Exploration of Zinc Oxide Nanoparticles for Efficient Photocatalytic Removal of Methylene Blue Dye: Synthesis, Characterization and Optimization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zinc Oxide Nanoparticles by Hydrothermal Method
2.3. Photocatalytic Experiments
3. Result and Discussion
3.1. Characterization of ZnO NPs
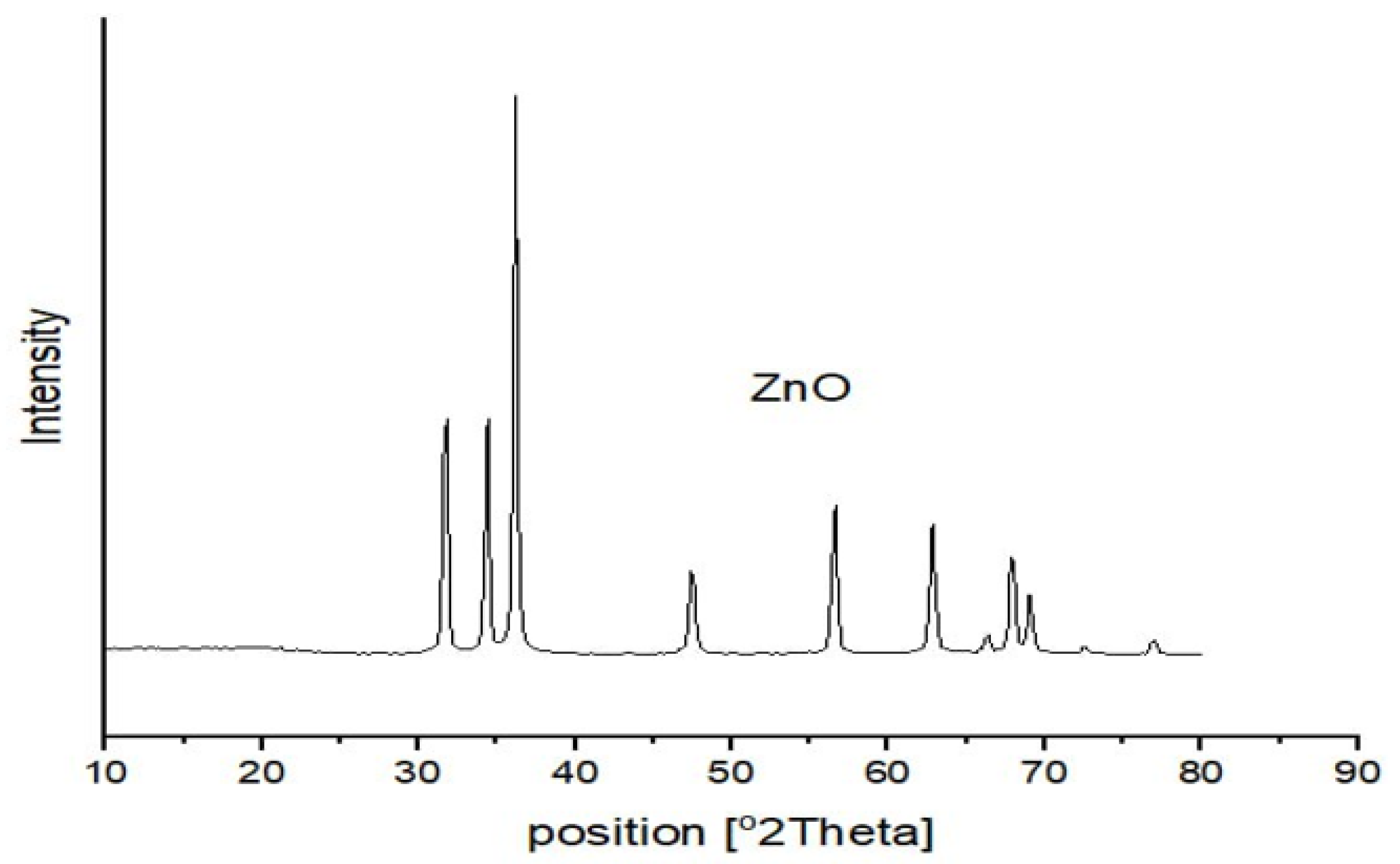
3.1.1. X-ray Diffraction Analysis
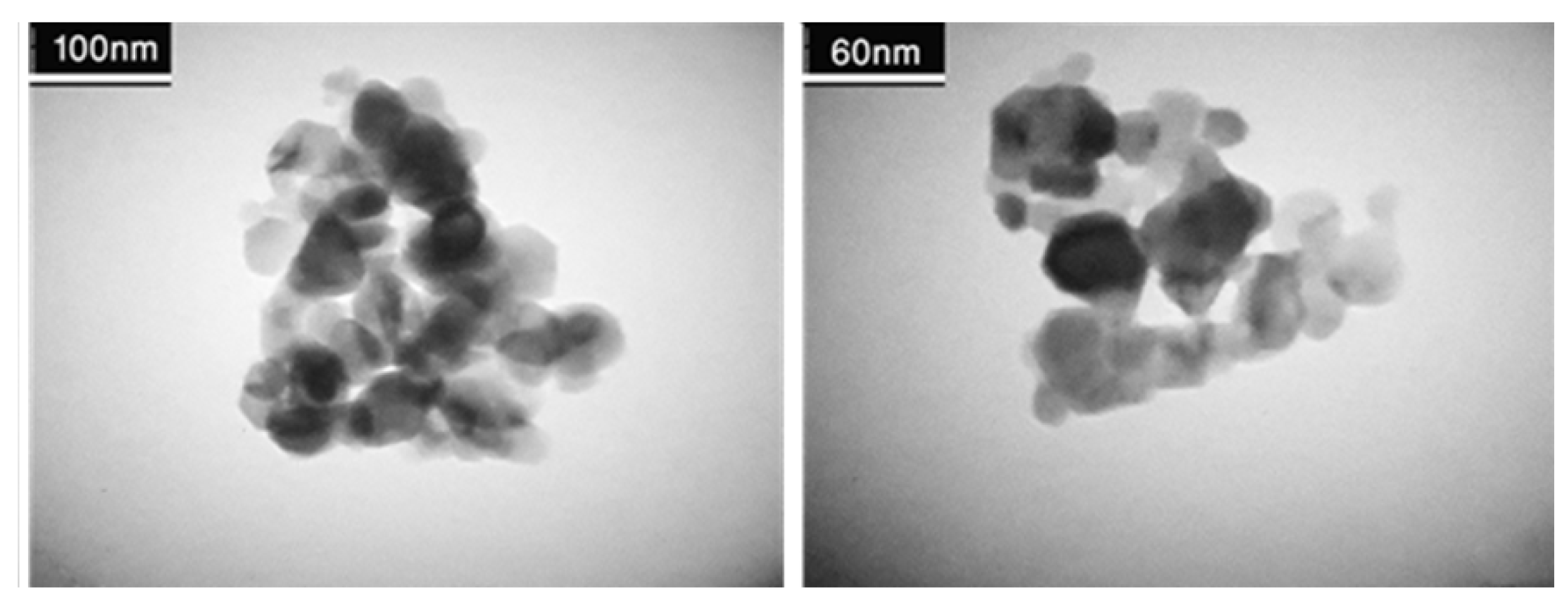
3.1.2. Transmittance Electron Microscopy (TEM) Analysis
3.1.3. FESEM Analysis
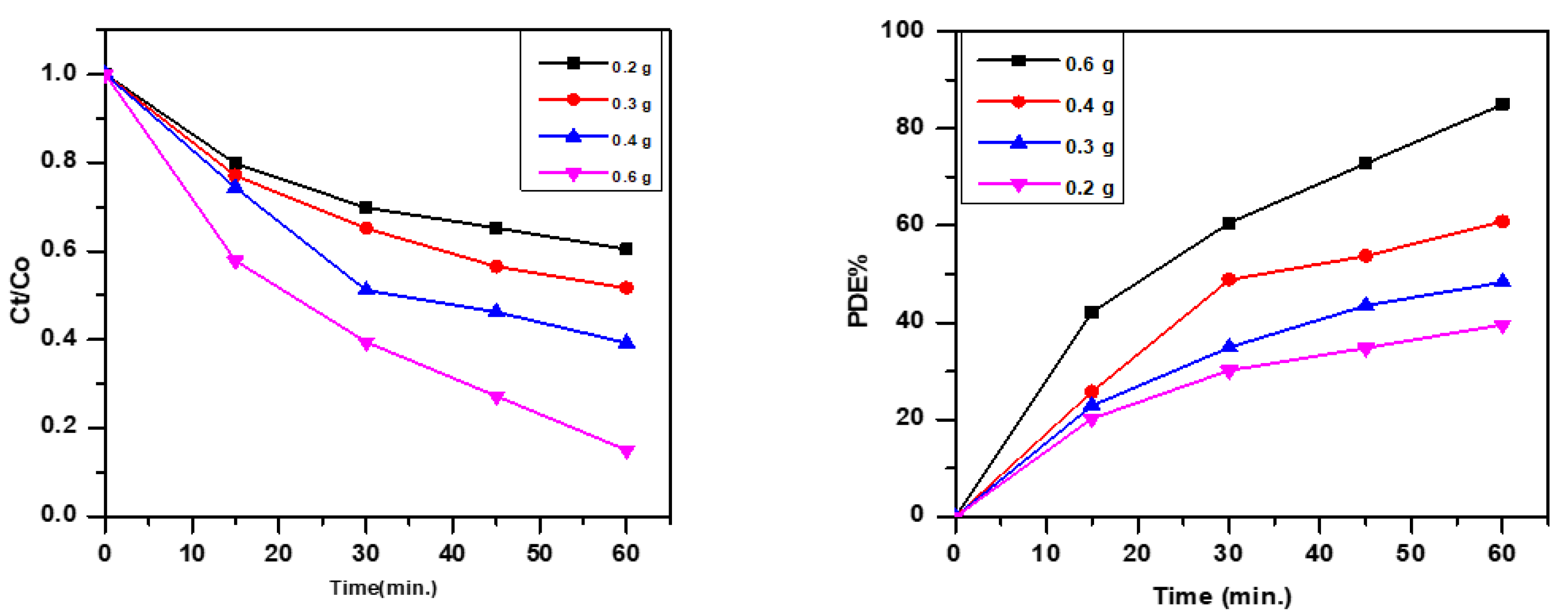
3.2. Effect of Mass Dosage
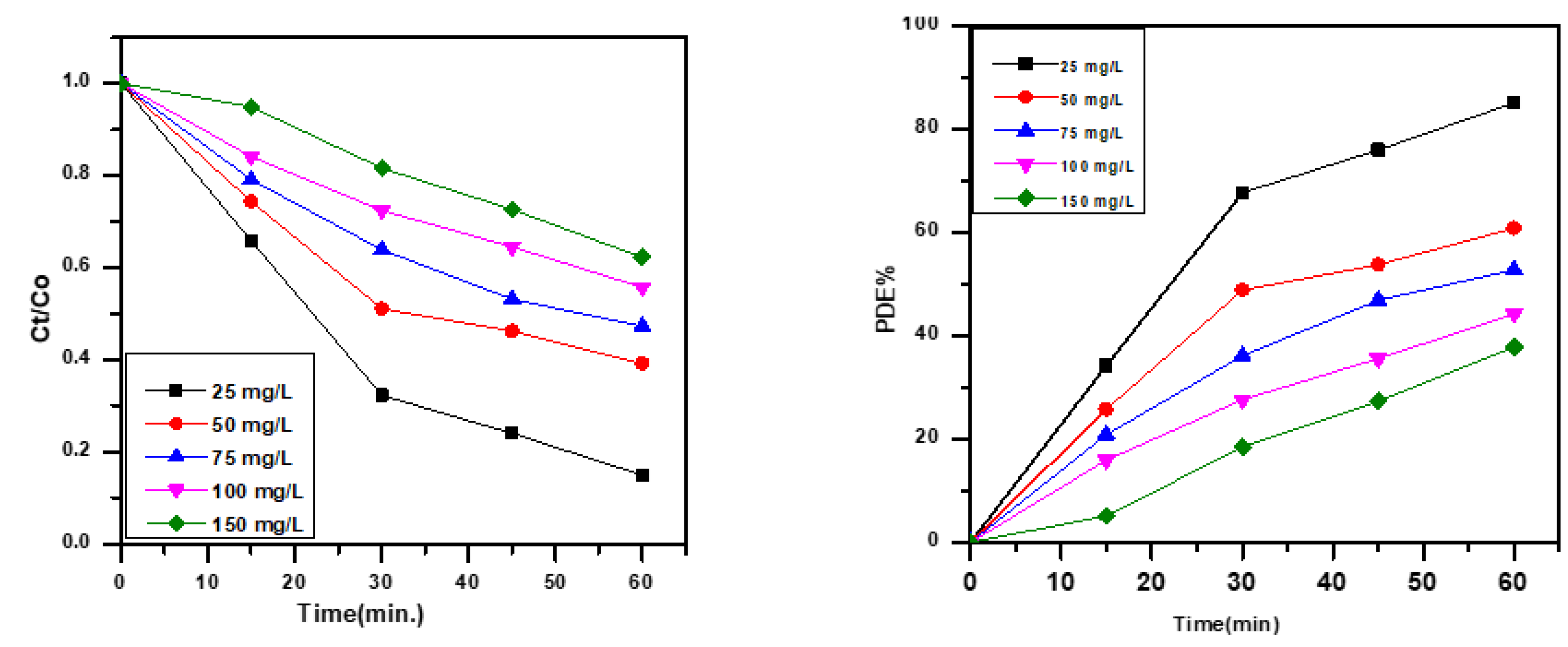
3.3. Effect of Concentration of MB Dye
3.4. Effect of Light Intensity (L.I.)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.S.; Tanaka, M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.M.; Alkaim, A.F. Comparative removal of three textile dyes from aqueous solutions by adsorption: As a model (corn-cob source waste) of plants role in environmental enhancement. Plant Arch. 2019, 19, 1613–1620. [Google Scholar]
- Rather, L.J.; Shahid-Ul-Islam; Akhter, S.; Qazi, P.; Mohammad, F. Chemistry of plant dyes: Applications and environmental implications of dyeing processes. Curr. Environ. Eng. 2017, 4, 103–120. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Achour, R.; Hassoune, H.; Lachehab, A.; el kacem Qaiss, A.; Bouhfid, R. Highly synergic adsorption/photocatalytic efficiency of Alginate/Bentonite impregnated TiO2 beads for wastewater treatment. J. Photochem. Photobiol. A Chem. 2021, 412, 113215. [Google Scholar] [CrossRef]
- El-Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Yap, P.; Liu, T.; Hamdani, M.; Akbour, R.A. Natural flocculants for the treatment of wastewaters containing dyes or heavy metals: A state-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 106060. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Stoller, M.; Vaiano, V.; Ciambelli, P.; Chianese, A.; Sannino, D. Photocatalytic degradation of organic dyes under visible light on N-doped TiO2 photocatalysts. Int. J. Photoenergy 2012, 2012, 626759. [Google Scholar] [CrossRef]
- Liu, Q. Pollution and treatment of dye wastewater. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Ajobree, A.M. White marble as an alternative surface for removal of toxic dyes (Methylene blue) from Aqueous solutions. Int. J. Adv. Sci. Technol. 2020, 29, 5470–5479. [Google Scholar]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Activated carbon (as a waste plant sources)-clay micro/nanocomposite as effective adsorbent: Process optimization for ultrasound-assisted adsorption removal of amoxicillin drug. Plant Arch. 2019, 19, 915–919. [Google Scholar]
- Rincón, N.C.; Hammouda, S.B.; Sillanpää, M.; Barrios, V.E. Enhanced photocatalytic performance of zinc oxide nanostructures via photoirradiation hybridisation with graphene oxide for the degradation of triclosan under visible light: Synthesis, characterisation and mechanistic study. J. Environ. Chem. Eng. 2018, 6, 6554–6567. [Google Scholar] [CrossRef]
- Salcedo, A.B.J. Synthesis and Performance of Nanocomposites Based on ZnO for the Photocatalytic Treatment of Water Contaminated with Endocrine Disruptor Compounds; Université de Lorraine: Nancy, France, 2014. [Google Scholar]
- Al-Gubury, H.Y.; Fairooz, N.Y.; Aljeboree, A.; Alqaraguly, M.B.; Alkaim, A.F. Photocatalytic degradation n-undecane using coupled ZnO-Co2O3. Int. J. Chem. Sci. 2015, 13, 863–874. [Google Scholar]
- Mahapatra, A.; Tripathy, M.; Hota, G. Polymer-Derived Electrospun Ceramic Nanofibers Adsorbents for Textile Wastewater Treatment. In Polymer Technology in Dye-Containing Wastewater: Volume 2; Springer: Berlin/Heidelberg, Germany, 2022; pp. 193–208. [Google Scholar]
- Al-Hussainawy, M.K.; Mehdi, Z.S.; Jasim, K.K.; Alshamsi, H.A.; Saud, H.R.; Kyhoiesh, H.A.K. A single rapid route synthesis of magnetite/chitosan nanocomposite: Competitive study. Results Chem. 2022, 4, 100567. [Google Scholar] [CrossRef]
- Kyhoiesh, H.A.K.; Al-Hussainawy, M.K.; Saud, H.R. Synthesis and Characterization of Polyacrylamide/Crotonic Acid and Its Composites with Carbon Nanotube and Rhodamine B. AIP Conf. Proc. 2022, 2398, 030018. [Google Scholar]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Hashim, F.S.; Alkaim, A.F.; Mahdi, S.M.; Alkhayatt, A.H.O. Photocatalytic degradation of GRL dye from aqueous solutions in the presence of ZnO/Fe2O3 nanocomposites. Compos. Commun. 2019, 16, 111–116. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H.; Hussein, F.H. Photocatalytic Hydrogen Production on Nanocomposite of Carbon Nanotubes and TiO2. J. Phys. Conf. Ser. 2018, 1032, 012056. [Google Scholar] [CrossRef]
- Hashim, F.S.; Alkaim, A.F.; Salim, S.J.; Alkhayatt, A.H.O. Effect of (Ag, Pd) doping on structural, and optical properties of ZnO nanoparticles: As a model of photocatalytic activity for water pollution treatment. Chem. Phys. Lett. 2019, 737, 136828. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H. Enhance photocatalytic activity of TiO2 by carbon nanotubes. Int. J. ChemTech Res. 2016, 9, 431–443. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, M.F.; Kareem, A.T.; Al-Majdi, K.; Omran, A.A. Exploration of Zinc Oxide Nanoparticles for Efficient Photocatalytic Removal of Methylene Blue Dye: Synthesis, Characterization and Optimization. Eng. Proc. 2023, 59, 120. https://doi.org/10.3390/engproc2023059120
Ramadan MF, Kareem AT, Al-Majdi K, Omran AA. Exploration of Zinc Oxide Nanoparticles for Efficient Photocatalytic Removal of Methylene Blue Dye: Synthesis, Characterization and Optimization. Engineering Proceedings. 2023; 59(1):120. https://doi.org/10.3390/engproc2023059120
Chicago/Turabian StyleRamadan, Montather F., Ashwaq Talib Kareem, Kadhum Al-Majdi, and Alaa A. Omran. 2023. "Exploration of Zinc Oxide Nanoparticles for Efficient Photocatalytic Removal of Methylene Blue Dye: Synthesis, Characterization and Optimization" Engineering Proceedings 59, no. 1: 120. https://doi.org/10.3390/engproc2023059120




