Water Treatment of Manganese Oxides and Organic Matter through Pre-Oxidation and Coagulation/Sedimentation †
Abstract
:1. Introduction
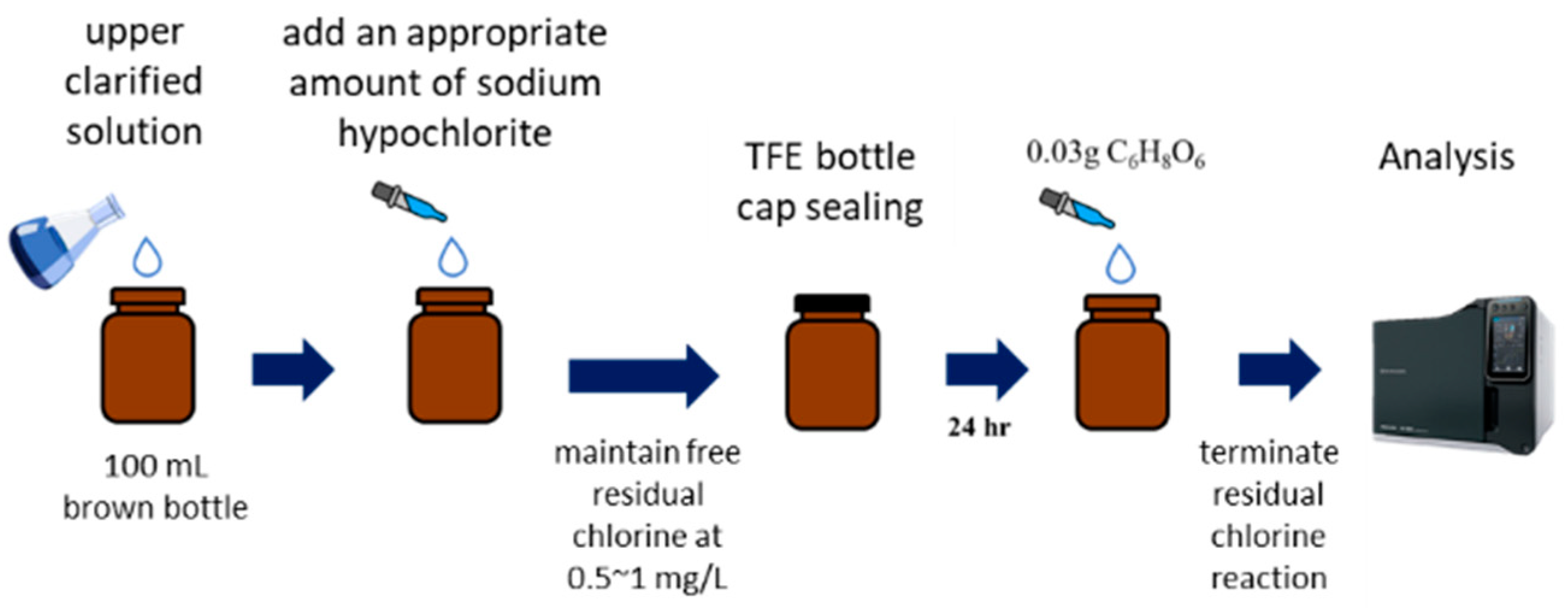
2. Materials and Methods
3. Results
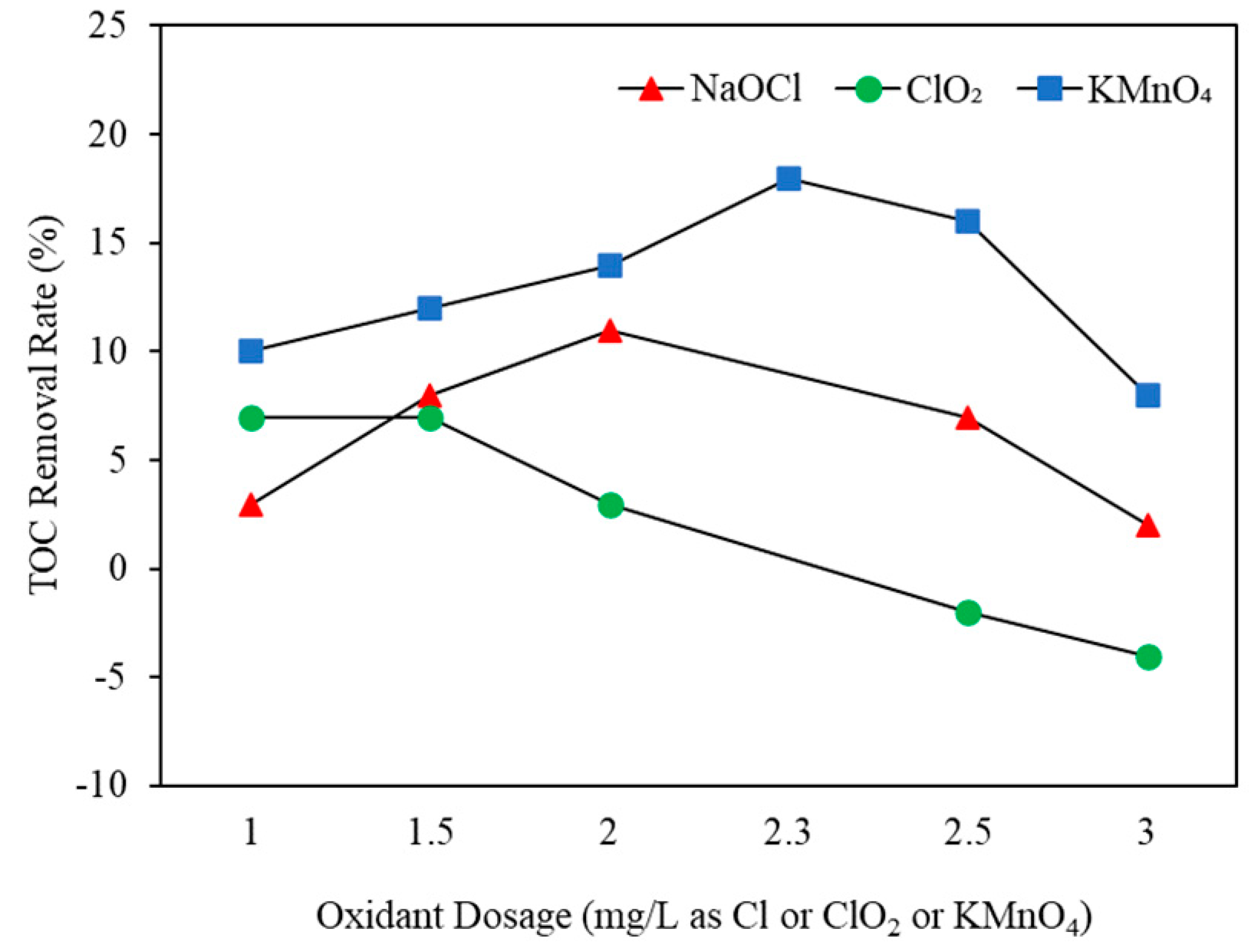
3.1. Effect of Oxidants on Organic Matter
3.1.1. Types and Doses
3.1.2. Effect of Oxidants on Coagulation and Sedimentation
3.2. Effect of Oxidants with Coagulants on Coagulation and Sedimentation
3.2.1. Manganese Removal
3.2.2. Turbidity Decrease
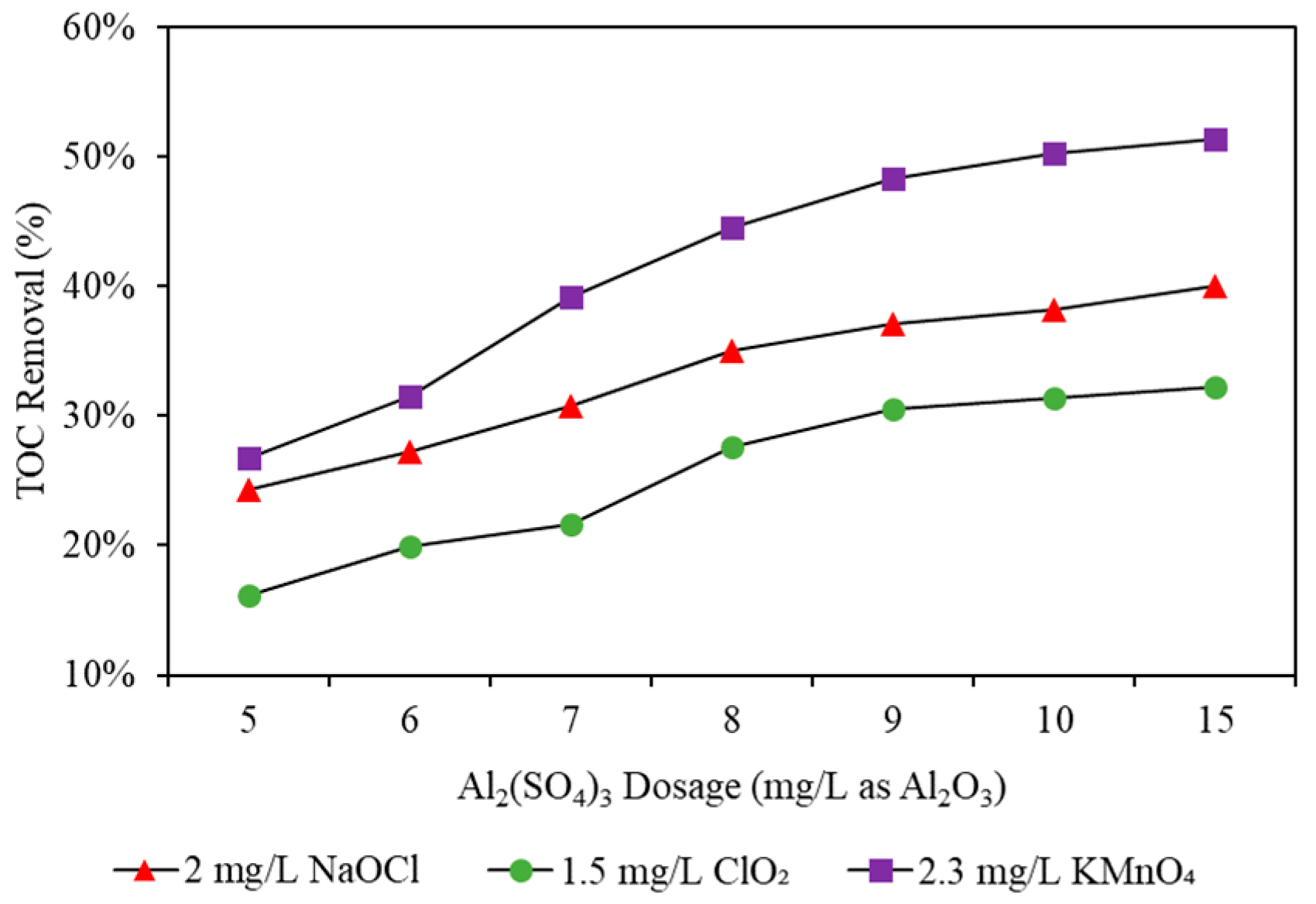
3.2.3. TOC Removal
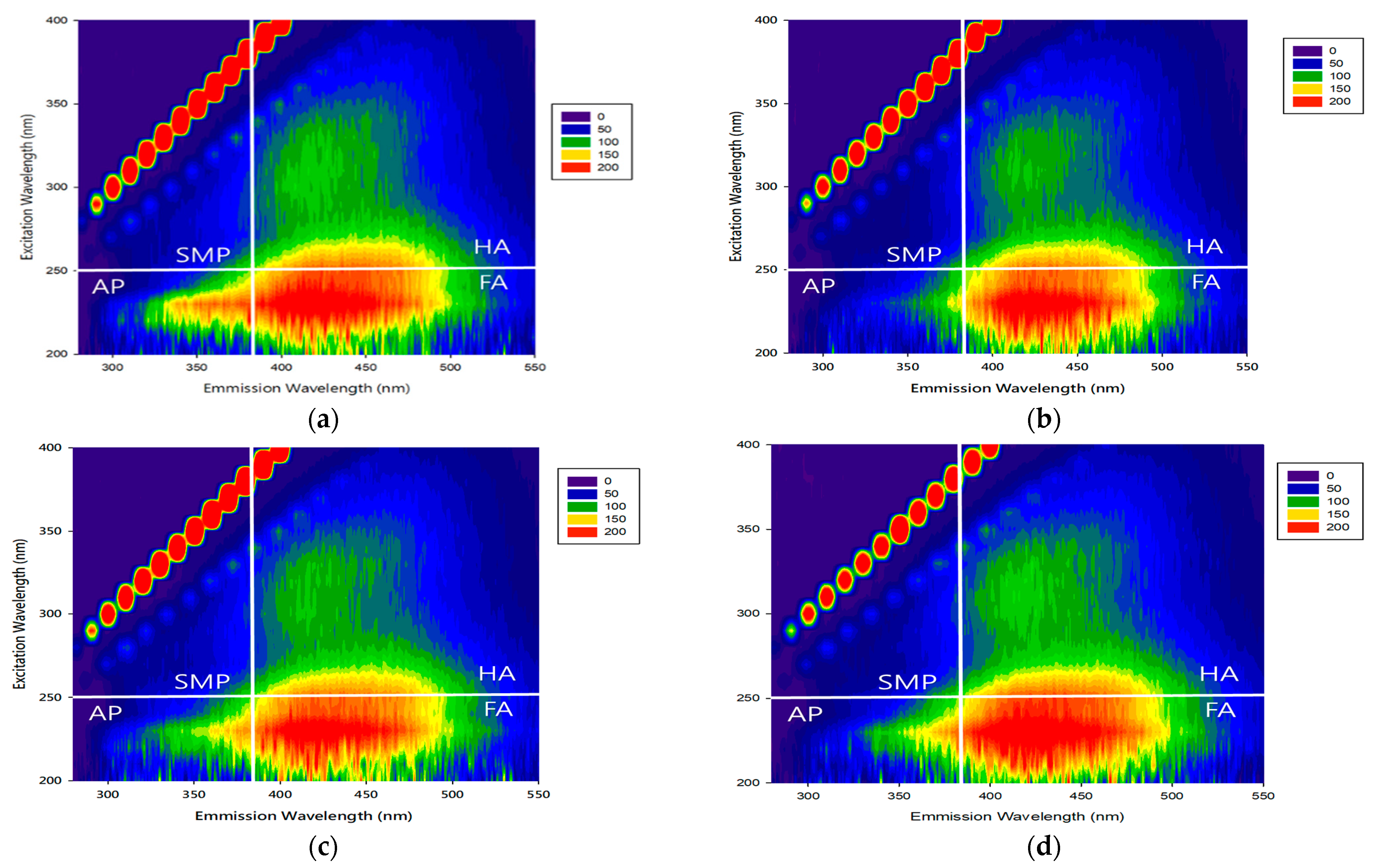
3.2.4. Effect of Organic Matter Composition on Its Removal
3.2.5. DBPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerrato, J.M. Biogeochemical Cycling of Manganese in Drinking Water Systems. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2010. [Google Scholar]
- Kohl, P.M.; Medlar, S.J. Occurrence of Manganese in Drinking Water and Manganese Control; AWWA: Denver, CO, USA, 2006. [Google Scholar]
- Uyguner Demirel, C.; Bekbolet, M.; Swietlik, J. Natural Organic Matter: Definitions and Characterization. Control of Disinfection By-Products in Drinking Water Systems; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2007; pp. 253–277. [Google Scholar]
- Dawson, J.J.C.; Malcolm, I.A.; Middlemas, S.J.; Tetzlaff, D.; Soulsby, C. Is the composition of dissolved organic carbon changing in upland acidic streams? Environ. Sci. Technol. 2009, 43, 7748–7753. [Google Scholar] [CrossRef] [PubMed]
- Aliverti, N.; Callegari, A.; Capodaglio, A.G.; Sauvignet, P. Nom removal from freshwater supplies by advanced separation technology. In Advanced Water Supply and Wastewater Treatment: A Road to Safer Society and Environment; Springer: Dordrecht, Germany, 2011. [Google Scholar]
- Delpla, I.; Jung, A.V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Yeh, H.H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Wang, C.C. Evaluation Metabolic Organic Products of Algae-Chlorella sp. and Assessing the Fate of Organic Compounds During Ozonation. Hung Kuang Unversity J. 2004, 43, 159–165. [Google Scholar]
- Tian, C.; Guo, T.T.; Liu, R.P.; Jefferson, W.; Liu, H.J.; Qu, J.H. Formation of disinfection by-products by Microcystis aeruginosa intracellular organic matter: Comparison between chlorination and bromination. Huan Jing Ke Xue 2013, 34, 4282–4289. [Google Scholar] [PubMed]
- Plummer, J.D.; Edzwald, J.K. Effects of chlorine and ozone on algal cell properties and removal of algae by coagulation. Water Supply Res. Technol.-Aqua 2002, 51, 307–318. [Google Scholar] [CrossRef]
- Chen, S.H. The Effect of ClO2 Pre-Oxidation on the Coagulation Efficiency of Drinking Water Treatment. Master’s Thesis, Department of Environmental Engineering and Science, Chia-Nan University of Pharmacy and Science, Tainan, Taiwan, 2003. [Google Scholar]
- Świetlik, J.; Raczyk-Stanisławiak, U.; Biłozor, S.; Ilecki, W.; Nawrocki, J. Adsorption of natural organic matter oxidized with ClO2 on granular activated carbon. Water Res. 2002, 36, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.C.; Huang, C.P.; Pan, J.R. Time requirement for rapid-mixing in coagulation. Colloids Surf. A Physicochem. Eng. Asp. 2002, 203, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Kang, J.; Shen, J.; Zhao, S.; Wang, B.; Zhang, X.; Chen, Z. EEM–PARAFAC characterization of dissolved organic matter and its relationship with disinfection by-products formation potential in drinking water sources of northeastern China. Sci. Total Environ. 2021, 774, 145297. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J. The Relationship between Organic Fractions of NOM and Disinfection by-Products Formation Potential. Master’s Thesis, Department of Environmental Engineering, National Cheng Kung University, Tainan, Taiwan, 2015. [Google Scholar]
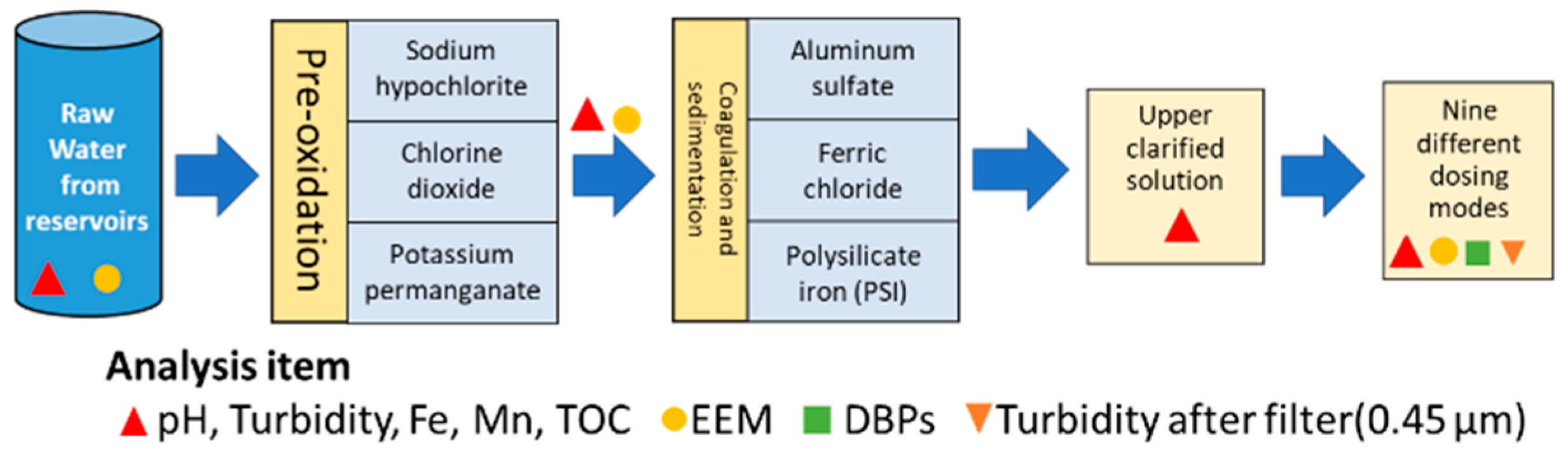


| Mode | Dosing Operation |
|---|---|
| A | 2 mg/L NaOCl + 8 mg/L Al2(SO4)3 |
| B | 2 mg/L NaOCl + 8 mg/L FeCl3 |
| C | 2 mg/L NaOCl + 3 mg/L PSI |
| D | 1.5 mg/L ClO2 + 8 mg/L Al2(SO4)3 |
| E | 1.5 mg/L ClO2 + 8 mg/L FeCl3 |
| F | 1.5 mg/L ClO2 + 3 mg/L PSI |
| G | 2.3 mg/L KMnO4 + 8 mg/L Al2(SO4)3 |
| H | 2.3 mg/L KMnO4 + 8 mg/L FeCl3 |
| I | 2.3 mg/L KMnO4 + 3 mg/L PSI |
| Mode | Mn Removal Rate (%) | Residual Mn (mg/L) |
|---|---|---|
| Raw water | N/A | 0.134 |
| NaOCl | 12% | 0.118 |
| A | 37% | 0.085 |
| B | 54% | 0.062 |
| C | 51% | 0.065 |
| ClO2 | 29% | 0.095 |
| D | 65% | 0.047 |
| E | 72% | 0.038 |
| F | 69% | 0.041 |
| KMnO4 | 71% | 0.039 |
| G | 84% | 0.022 |
| H | 92% | 0.011 |
| I | 89% | 0.015 |
| Mode | Turbidity Removal Rate (%) | Residual Turbidity (NTU) |
|---|---|---|
| Raw water | N/A | 213 |
| Only NaOCl | 3% | 206 |
| A | 99% | 2.86 |
| B | 98% | 3.96 |
| C | 98% | 4.22 |
| Only ClO2 | 20% | 170 |
| D | 99% | 1.43 |
| E | 98% | 3.71 |
| F | 98% | 3.34 |
| Only KMnO4 | 36% | 136 |
| G | 100% | 0.11 |
| H | 99% | 2.92 |
| I | 99% | 2.69 |
| Mode | TOC Removal Rate (%) | Residual TOC (mg/L) | |
|---|---|---|---|
| Raw water | N/A | 2.82 | |
| NaOCl | A | 46% | 1.51 |
| B | 30% | 1.98 | |
| C | 28% | 2.03 | |
| ClO2 | D | 38% | 1.75 |
| E | 23% | 2.17 | |
| F | 24% | 2.13 | |
| KMnO4 | G | 55% | 1.26 |
| H | 40% | 1.69 | |
| I | 37% | 1.78 | |
| Organic Matter Type | Raw Water | Mode A | Mode B | Mode C | |||
| Spectral Intensity (E0) | Spectral Intensity (E0) | Removal Rate * (%) | Spectral Intensity (E0) | Removal Rate (%) | Spectral Intensity (E0) | Removal Rate (%) | |
| SMP | 51.172 | 34.842 | 32% | 36.286 | 29% | 34.971 | 32% |
| HA | 75.366 | 34.634 | 54% | 49.108 | 35% | 48.607 | 36% |
| FA | 128.23 | 63.388 | 51% | 88.975 | 31% | 88.355 | 31% |
| AP | 64.506 | 49.504 | 23% | 49.140 | 24% | 46.599 | 28% |
| Organic Matter Type | Mode D | Mode E | Mode F | ||||
| Spectral intensity (E0) | Removal rate * (%) | Spectral intensity (E0) | Removal Rate (%) | Spectral intensity (E0) | Removal Rate (%) | ||
| SMP | 33.550 | 34% | 42.301 | 17% | 39.552 | 23% | |
| HA | 35.454 | 53% | 51.184 | 32% | 51.952 | 31% | |
| FA | 62.282 | 51% | 95.687 | 25% | 90.628 | 29% | |
| AP | 43.605 | 32% | 66.207 | −3% | 46.727 | 28% | |
| Organic Matter Type | Mode G | Mode H | Mode I | ||||
| Spectral intensity (E0) | Removal rate * (%) | Spectral intensity (E0) | Removal Rate (%) | Spectral intensity (E0) | Removal Rate (%) | ||
| SMP | 29.337 | 43% | 32.914 | 36% | 31.947 | 38% | |
| HA | 29.959 | 60% | 49.305 | 35% | 47.358 | 37% | |
| FA | 56.034 | 56% | 90.962 | 29% | 87.158 | 32% | |
| AP | 39.681 | 38% | 44.134 | 32% | 39.896 | 38% | |
| Mode | Disinfection By-Product | |
|---|---|---|
| TTHMs | HAAs | |
| A | 0.077 | 0.066 |
| B | 0.119 | 0.091 |
| C | 0.129 | 0.088 |
| D | 0.079 | 0.052 |
| E | 0.115 | 0.074 |
| F | 0.119 | 0.079 |
| G | 0.071 | 0.045 |
| H | 0.107 | 0.078 |
| I | 0.117 | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Kan, C.-C.; Lee, S.-C.; Yang, F.-Y. Water Treatment of Manganese Oxides and Organic Matter through Pre-Oxidation and Coagulation/Sedimentation. Eng. Proc. 2023, 55, 59. https://doi.org/10.3390/engproc2023055059
Wu Y-C, Kan C-C, Lee S-C, Yang F-Y. Water Treatment of Manganese Oxides and Organic Matter through Pre-Oxidation and Coagulation/Sedimentation. Engineering Proceedings. 2023; 55(1):59. https://doi.org/10.3390/engproc2023055059
Chicago/Turabian StyleWu, Yi-Chang, Chi-Chuan Kan, Shih-Chieh Lee, and Feng-Yu Yang. 2023. "Water Treatment of Manganese Oxides and Organic Matter through Pre-Oxidation and Coagulation/Sedimentation" Engineering Proceedings 55, no. 1: 59. https://doi.org/10.3390/engproc2023055059






