Fabrication of Titanium Oxide Thin-Film Electrodes with Photocatalytic Activities and an Evaluation of Their Photoelectrochemical Properties †
Abstract
:1. Introduction
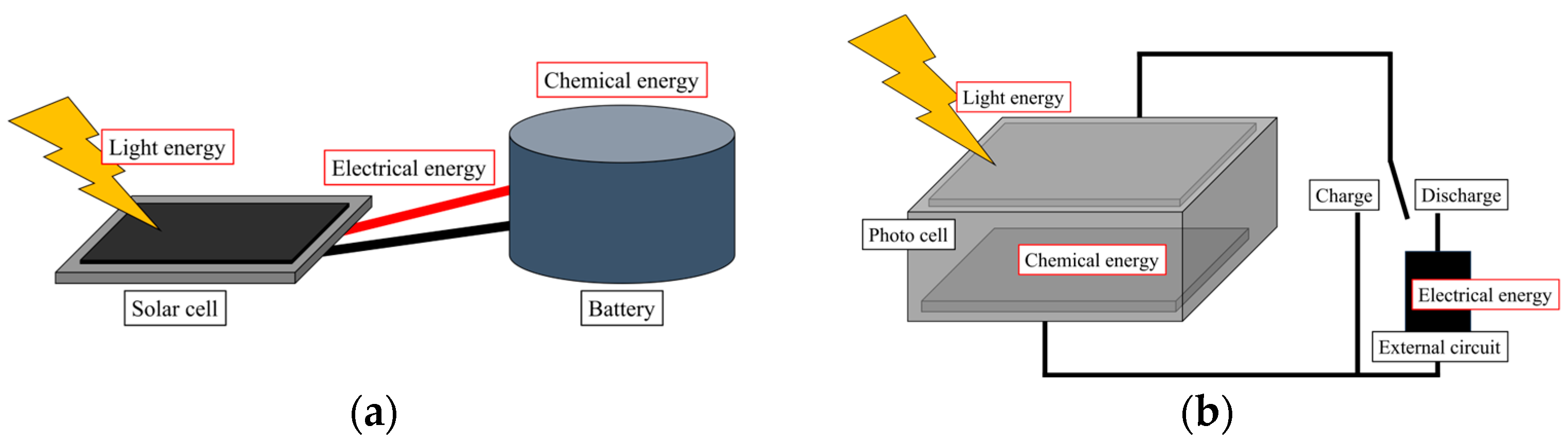
Purpose
2. Material and Method
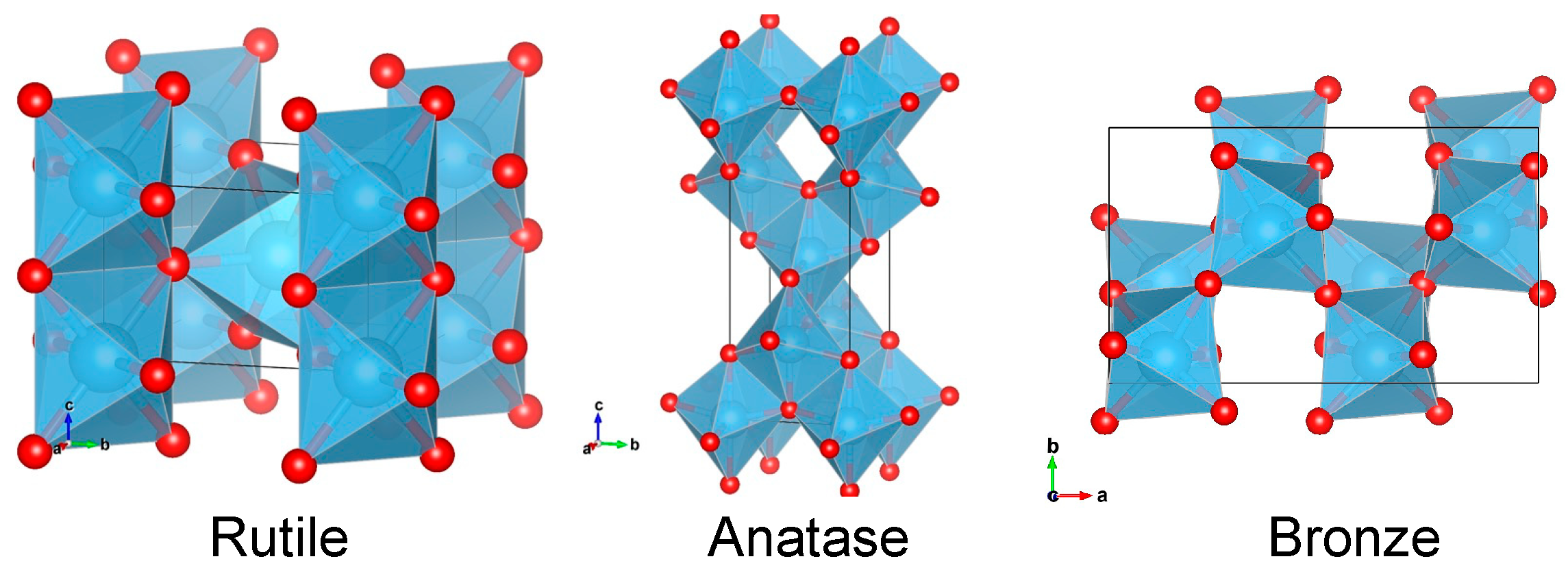
2.1. Sample Synthesis
2.2. Sample Analysis
2.3. Fabrication of Thin-Film Electrodes
2.4. Photoelectrochemical Properties
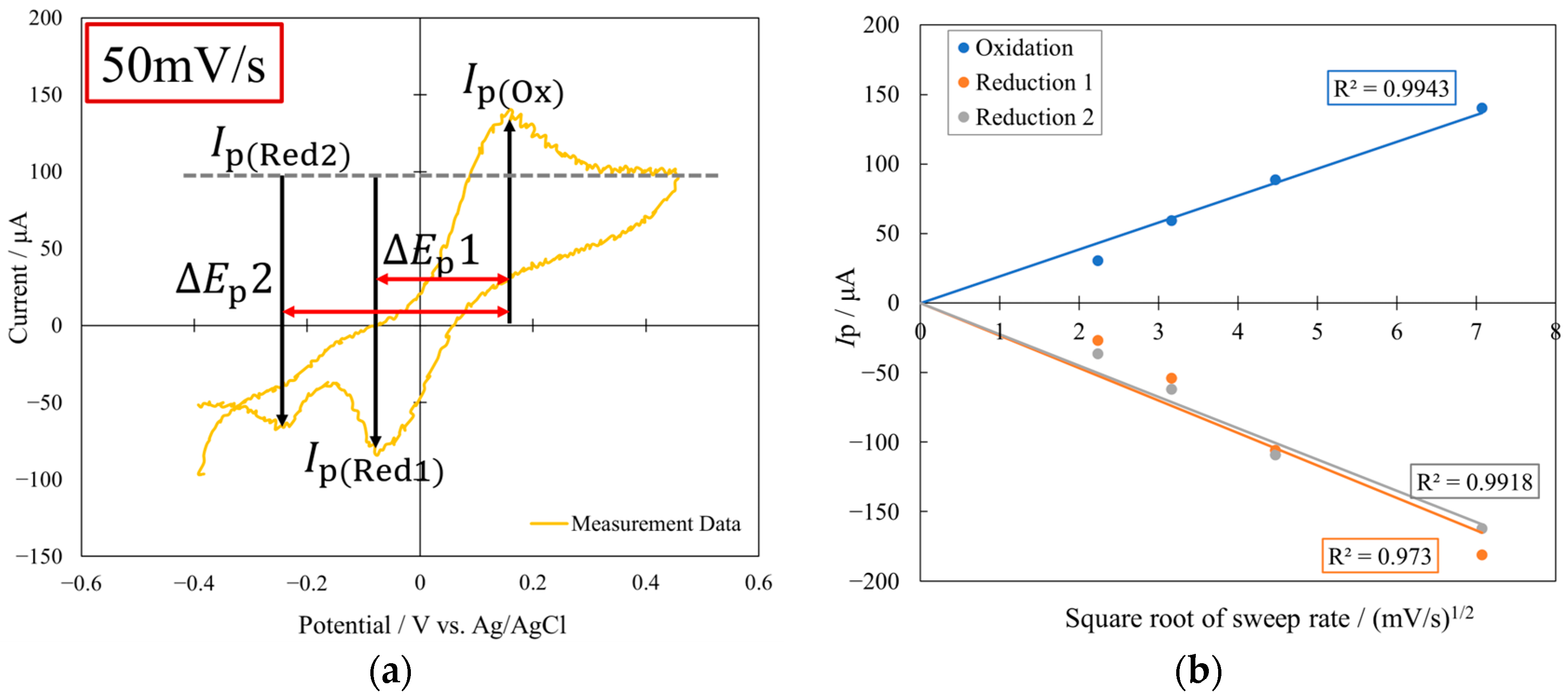
3. Results and Discussion
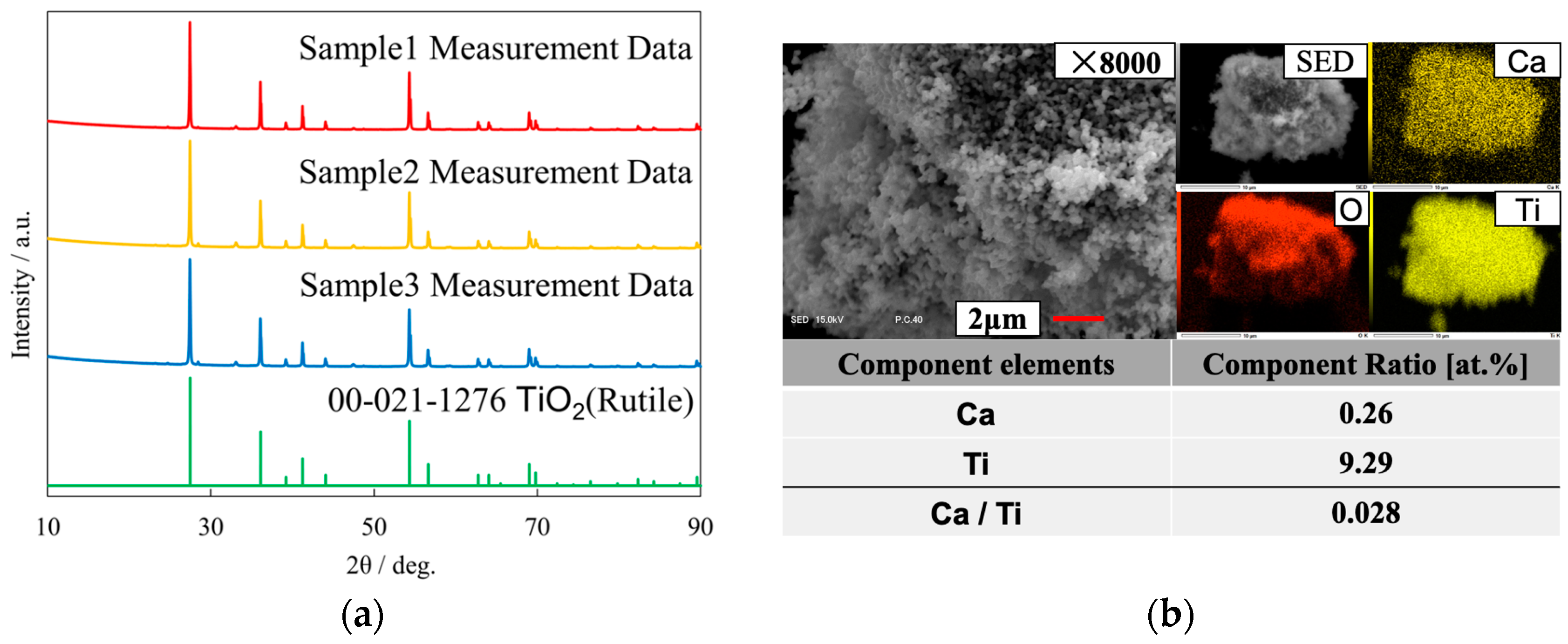
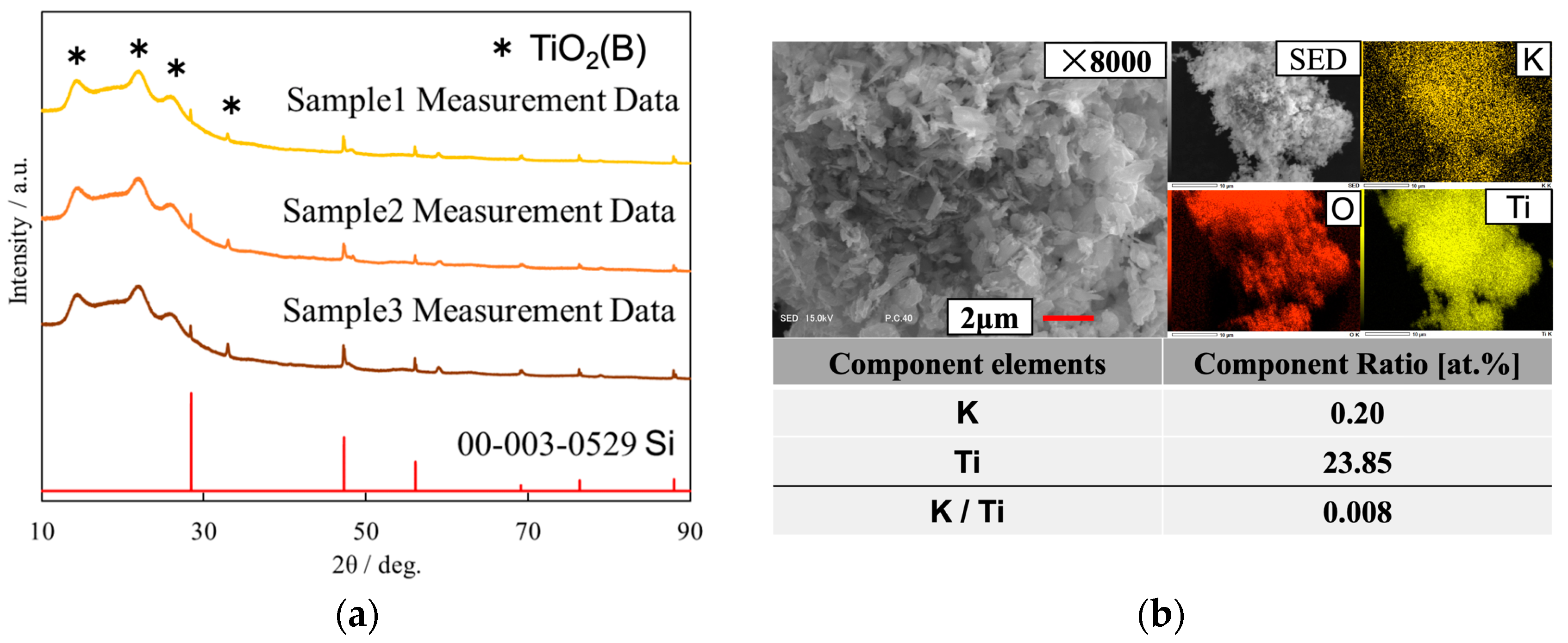
3.1. Sample Analysis
3.2. Photoelectrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Abraham, C.; Ma, Y.; Lee, M.; Helfrick, E.; Oh, D.; Lee, D. Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films. Appl. Sci. 2020, 10, 4727. [Google Scholar] [CrossRef]
- Huang, X.; Cui, S.; Wieboldt, R.C.; Hallac, P.B.; Fell, C.R.; Metz, B.; Jiang, J.; Chen, J. Hollow TiO2 as an Anode for Lithium Ion Batteries: Synthesis and In Situ Visualization of State of Charge. Adv. Electron. Mater. 2015, 1, 1500256. [Google Scholar] [CrossRef]
- Kolen’Ko, Y.V.; Kovnir, K.A.; Gavrilov, A.I.; Garshev, A.V.; Frantti, J.; Lebedev, O.I.; Churagulov, B.R.; Van Tendeloo, G.; Yoshimura, M. Hydrothermal synthesis and characterization of nanorods of various titanates and titanium dioxide. J. Phys. Chem. B 2006, 110, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- d’Elia, D.; Beauger, C.; Hochepied, J.F.; Rigacci, A.; Berger, M.H.; Keller, N.; Keller-Spitzer, V.; Suzuki, Y.; Valmalette, J.C.; Benabdesselam, M.; et al. Impact of three different TiO2 morphologies on hydrogen evolution by methanol-assisted water splitting: Nanoparticles, nanotubes and aerogels. Int. J. Hydrogen Energy 2011, 36, 14360–14373. [Google Scholar] [CrossRef]


| PVdF [g] | HSTR [g] | wt. Ratio |
|---|---|---|
| 0.05 | non | 1:0 |
| 0.05 | 0.05 | 1:1 |
| 0.05 | 0.02 | 1:0.4 |
| 0.05 | 0.01 | 1:0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakatsuki, N.; Tojo, T. Fabrication of Titanium Oxide Thin-Film Electrodes with Photocatalytic Activities and an Evaluation of Their Photoelectrochemical Properties. Eng. Proc. 2023, 55, 57. https://doi.org/10.3390/engproc2023055057
Wakatsuki N, Tojo T. Fabrication of Titanium Oxide Thin-Film Electrodes with Photocatalytic Activities and an Evaluation of Their Photoelectrochemical Properties. Engineering Proceedings. 2023; 55(1):57. https://doi.org/10.3390/engproc2023055057
Chicago/Turabian StyleWakatsuki, Naoya, and Tomohiro Tojo. 2023. "Fabrication of Titanium Oxide Thin-Film Electrodes with Photocatalytic Activities and an Evaluation of Their Photoelectrochemical Properties" Engineering Proceedings 55, no. 1: 57. https://doi.org/10.3390/engproc2023055057







