First-Principle Calculation Analysis on Electronic Structures and Molecular Dynamics of Gadolinium-Doped FAPbI3 †
Abstract
:1. Introduction
2. Calculation
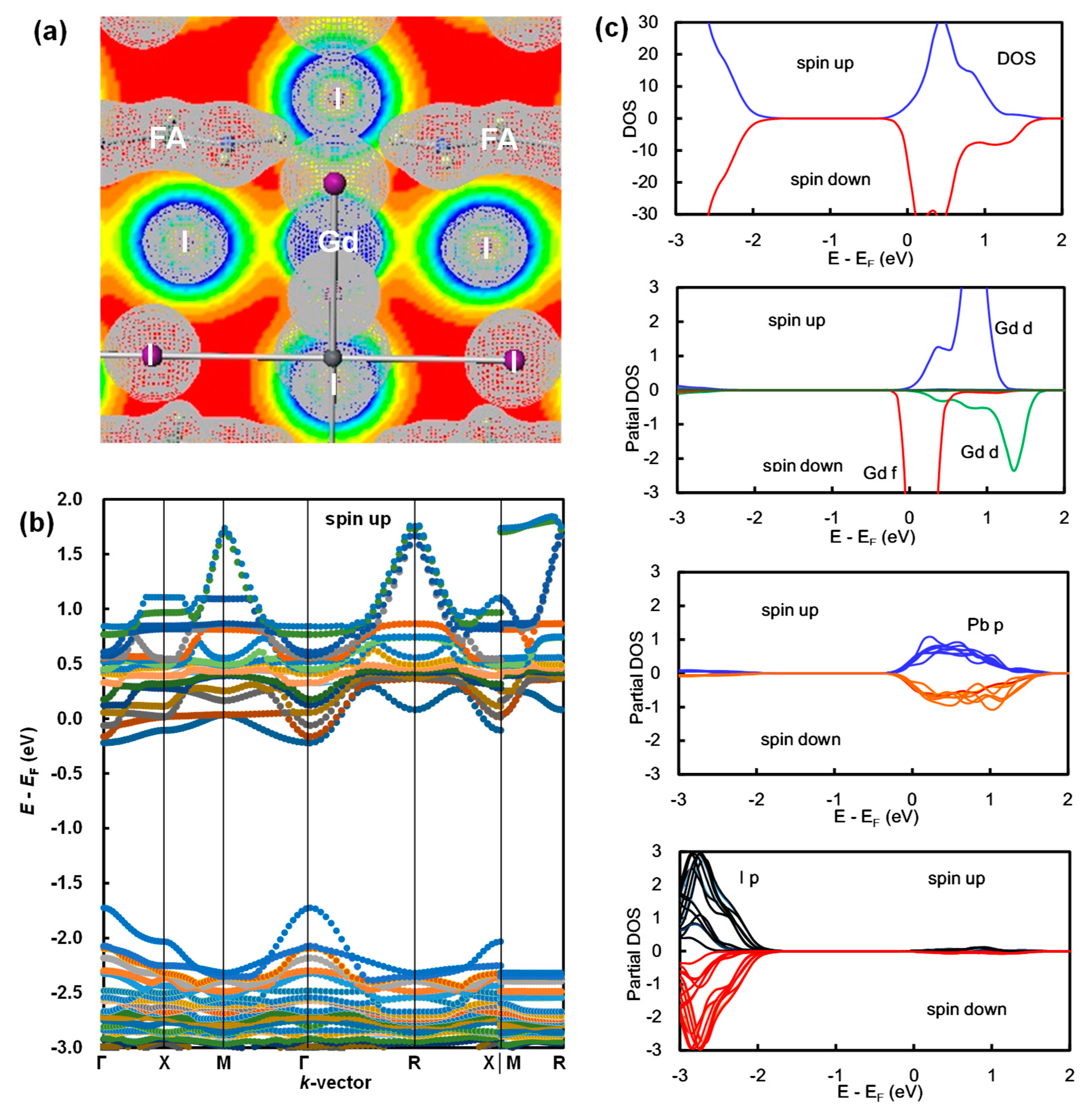
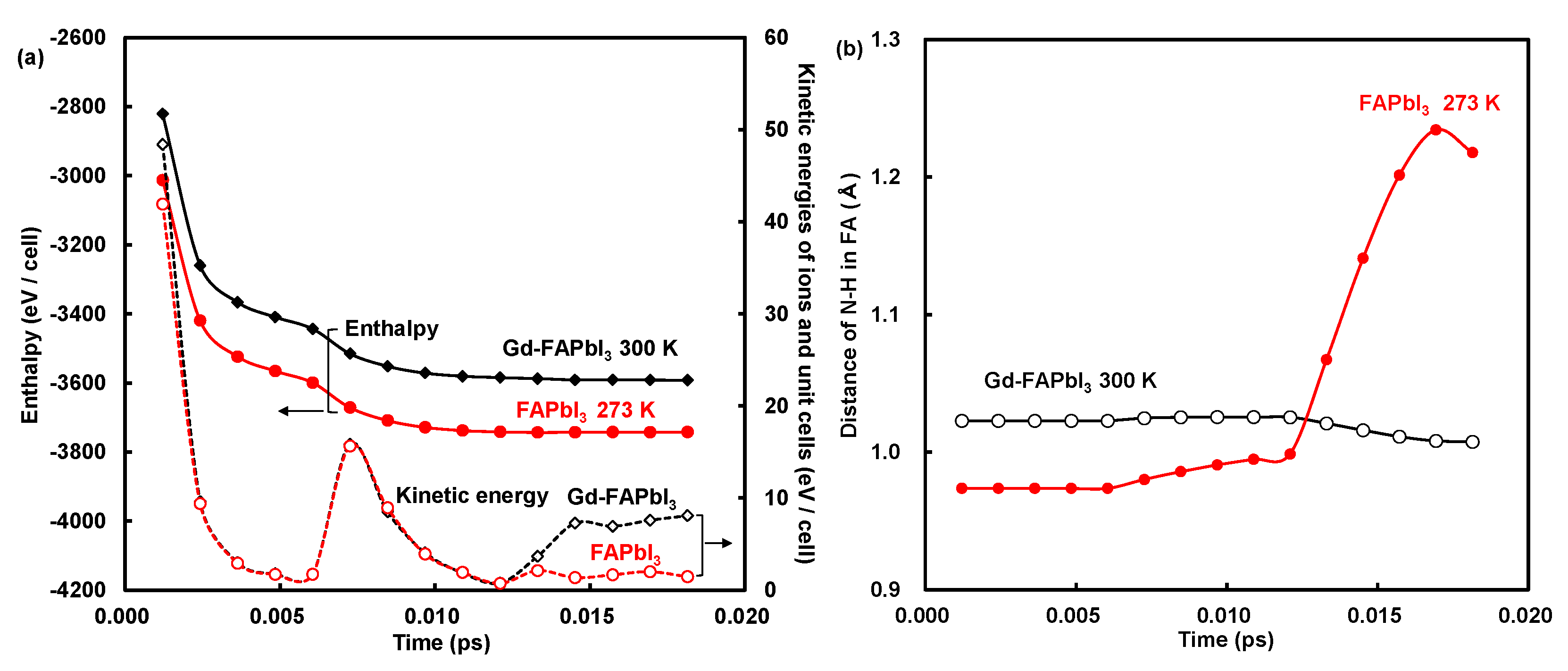
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Wang, X.; Bi, E.; Jiang, F.; Park, S.M.; Li, Y.; Chen, L.; Wang, Z.; Zeng, L.; Chen, H.; et al. Rational design of Lewis base molecules for stable and efficient inverted perovskite solar cells. Science 2023, 379, 690–694. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, Z.; Shao, Z.; Hao, L.; Rao, Y.; Chen, C.; Liu, D.; Zhao, Q.; Sun, X.; et al. Ammonia for post-healing of formamidinium-based Perovskite films. Nat. Commun. 2022, 13, 4417. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Chen, Y.; Qiu, W.; Peng, Q. Stable perovskite solar cells with 25.17% efficiency enabled by improving crystallization and passivating defects synergistically. Energy Environ. Sci. 2022, 15, 4700–4709. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, D.; Wang, X.; Jiang, X.; Liu, B.; Li, B.; Li, Z.; Gao, D.; Zhang, C.; Wang, Y.; et al. Eco-friendly perovskite solar cells: From materials design to device processing and recycling. EcoMat 2023, 5, e12352. [Google Scholar] [CrossRef]
- Ono, I.; Oku, T.; Suzuki, A.; Asakawa, Y.; Terada, S.; Okita, M.; Fukunishi, S.; Tachikawa, T. Fabrication and characterization of CH3NH3PbI3 solar cells with added guanidinium and inserted with decaphenylpentasilane. Jpn. J. Appl. Phys. 2022, 61, SB1024. [Google Scholar] [CrossRef]
- Okumura, R.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of adding alkali metals and organic cations to Cu-based perovskite solar cells. Appl. Sci. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cu, K and guanidinium addition to CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2022, 51, 4317–4328. [Google Scholar] [CrossRef]
- Asakawa, Y.; Oku, T.; Kido, M.; Suzuki, A.; Okumura, R.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Fabrication and characterization of SnCl2- and CuBr-added perovskite photovoltaic devices. Technologies 2022, 10, 112. [Google Scholar] [CrossRef]
- Terada, S.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Ethylammonium bromide- and potassium-added CH3NH3PbI3 perovskite solar cells. Photonics 2022, 9, 791. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. First-principles calculations and device characterizations of formamidinium-cesium lead triiodide perovskite crystals stabilized by germanium or copper. Jpn. J. Appl. Phys. 2023, 62, SK1015. [Google Scholar] [CrossRef]
- Hossain, M.K.; Toki, G.F.I.; Kuddus, A.; Rubel, M.H.K.; Hossain, M.M.; Bencherif, H.; Rahman, M.F.; Islam, M.R.; Mushtaq, M. An extensive study on multiple ETL and HTL layers to design and simulation of high-performance lead-free CsSnCl3-based perovskite solar cells. Sci. Rep. 2023, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Mohammed, M.K.A.; Pandey, R.; Arnab, A.A.; Rubel, M.H.K.; Hossain, K.M.; Ali, M.H.; Rahman, M.F.; Bencherif, H.; Madan, J.; et al. Numerical analysis in DFT and SCAPS-1D on the influence of different charge transport layers of CsPbBr3 perovskite solar cells. Energy Fuels 2023, 37, 6078. [Google Scholar] [CrossRef]
- Hossain, M.K.; Toki, G.F.I.; Samajdar, D.P.; Mushtaq, M.; Rubel, M.H.K.; Pandey, R.; Madan, J.; Mohammed, M.K.A.; Islam, M.R.; Rahman, M.F.; et al. Deep insights into the coupled optoelectronic and photovoltaic analysis of lead-free CsSnI3 perovskite-based solar cell using DFT calculations and SCAPS-1D simulations. ACS Omega 2023, 8, 22466–22485. [Google Scholar] [CrossRef]
- Diao, X.; Diao, Y.; Tang, Y.; Zhao, G.; Gu, Q.; Xie, Y.; Shi, Y.; Zhu, P.; Zhang, L. High-throughput screening of stable and efficient double inorganic halide perovskite materials by DFT. Sci. Rep. 2022, 12, 12633. [Google Scholar] [CrossRef]
- Ma, C.; Eickemeyer, F.T.; Lee, S.H.; Kang, D.H.; Kwon, S.J.; Grätzel, M.; Park, N.G. Unveiling facet-dependent degradation and facetengineering for stable perovskite solar cells. Science 2023, 379, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chin, X.Y.; Turkay, D.; Steele, J.A.; Tabean, S.; Eswara, S.; Mensi, M.; Fiala, P.; Wolff, C.M.; Paracchino, A.; Artuk, K.; et al. Interface passivation for 31.25%-efficient perovskite/silicon tandem solar cells. Science 2023, 381, 59–63. [Google Scholar] [CrossRef]
- Xia, J.; Liang, C.; Gu, H.; Mei, S.; Li, S.; Zhang, N.; Chen, S.; Cai, Y.; Xing, G. Surface passivation toward efficient and stable perovskite solar cells. Energy Environ. Mater. 2023, 6, e12296. [Google Scholar] [CrossRef]
- Ren, J.; Liu, T.; He, B.; Wu, G.; Gu, H.; Wang, B.; Li, J.; Mao, Y.; Chen, S.; Xing, G. Passivating defects at the bottom interface of perovskite by ethylammonium to improve the performance of perovskite solar cells. Small 2022, 18, 2203536. [Google Scholar] [CrossRef] [PubMed]
- Azmi, R.; Ugur, E.; Seitkhan, A.; Aljamaan, F.; Subbiah, A.S.; Liu, J.; Harrison, G.T.; Nugraha, M.I.; Eswaran, M.K.; Babics, M.; et al. Damp heat–stable perovskite solar cells with tailored-dimensionality 2D/3D heterojunctions. Science 2022, 376, 73–77. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Y.; Xing, G.; Cao, D. Surface passivation of perovskite with organic hole transport materials for highly efficient and stable perovskite solar cells. Mater. Mater. Today Adv. 2022, 16, 100300. [Google Scholar] [CrossRef]
- Lin, R.; Xu, J.; Wei, M.; Wang, Y.; Qin, Z.; Liu, Z.; Wu, J.; Xiao, K.; Chen, B.; Park, S.M.; et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 2022, 603, 73–78. [Google Scholar] [CrossRef]
- Suzuki, A.; Hasegawa, R.; Funayama, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Additive effects of CuPcX4-TCNQ on CH3NH3PbI3 perovskite solar cells. J. Mater. Sci. Mater. Electron. 2023, 34, 588. [Google Scholar] [CrossRef]
- Ogawa, C.; Suzuki, A.; Oku, T.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Metallophtalocyanine used interface engineering for improving long-term stability of methylammonium lead triiodide perovskite. Phys. Status Solidi A 2023, 220, 2300038. [Google Scholar] [CrossRef]
- Kachhap, S.; Singh, S.; Singh, A.K.; Singh, S.K. Lanthanide-doped inorganic halide perovskites (CsPbX3): Novel properties and emerging applications. J. Mater. Chem. C 2022, 10, 3647. [Google Scholar] [CrossRef]
- Samanta, T.; Mukurala, N.; Viswanath, N.S.M.; Han, J.H.; Cho, H.B.; Min, J.W.; Jung, S.W.; Park, Y.; Chung, W.J.; Im, W.B. Recent progress in lanthanide-based metal halide perovskites: Synthesis, properties, and applications. Opt. Mater. X 2023, 18, 100238. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L.; et al. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mir, W.J.; Sheikh, T.; Arfin, H.; Xia, Z.; Nag, A. Lanthanide doping in metal halide perovskite nanocrystals: Spectral shifting, quantum cutting and optoelectronic applications. NPG Asia Mater. 2020, 12, 9. [Google Scholar] [CrossRef]
- Lee, M.; Lee, D.H.D.; Hong, S.V.; Woo, H.Y.; Chae, J.-Y.; Lee, D.W.; Han, M.J.; Paik, T. Highly luminescent and multifunctional zero-dimensional cesium lanthanide chloride (Cs3LnCl6) colloidal nanocrystals. Adv. Opt. Mater. 2022, 10, 2102727. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, M. Lanthanide doped lead-free double perovskites as the promising next generation ultra-broadband light sources. Light Sci. Appl. 2022, 11, 99. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Wang, X.; Guo, Q.; Liu, X.; Sun, W.; Wei, Y.; Huang, Y.; Lan, Z.; Huang, M.; et al. Suppressing vacancy defects and grain boundaries via ostwald ripening for high-performance and stable perovskite solar cells. Adv. Mater. 2020, 32, 1904347. [Google Scholar] [CrossRef]
- Suzuki, A.; Kishimoto, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effect of lanthanide compounds into perovskite layer on photovoltaic properties and electronic structures. Synth. Met. 2022, 287, 117092. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Electronic structures and molecular dynamics of gadolinium-doped FAPbI3 perovskite crystals. Jpn. J. Appl. Phys. 2023, 62, SK1006. [Google Scholar] [CrossRef]
- Fransson, E.; Rosander, P.; Eriksson, F.; Rahm, J.M.; Tadano, T.; Erhart, P. Limits of the phonon quasi-particle picture at the cubic-to-tetragonal phase transition in halide perovskites. Commun. Phys. 2023, 6, 173. [Google Scholar] [CrossRef]
- Kaiser, W.; Mosconi, E.; Alothman, A.A.; Meggiolaro, D.; Gagliardi, A.; Angelis, F.D. Halide-driven formation of lead halide perovskites: Insight from ab initio molecular dynamics simulations. Mater. Adv. 2021, 2, 3915–3926. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, L.; Lei, W.; Wang, S.; Xi, J.; Yang, J. Temperature-dependence of the band gap in the all-inorganic perovskite CsPbI3 from room to high temperatures. Phys. Chem. Chem. Phys. 2022, 24, 16003–16010. [Google Scholar] [CrossRef]
- Kaiser, W.; Ricciarelli, D.; Mosconi, E.; Alothman, A.A.; Ambrosio, F.; Angelis, F.D. Stability of tin-versus lead-halide perovskites: Ab initio molecular dynamics simulations of perovskite/water interfaces. J. Phys. Chem. Lett. 2022, 13, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-L.; Adesiyan, A.; Gassoumi, A.; Gorji, N.E. A molecular dynamics study of water confined in between two graphene sheets under compression. J. Nanopart. Res. 2023, 25, 51. [Google Scholar] [CrossRef]
- Arora, N.; Greco, A.; Meloni, S.; Hinderhofer, A.; Mattoni, A.; Rothlisberger, U.; Hagenlocher, J.; Caddeo, C.; Zakeeruddin, S.M.; Schreiber, F.; et al. Kinetics and energetics of metal halide perovskite conversion reactions at the nanoscale. Commun. Mater. 2022, 3, 22. [Google Scholar] [CrossRef]
- Shi, R.; Guo, M.; Long, R. Improved defect tolerance and charge carrier lifetime in tin–lead mixed perovskites: Ab Initio quantum dynamics, J. Phys. Chem. Lett. 2023, 14, 499–507. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic perovskite structure of black formamidinium lead iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Mashiyama, H.; Kurihara, Y.; Azetsu, T. Disordered cubic perovskite structure of CH3NH3PbX3 (X = Cl, Br, I). J. Korean Phys. Soc. 1998, 32, S156–S158. [Google Scholar]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, A.; Oku, T. First-Principle Calculation Analysis on Electronic Structures and Molecular Dynamics of Gadolinium-Doped FAPbI3. Eng. Proc. 2023, 56, 33. https://doi.org/10.3390/ASEC2023-15332
Suzuki A, Oku T. First-Principle Calculation Analysis on Electronic Structures and Molecular Dynamics of Gadolinium-Doped FAPbI3. Engineering Proceedings. 2023; 56(1):33. https://doi.org/10.3390/ASEC2023-15332
Chicago/Turabian StyleSuzuki, Atsushi, and Takeo Oku. 2023. "First-Principle Calculation Analysis on Electronic Structures and Molecular Dynamics of Gadolinium-Doped FAPbI3" Engineering Proceedings 56, no. 1: 33. https://doi.org/10.3390/ASEC2023-15332





