Effectiveness of Small Amount of Surface Penetrant against Chloride Ion Penetration †
Abstract
:1. Introduction
2. Material and Methods
2.1. Properties of Fine Aggregate and Binding Materials
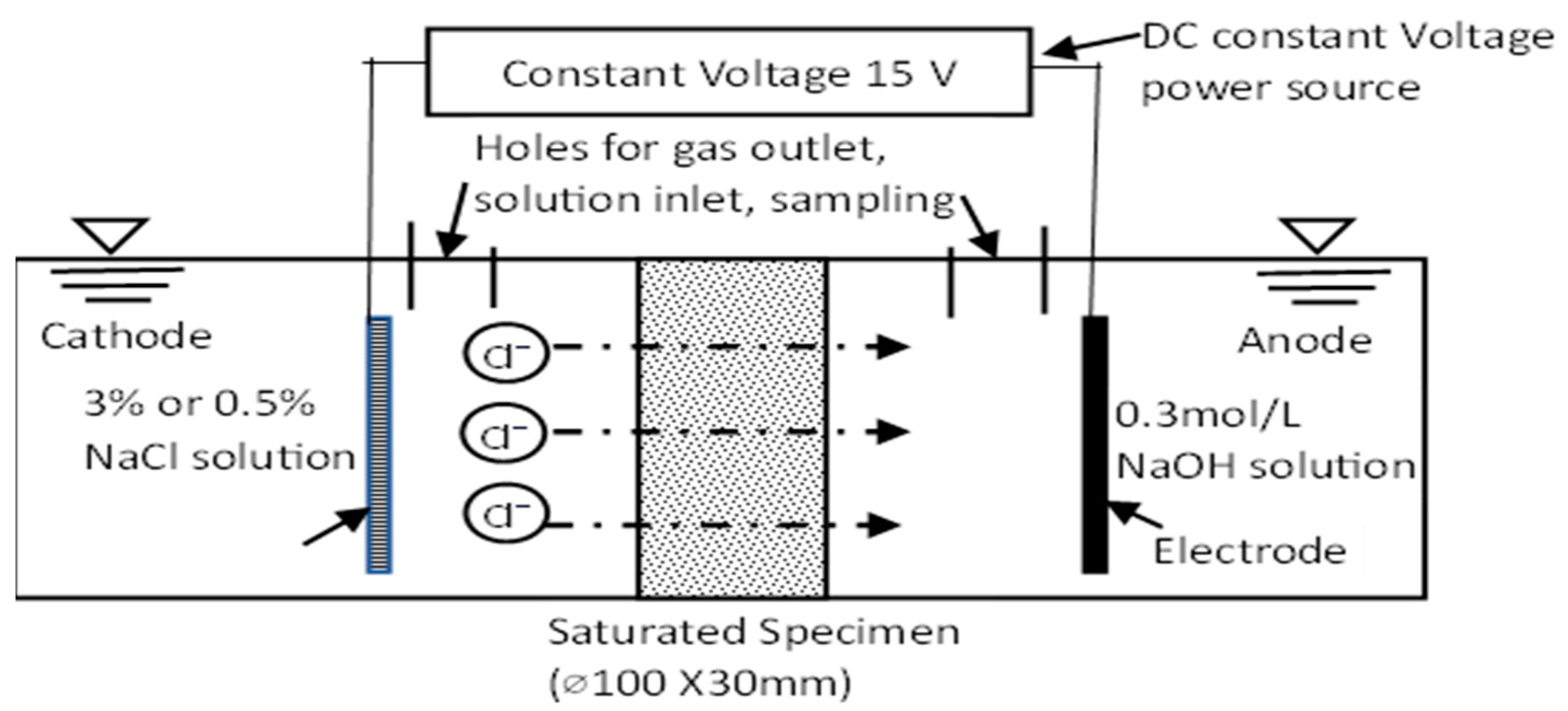
2.2. Research Methodology
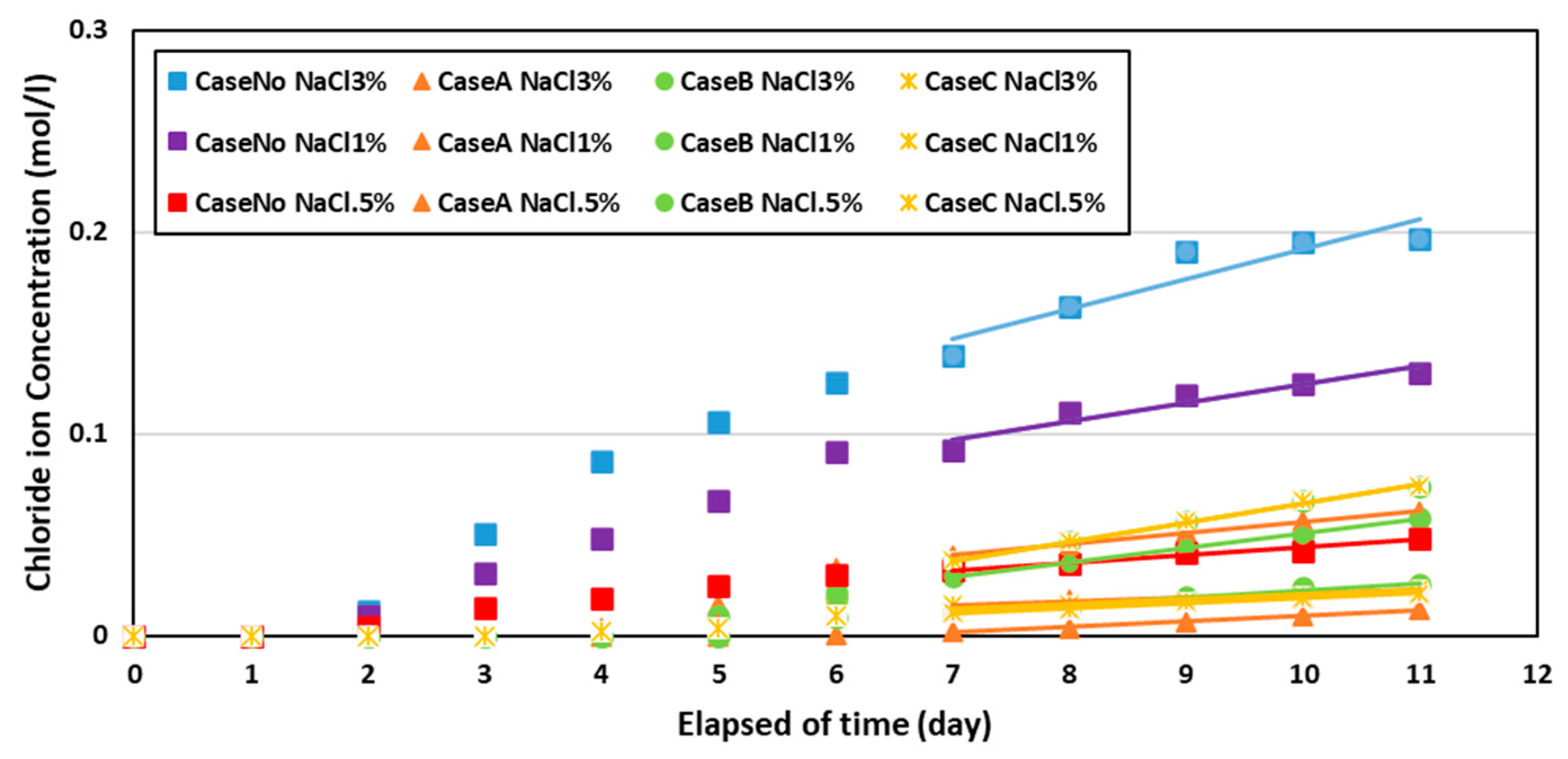
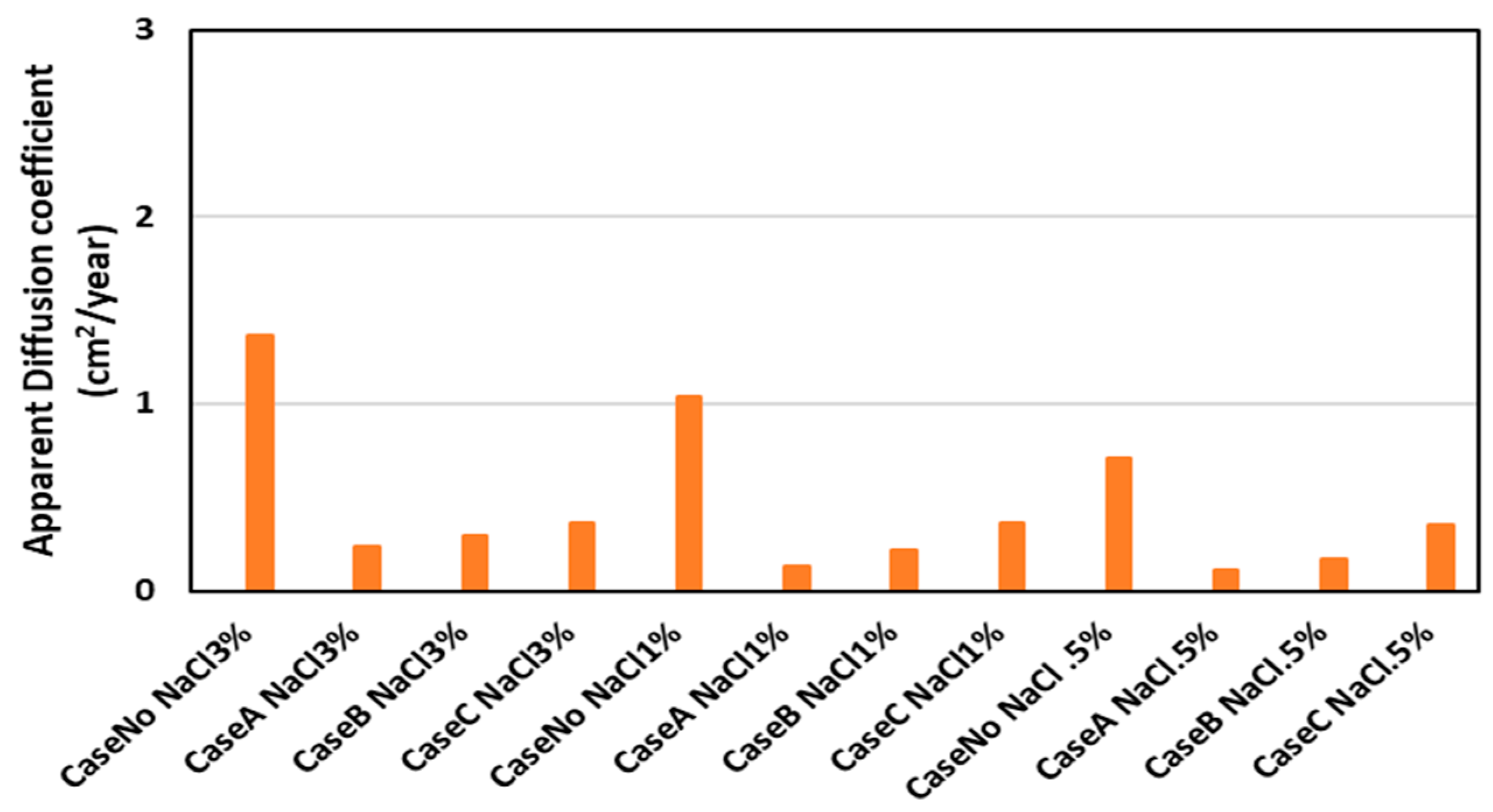
3. Result and Discussion
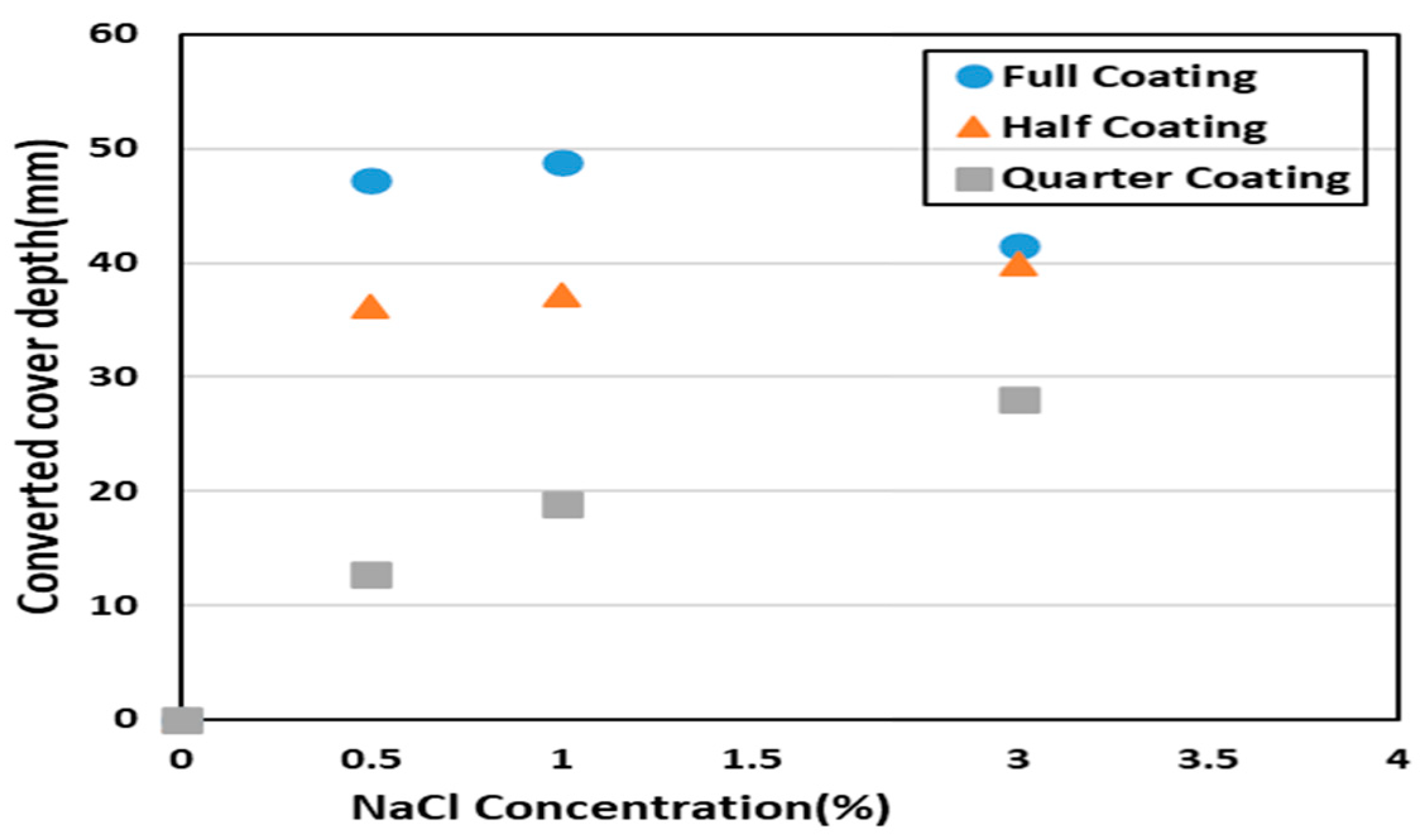
4. Simulation of Corrosion Initiation Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanaoka, D.; Amino, T.; Habuchi, T.; Miyazato, S.; Tabata, S. A study on prediction method of chloride ion penetration into concrete with surface penetrants. In Life-Cycle of Structural Systems: Design, Assessment, Maintenance and Management, Procceedings of the 4th International Symposium on Life-Cycle Civil Engineering, IALCCE 2014, Tokyo, Japan, 16–19 November 2014; Taylor & Francis: Abingdon, UK, 2014; pp. 950–955. [Google Scholar] [CrossRef]
- Moon, H.Y.; Shin, D.G.; Choi, D.S. Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system. Constr. Build. Mater. 2007, 21, 362–369. [Google Scholar] [CrossRef]
- Swamy, R.N.; Tanikawa, S. Surface coatings to preserve concrete durability. In Protection of Concrete; Dhir, R.K., Green, J.W., Spon, F.N.E., Eds.; CRC Press: London, UK, 1990; Volume 1, pp. 149–165. [Google Scholar]
- Kuroiwa, D.; Miyazato, S. Proposal of Estimation Method for Apparent Chloride Ion Diffusion Coefficient At Reform Part By Silicate Type Surface Penetrant and Trial Calculation for Corrosion Occurrence Time. J. Jpn. Soc. Civ. Eng. Ser. E2 (Mater. Concr. Struct.) 2015, 71, 124–134. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Almusallam, A.A. Effectiveness of concrete surface treatment materials in reducing chloride-induced reinforcement corrosion. Constr. Build. Mater. 1997, 11, 443–451. [Google Scholar] [CrossRef]
- Japan Society of Civil Engineers. Standard Specifications for Concrete Structures—2007 “Materials and Construction”; Japan Society of Civil Engineers: Tokyo, Japan, 2010. [Google Scholar]
- Yoshitani, T.; Miyazato, S. Estimation of Chloride ion Diffusion coefficient of concrete surface layer modified by Silane-based surface impregnation. In Proceedings of the 70th Annual Conference of the Japan Society of Engineers, Okayama, Japan, 16–17 September 2015; pp. 1173–1174. [Google Scholar]
- Uomoto, T.; Ishibashi, T.; Nobuta, Y.; Satoh, T.; Kawano, H.; Takewaka, K.; Uji, K. Standard Specifications for Concrete Structures-2007 by Japan Society of Civil Engineers. Concr. J. 2008, 46, 3–14. [Google Scholar] [CrossRef]

| W/C | S/C | Unit Weight (kg/m3) | ||
|---|---|---|---|---|
| W | C * | S ** | ||
| 0.50 | 2.5 | 12.296 | 24.591 | 61.5 |
| Case | Classification-Type | Application Quantity (g/m2) |
|---|---|---|
| Case No. | No penetrant | 0 |
| Case A | Silane-type | 200 |
| Case B | 100 | |
| Case C | 50 |
| Cases | Effective Diffusion (cm2/Year) | Apparent Diffusion (cm2/Year) | ) Penetrant (cm2/Year) |
|---|---|---|---|
| Case No NaCl 3% | 2.67 | 1.36 | |
| Case A NaCl 3% | 0.47 | 0.24 | 0.0062 |
| Case B NaCl 3% | 0.59 | 0.30 | 0.0055 |
| Case C NaCl 3% | 0.71 | 0.36 | 0.0025 |
| Case No NaCl 1% | 2.03 | 1.04 | |
| Case A NaCl 1% | 0.26 | 0.13 | 0.0029 |
| Case B NaCl 1% | 0.43 | 0.22 | 0.0040 |
| Case C NaCl 1% | 0.71 | 0.36 | 0.0035 |
| Case No NaCl 0.5% | 1.40 | 0.71 | |
| Case A NaCl 0.5% | 0.21 | 0.11 | 0.0025 |
| Case B NaCl 0.5% | 0.34 | 0.17 | 0.0034 |
| Case C NaCl 0.5% | 0.69 | 0.35 | 0.0059 |
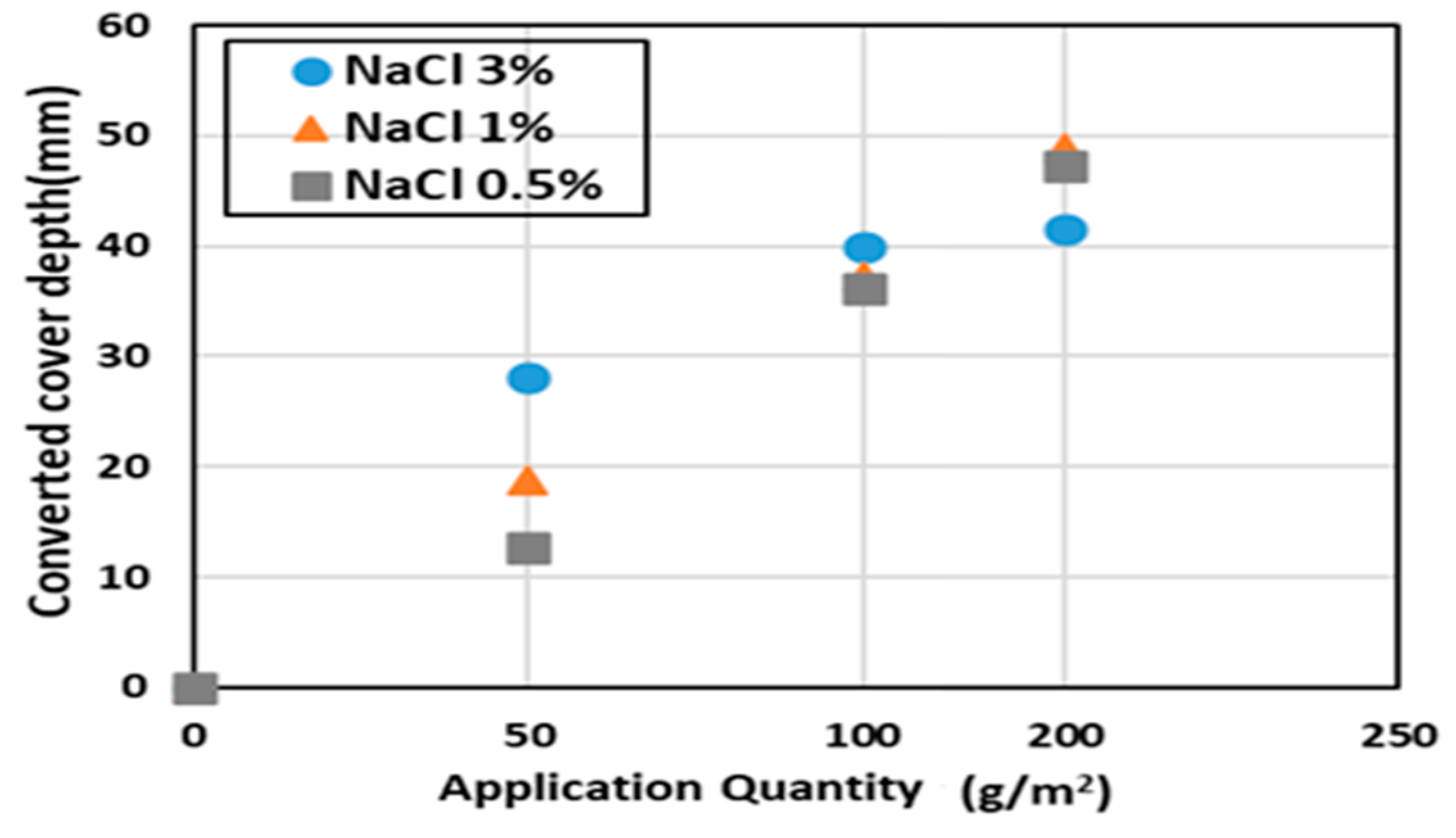
| Case | Converted Cover Depth (mm) |
|---|---|
| Case A NaCl 3% | 41.50 |
| Case B NaCl 3% | 39.94 |
| Case C NaCl 3% | 28.08 |
| Case A NaCl 1% | 48.84 |
| Case B NaCl 1% | 37.27 |
| Case C NaCl 1% | 18.84 |
| Case A NaCl 0.5% | 47.27 |
| Case B NaCl 0.5% | 36.25 |
| Case C NaCl 0.5% | 12.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyeltshen, R.; Miyazato, S. Effectiveness of Small Amount of Surface Penetrant against Chloride Ion Penetration. Eng. Proc. 2023, 55, 18. https://doi.org/10.3390/engproc2023055018
Gyeltshen R, Miyazato S. Effectiveness of Small Amount of Surface Penetrant against Chloride Ion Penetration. Engineering Proceedings. 2023; 55(1):18. https://doi.org/10.3390/engproc2023055018
Chicago/Turabian StyleGyeltshen, Rinchen, and Shinichi Miyazato. 2023. "Effectiveness of Small Amount of Surface Penetrant against Chloride Ion Penetration" Engineering Proceedings 55, no. 1: 18. https://doi.org/10.3390/engproc2023055018






