Cytotoxic Activity of Metal Nanoparticle Complexes †
Abstract
:1. Introduction
2. Metal-Nanoparticle Complexes
3. Anticancer Activity of MNP Complexes
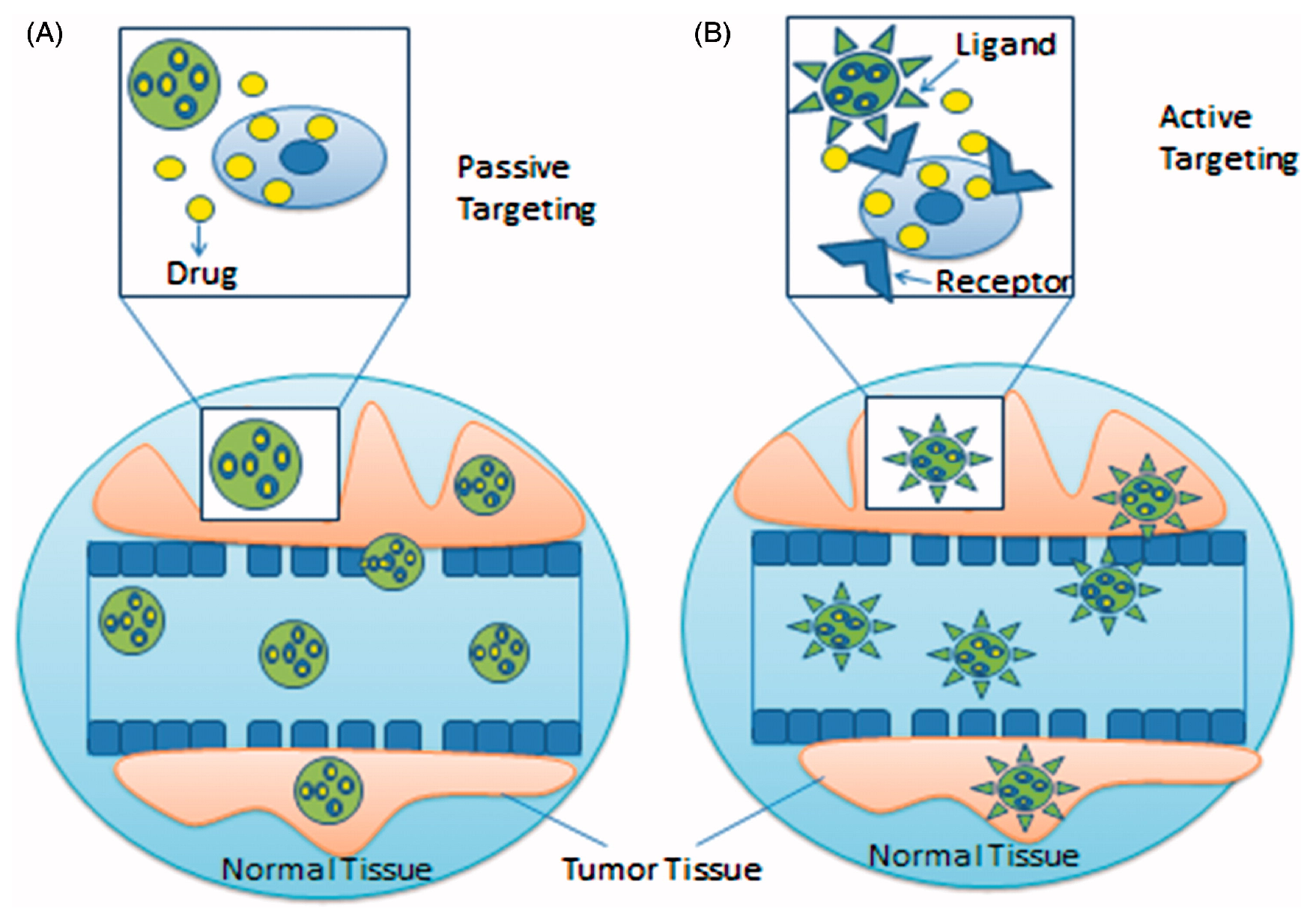
4. Targeting Strategies of Drug-Loaded NPs
4.1. Passive Targeting
4.2. Active Targeting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudi, S.Y.; Usman, M.T.; Ibrahim, S. Clinical and Industrial Application of Organometallic Compounds and Complexes: A Review. Am. J. Chem. Appl. 2015, 2, 151–158. [Google Scholar]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 4972. [Google Scholar] [CrossRef]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Meng, R.D.; Shelton, C.C.; Li, Y.M.; Qin, L.X.; Notterman, D.; Paty, P.B.; Schwartz, G.K. Gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009, 69, 573–582. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Medicinal Properties of Organometallic Compounds. In Bioorganometallic Chemistry, 1st ed.; Simonneaux, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 17, pp. 177–210. [Google Scholar]
- Hashmi, K.; Satya; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal Applications of Vanadium Complexes with Schiff Bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar] [CrossRef]
- Satya; Hashmi, K.; Gupta, S.; Singh, N.; Khan, T.; Joshi, S. Nanofabrication of Metals and Their Compounds for Effective Medicinal and Environmental Applications (A Review). Russ. J. Gen. Chem. 2023, 93, 635–665. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Challenges for Metals in Medicine: How Nanotechnology May Help To Shape the Future. ACS Nano 2013, 7, 5654–5659. [Google Scholar] [CrossRef]
- Maldonado, C.R.; Salassa, L.; Gomez-Blanco, N.; Mareque-Rivas, J.C. Nano-functionalization of metal complexes for molecular imaging and anticancer therapy. Coord. Chem. Rev. 2013, 257, 2668–2688. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Taleghani, A.S.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2022, 27, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Balva, M.; Legeai, S.; Garoux, L.; Leclerc, N.; Meux, E. Dismantling and chemical characterization of spent Peltier thermoelectric devices for antimony, bismuth and tellurium recovery. Environ. Technol. 2016, 38, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Yang, M.; Li, M.X.; Yu, H.; Wu, H.C.; Xie, S.Q. Synthesis, crystal structure and biological evaluation of a main group seven-coordinated bismuth (III) complex with 2-acetylpyridine N4-phenylthiosemicarbazone. Bioorganic Med. Chem. Lett. 2013, 23, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Rodrigues, B.L.; Marzano, I.M.; Perreira-Maia, E.C.; Dittz, D.; Paz Lopes, M.T.; Ishfaq, M.; Frézard, F.; Demicheli, C. Cytotoxicity and apoptotic activity of novel organobismuth(V) and organoantimony(V) complexes in different cancer cell lines. Eur. J. Med. Chem. 2016, 109, 254–267. [Google Scholar] [CrossRef]
- Božić, A.R.; Bjelogrlić, S.K.; Novaković, I.T.; Filipović, N.R.; Petrović, P.M.; Marinković, A.D.; Todorović, T.R.; Cvijetić, I.N. Antimicrobial Activity of Thiocarbohydrazones: Experimental Studies and Alignment-Independent 3D QSAR Models. ChemistrySelect 2018, 3, 2215–2221. [Google Scholar] [CrossRef]
- El-Wakiel, N. Nano-sized metal complexes of azo L-histidine: Synthesis, characterization and their application for catalytic oxidation of 2-amino phenol. Appl. Organomet. Chem. 2018, 32, e4229. [Google Scholar] [CrossRef]
- Jawoor, S.S.; Patil, S.A.; Kumbar, M.; Ramawadgi, P.B. Green synthesis of nano sized transition metal complexes containing heterocyclic Schiff base: Structural and morphology characterization and bioactivity study. J. Mol. Struct. 2018, 1164, 378–385. [Google Scholar] [CrossRef]
- Mahmoud, W.; Refaat, A.M.; Mohamed, G.G. Nano Schiff Base and Its Metal Complexes: Synthesis, Characterization Tools, Biological Applications and Molecular Docking Studies. Egypt. J. Chem. 2020, 63, 2157–2176. [Google Scholar]
- Chattopadhyay, S.; Chakraborty, S.P.; Laha, D.; Baral, R.; Pramanik, P.; Roy, S. Surface-modified cobalt oxide nanoparticles: New opportunities for anti-cancer drug development. Cancer Nanotechnol. 2012, 3, 13–23. [Google Scholar] [CrossRef]
- Adwin Jose, P.; Sankarganesh, M.; Dhaveethu Raja, J.; Senthilkumar, G.S.; Nandini Asha, R.; Raja, S.J.; Sheela, C.D. Bio-inspired nickel nanoparticles of pyrimidine-Schiff base: In vitro anticancer, BSA and DNA interactions, molecular docking and antioxidant studies. J. Biomol. Struct. Dyn. 2022, 40, 10715–10729. [Google Scholar] [CrossRef]
- Jose, P.A.; Raja, J.D.; Sankarganesh, M.; Rajesh, J. Evaluation of antioxidant, DNA targeting, antimicrobial and cytotoxic studies of imine capped copper and nickel nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 178, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Harish, R.; Nisha, K.D.; Prabakaran, S.; Sridevi, B.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Vinniee, C.; Ganesh, M.R. Cytotoxicity assessment of chitosan coated CdS nanoparticles for bio-imaging applications. Appl. Surf. Sci. 2020, 499, 143817. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Patil, R.V.; Havaldar, D.V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnology 2021, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, Q.; Qin, X.; Bhavsar, D.; Yang, L.; Chen, Q.; Zheng, W.; Chen, L.; Liu, J. Improving the anticancer efficacy of laminin receptor-specific therapeutic ruthenium nanoparticles (RuBB-loaded EGCG-RuNPs) via ROS-dependent apoptosis in SMMC-7721 cells. ACS Appl. Mater. Interfaces 2017, 8, 15000–15012. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Charmi, J.; Salehiabar, M.; Abhari, F.; Danafar, H. Tumor targeted albumin coated bismuth sulfide nanoparticles (Bi2S3) as radiosensitizers and carriers of curcumin for enhanced chemoradiation therapy. ACS Biomater. Sci. Eng. 2019, 5, 4416–4424. [Google Scholar] [CrossRef] [PubMed]
- Jarestan, M.; Khalatbari, K.; Pouraei, A.; Sadat Shandiz, S.A.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech 2020, 10, 230. [Google Scholar] [CrossRef]
- Binu, N.M.; Prema, D.; Prakash, J.; Balagangadharan, K.; Balashanmugam, P.; Selvamurugan, N.; Venkatasubbu, G.D. Folic acid decorated pH sensitive polydopamine coated honeycomb structured nickel oxide nanoparticles for targeted delivery of quercetin to triple negative breast cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127609. [Google Scholar] [CrossRef]
- Akturk, O. The anticancer activity of doxorubicin-loaded levan-functionalized gold nanoparticles synthesized by laser ablation. Int. J. Biol. Macromol. 2022, 196, 72–85. [Google Scholar] [CrossRef]
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Zalba, S.; Navarro, I.; de Ilarduya, C.T.; Garrido, M.J. Pharmacodynamics of cisplatin-loaded PLGA nanoparticles administered to tumor-bearing mice. Eur. J. Pharm. Biopharm. 2010, 74, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, H.; Mosavian, M.H.; Sabouri, Z.; Khazaei, M.; Darroudi, M. pH-responsive and CD44-targeting by Fe3O4/MSNs-NH2 nanocarriers for oxaliplatin loading and colon cancer treatment. Inorg. Chem. Commun. 2021, 125, 108430. [Google Scholar] [CrossRef]
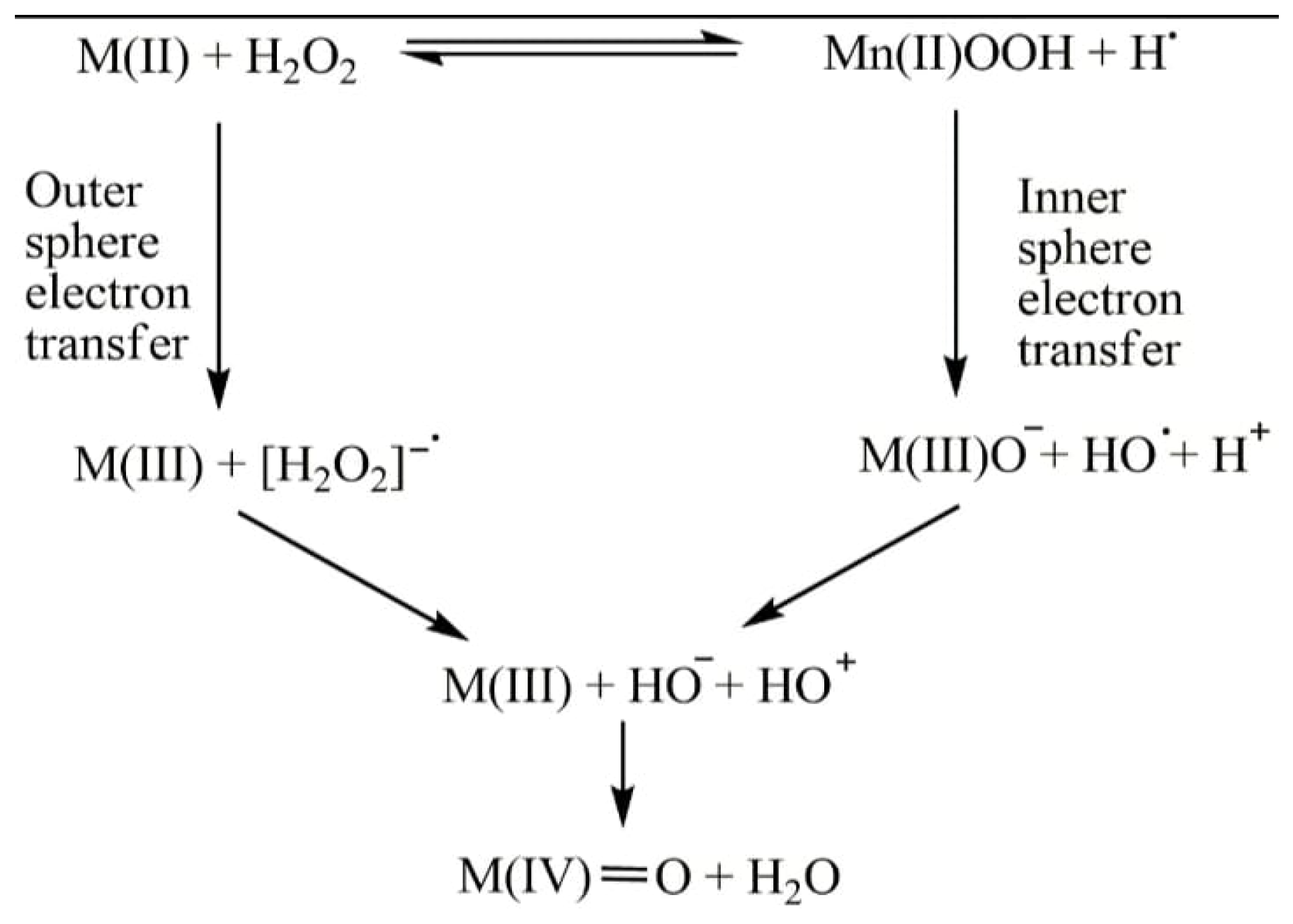
| MNPs | Functionalized Ligand | Cancer Cell Lines | Reference |
|---|---|---|---|
| CoONPs | phosphonomethyl iminodiacetic acid | Jurkat (T-cell lymphoma) and KB (oral carcinoma) cell lines | [20] |
| NiNPs | 2-(4,6-dimethoxypyrimidin-2-ylimino)methyl)-6-methoxyphenol (DMPMM) | human breast cancer (MCF-7), human lung cancer (A549) and human liver cancer(HepG2) cell lines | [21] |
| CuNPs | DMPMM | human breast cancer cell line (MCF-7) | [22] |
| CdSNPs | Chitosan | Human Jurkat cell and erythrocytes cell lines | [23] |
| AuNPs | quercetin | human breast cancer cell line (MCF-7) | [24] |
| RuNPs | epigallocatechin gallate | human liver cancer(HepG2) cell line | [25] |
| BiSNPs | Curcumin | Mouse breast cancer cell line(4T1) | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Satya; Hashmi, K.; Gupta, S.; Joshi, S. Cytotoxic Activity of Metal Nanoparticle Complexes. Eng. Proc. 2023, 56, 27. https://doi.org/10.3390/ASEC2023-15242
Singh N, Satya, Hashmi K, Gupta S, Joshi S. Cytotoxic Activity of Metal Nanoparticle Complexes. Engineering Proceedings. 2023; 56(1):27. https://doi.org/10.3390/ASEC2023-15242
Chicago/Turabian StyleSingh, Nidhi, Satya, Kulsum Hashmi, Sakshi Gupta, and Seema Joshi. 2023. "Cytotoxic Activity of Metal Nanoparticle Complexes" Engineering Proceedings 56, no. 1: 27. https://doi.org/10.3390/ASEC2023-15242




