Performance Evaluation of Lithium Nitrite-Based Gel against Corrosion of Rebar with Partial Short Cover Depth in Chloride Environment †
Abstract
:1. Introduction
2. Materials and Methods
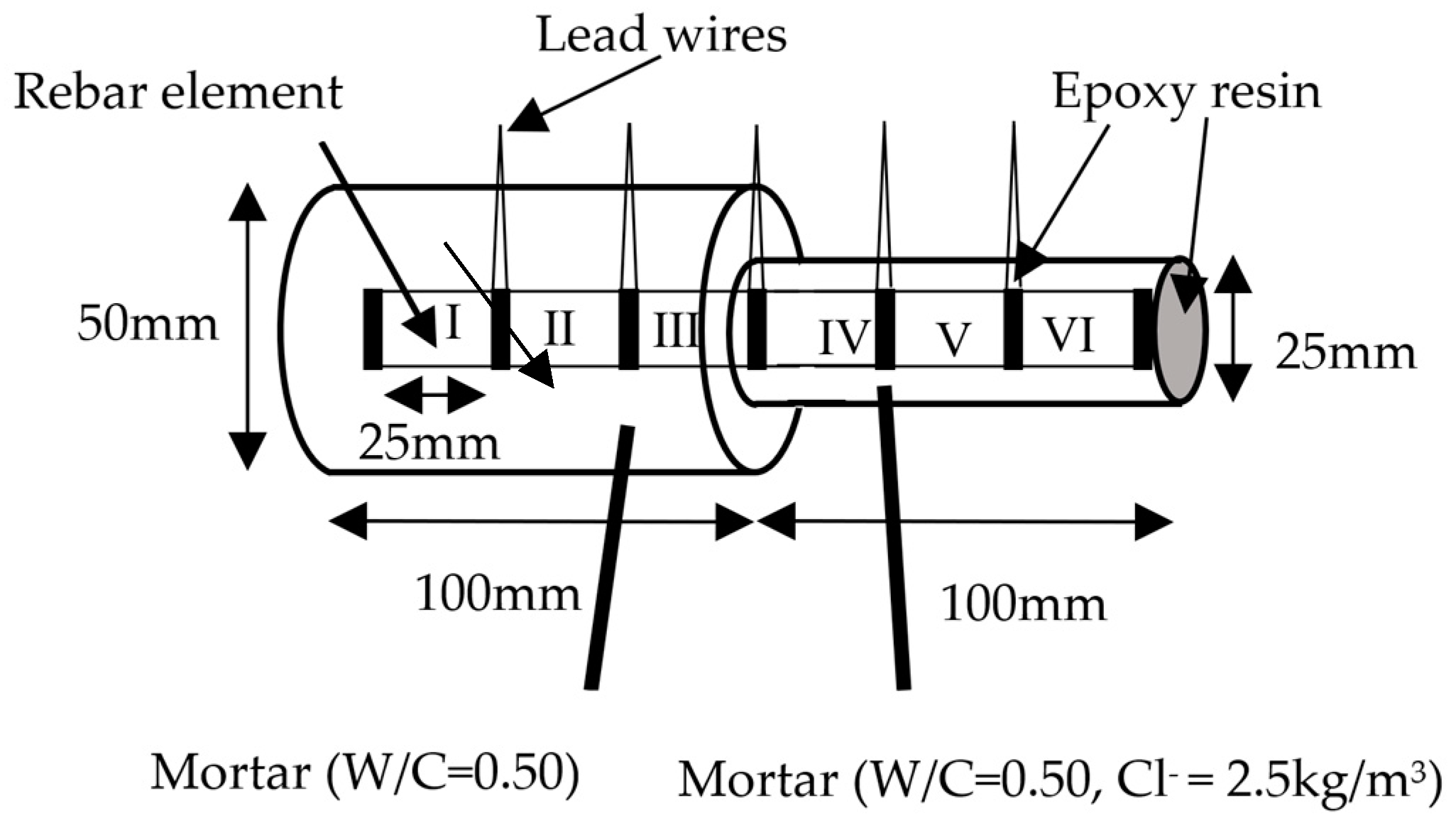
2.1. Specimen
2.2. Electrochemical Measurement
3. Results and Discussions
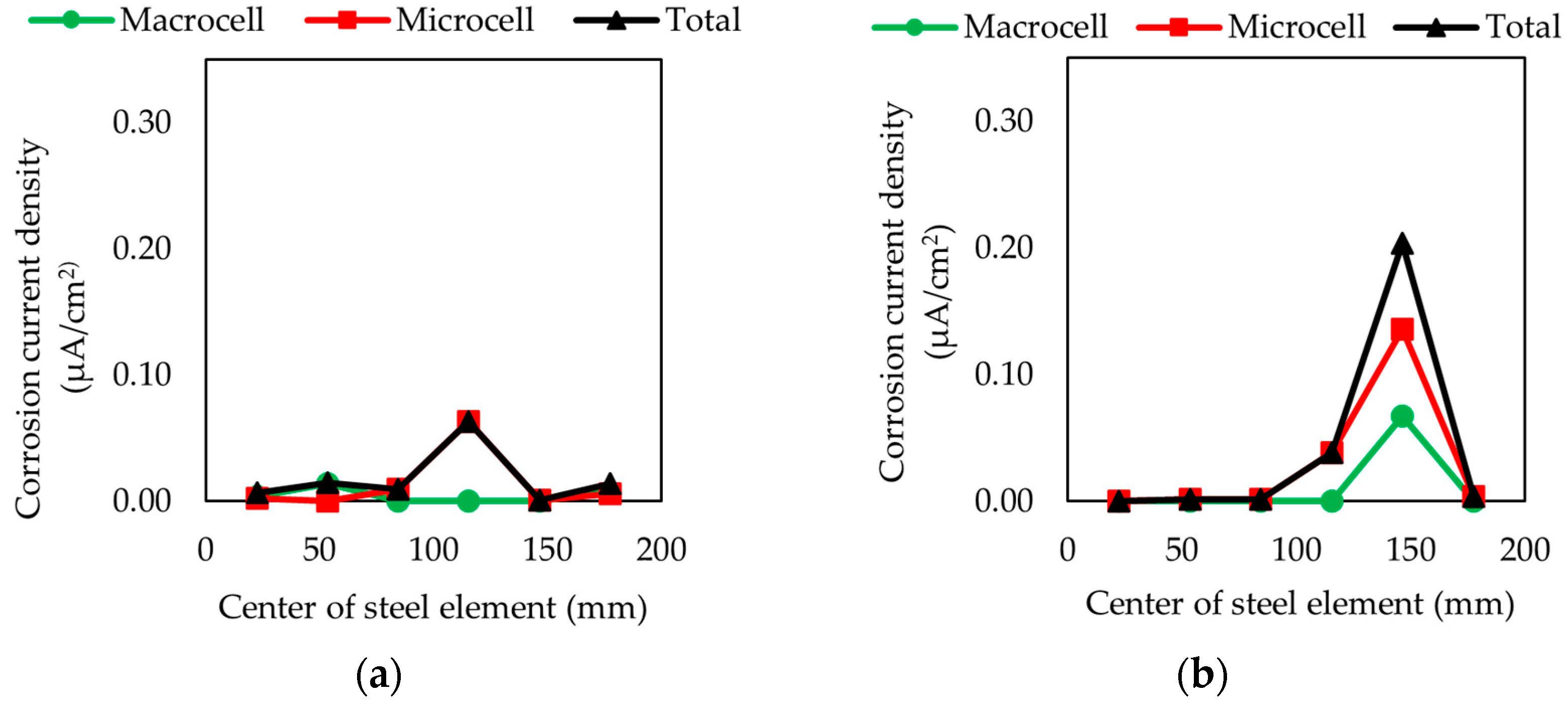
3.1. Corrosion Current Density
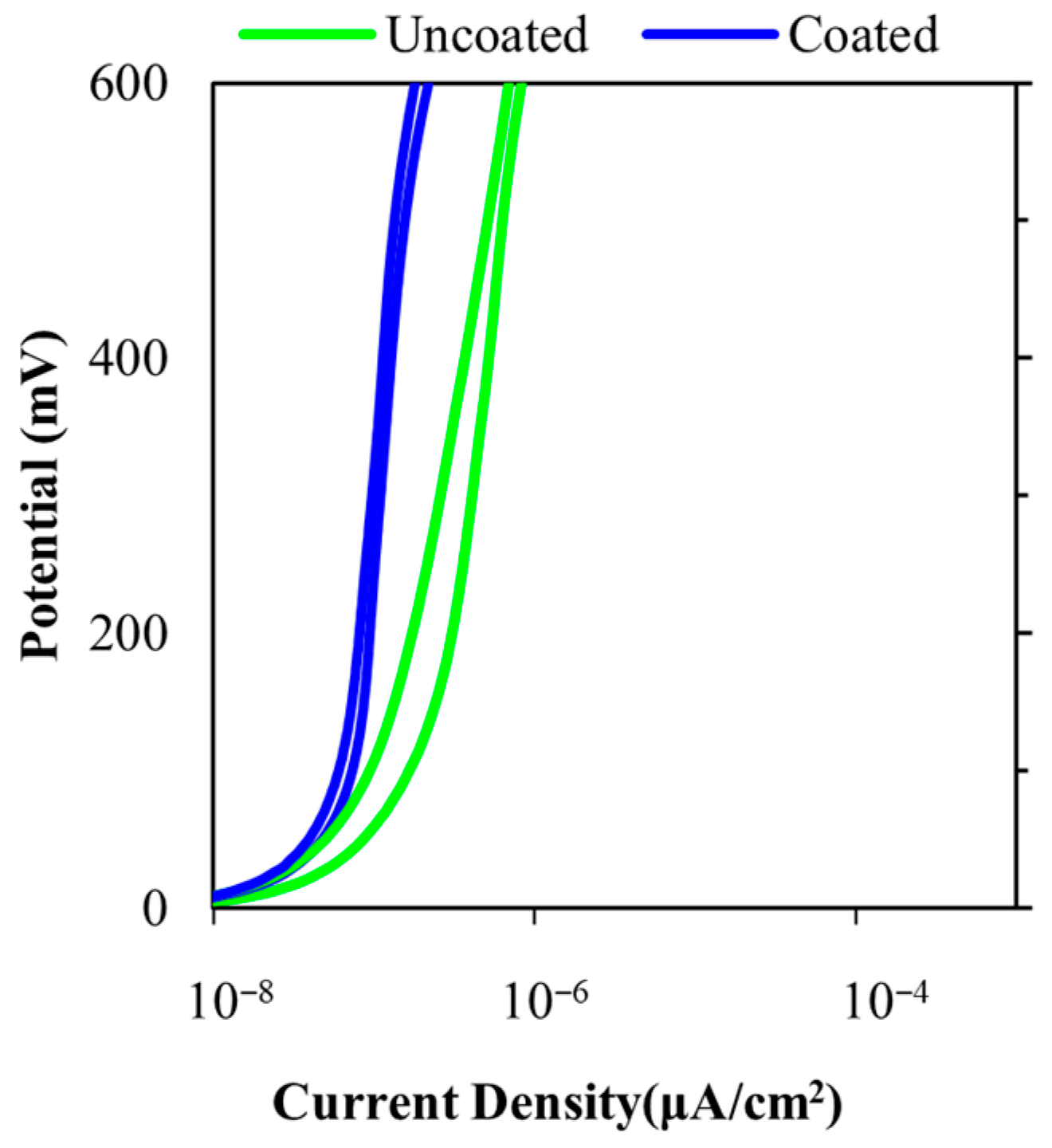
3.2. Anodic Polarization Curves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, A.K.; Miyazato, S.; Sugawara, N. Influence of Cover depth and mortar quality on Rebar corrosion under aggressive chloride environment. In Proceedings of the 4th Asian Concrete Federation Symposium on Emerging Technologies for Structural Longevity (ACF2023_ETSL), Shenzhen, China, 11–13 March 2023. [Google Scholar]
- Lee, H.S.; An, J.M.; Shin, S.W. Study on the evaluation of corrosion protection effect with LiNO2, inhibitor. J. Korea inst. Struct. Maint. Insp. 2002, 6, 339–342. [Google Scholar]
- Miyaguchi, K.; Takai, K.; Miyazato, S. Corrosion inhibition effects of reinforcing steel bars of reinforced concrete structures subjected to salt damage by Gel coating material containing Anti-corrosive. In Proceedings of the 77th Annual Lecture of Civil Engineering Society National Conference, Kyoto University Yoshida campus, Kyoto, Japan, 15–16 September 2022; Volume 548. (In Japanese). [Google Scholar]
- Miyaguchi, K.; Takai, K.; Miyazato, S. Corrosion prevention effect of Reinforced concrete Using Gel composed Inhibitor against chloride attack, carbonation and composite deterioration. Proc. Concr. Struct. Scenar. JSMS 2022, 22, 1–6. Available online: https://cir.nii.ac.jp/crid/1520576137321497472 (accessed on 30 November 2023). (In Japanese).
- Miyazato, S.; Otsuki, N. Steel corrosion induced by chloride or carbonation in mortar with bending cracks or joints. J. Adv. Concr. Technol. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Hansson, C.M.; Poursaee, A.; Laurent, A. Macrocell and microcell corrosion of steel in ordinary Portland cement and high-performance concretes. Cem. Concr. Res. 2006, 36, 2098–2102. [Google Scholar] [CrossRef]
- Miyazato, S.; Otsuki, N. Measurement Method for Macrocell Corrosion in Concrete Specimen using a Segmented Steel Bar. J. Adv. Concr. Technol. 2022, 20, 222–235. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]

| W/C | S/C | Unit Weight (kg/m³) | ||
|---|---|---|---|---|
| 0.50 | 2.5 | W | C | S |
| 276 | 553 | 1384 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.A.; Miyazato, S.; Mizuguchi, H.; Miyaguchi, K. Performance Evaluation of Lithium Nitrite-Based Gel against Corrosion of Rebar with Partial Short Cover Depth in Chloride Environment. Eng. Proc. 2023, 55, 35. https://doi.org/10.3390/engproc2023055035
Khalid MA, Miyazato S, Mizuguchi H, Miyaguchi K. Performance Evaluation of Lithium Nitrite-Based Gel against Corrosion of Rebar with Partial Short Cover Depth in Chloride Environment. Engineering Proceedings. 2023; 55(1):35. https://doi.org/10.3390/engproc2023055035
Chicago/Turabian StyleKhalid, Muhammad Afaq, Shinichi Miyazato, Hibiki Mizuguchi, and Katsuichi Miyaguchi. 2023. "Performance Evaluation of Lithium Nitrite-Based Gel against Corrosion of Rebar with Partial Short Cover Depth in Chloride Environment" Engineering Proceedings 55, no. 1: 35. https://doi.org/10.3390/engproc2023055035






