.-J. Damage to pBR322 DNA photosensitized by hypocrellin A in liposomes and its derivative in solution. J. Photochem. Photobiol. B Biol. 1996, 33, 73–78. [Google Scholar] [CrossRef]
- Kahn, G.; Fleischaker, B.I. Red blood cell hemolysis by photosensitizing compounds. J. Investig. Dermatol. 1971, 56, 85–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).