Electrical and Optical Properties of Controlled Reduced Graphene Oxide Prepared by a Green and Facile Route †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
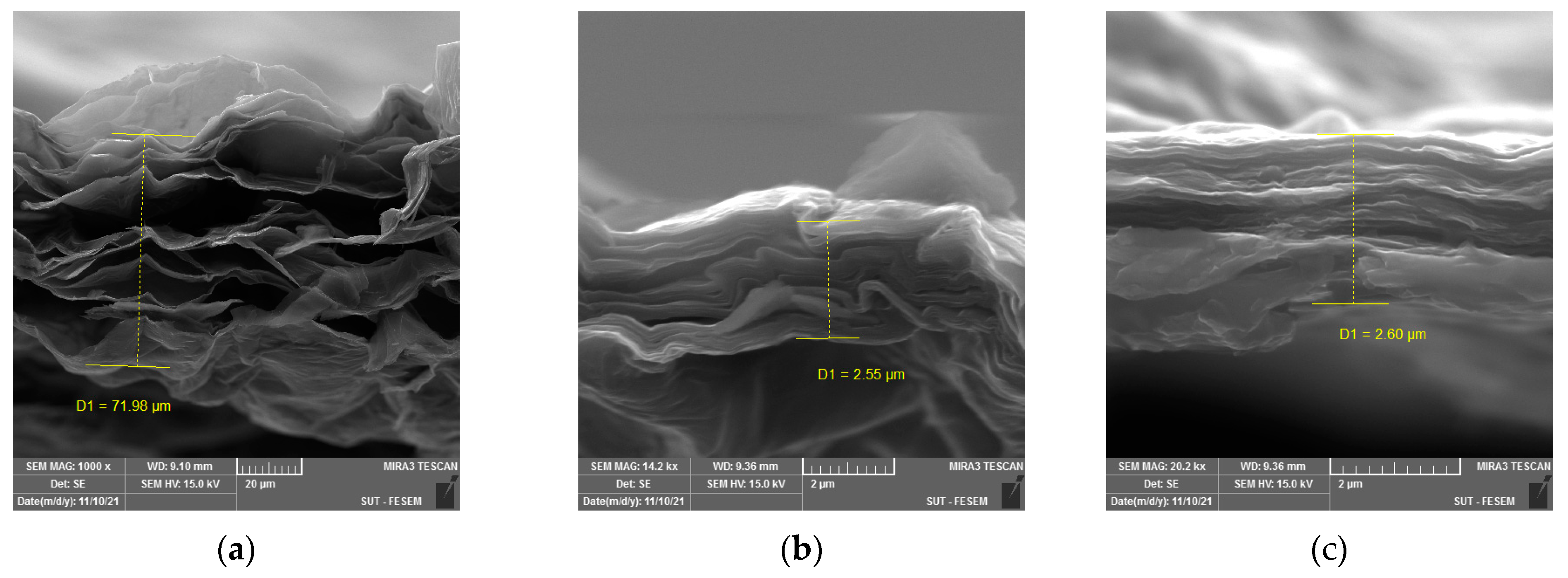
3.1. Structural Properties
3.2. Electrical Properties
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Rokmana, A.W.; Asriani, A.; Suhendar, H.; Triyana, K.; Kusumaatmaja, A.; Santoso, I. The optical properties of thin film reduced graphene oxide/poly (3, 4 ethylenedioxtriophene): Poly (styrene sulfonate)(pedot: Pss) fabricated by spin coating. Proc. J. Phys. Conf. Ser. 2018, 1011, 012007. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, T.; Yao, Y.; Li, T.; Hu, L.; Marconnet, A. Thermally conductive reduced graphene oxide thin films for extreme temperature sensors. Adv. Funct. Mater. 2019, 29, 1901388. [Google Scholar] [CrossRef]
- Liu, G.; Tan, Q.; Kou, H.; Zhang, L.; Wang, J.; Lv, W.; Dong, H.; Xiong, J. A flexible temperature sensor based on reduced graphene oxide for robot skin used in internet of things. Sensors 2018, 18, 1400. [Google Scholar] [CrossRef]
- Rana, K.; Singh, J.; Ahn, J.-H. A graphene-based transparent electrode for use in flexible optoelectronic devices. J. Mater. Chem. C 2014, 2, 2646–2656. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Yu, H.; Chen, C.; Sun, J.; Zhang, H.; Feng, Y.; Qin, M.; Feng, W. Highly thermally conductive polymer/graphene composites with rapid room-temperature self-healing capacity. Nano-Micro Lett. 2022, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, N.; Higgins, D.; Li, D.; Li, Q.; Xu, H.; Spendelow, J.S.; Wu, G. Self-assembled reduced graphene oxide/polyacrylamide conductive composite films. ACS Appl. Mater. Interfaces 2014, 6, 19783–19790. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sudhagar, P.; Kang, Y.S.; Choi, W. Graphene synthesis and application for solar cells. J. Mater. Res. 2014, 29, 299–319. [Google Scholar] [CrossRef]
- Hsu, A.L.; Herring, P.K.; Gabor, N.M.; Ha, S.; Shin, Y.C.; Song, Y.; Chin, M.; Dubey, M.; Chandrakasan, A.P.; Kong, J. Graphene-based thermopile for thermal imaging applications. Nano Lett. 2015, 15, 7211–7216. [Google Scholar] [CrossRef] [PubMed]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y.-k. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Cui, T.-R.; Li, D.; Huang, X.-R.; Yan, A.-Z.; Dong, Y.; Xu, J.-D.; Guo, Y.-Z.; Wang, Y.; Chen, Z.-K.; Shao, W.-C. Graphene-based flexible electrode for electrocardiogram signal monitoring. Appl. Sci. 2022, 12, 4526. [Google Scholar] [CrossRef]
- Paulchamy, B.; Arthi, G.; Lignesh, B. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.; Sachdev, K. Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Z.; Liu, Z.; Ren, G. Mechanism of oxidization of graphite to graphene oxide by the hummers method. ACS Omega 2022, 7, 23503–23510. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, S.; Maiyalagan, T. CuCo2S4@B, N-doped reduced graphene oxide hybrid as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. ACS Omega 2022, 7, 19183–19192. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning electron microscopy: Principle and applications in nanomaterials characterization. In Handbook of Materials Characterization; Springer: Cham, Switzerland, 2018; pp. 113–145. [Google Scholar]
- Smith, E.; Dent, G. Introduction, Basic Theory and Principles. In Modern Raman Spectroscopy—A Practical Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–21. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Aiyappa, H.B.; Kurungot, S. An efficient oxygen reduction electrocatalyst from graphene by simultaneously generating pores and nitrogen doped active sites. J. Mater. Chem. 2012, 22, 23799–23805. [Google Scholar] [CrossRef]
- Sibilia, S.; Bertocchi, F.; Chiodini, S.; Cristiano, F.; Ferrigno, L.; Giovinco, G.; Maffucci, A. Temperature-dependent electrical resistivity of macroscopic graphene nanoplatelet strips. Nanotechnology 2021, 32, 275701. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshyar, P.; Zamani, A.; Rezapour Kiani, D.; Fakhraeelotfabadi, S.; Fardmanesh, M. Electrical and Optical Properties of Controlled Reduced Graphene Oxide Prepared by a Green and Facile Route. Eng. Proc. 2023, 58, 68. https://doi.org/10.3390/ecsa-10-16175
Hooshyar P, Zamani A, Rezapour Kiani D, Fakhraeelotfabadi S, Fardmanesh M. Electrical and Optical Properties of Controlled Reduced Graphene Oxide Prepared by a Green and Facile Route. Engineering Proceedings. 2023; 58(1):68. https://doi.org/10.3390/ecsa-10-16175
Chicago/Turabian StyleHooshyar, Parsa, Atieh Zamani, Deniz Rezapour Kiani, Shayan Fakhraeelotfabadi, and Mehdi Fardmanesh. 2023. "Electrical and Optical Properties of Controlled Reduced Graphene Oxide Prepared by a Green and Facile Route" Engineering Proceedings 58, no. 1: 68. https://doi.org/10.3390/ecsa-10-16175




