Hybrid Feature Selection and Classifying Stages through Electrocardiogram (ECG) Signal for Heart Disease Prediction †
Abstract
:1. Introduction
2. Literature Review
3. Proposed Methodology
3.1. ECG Data Source
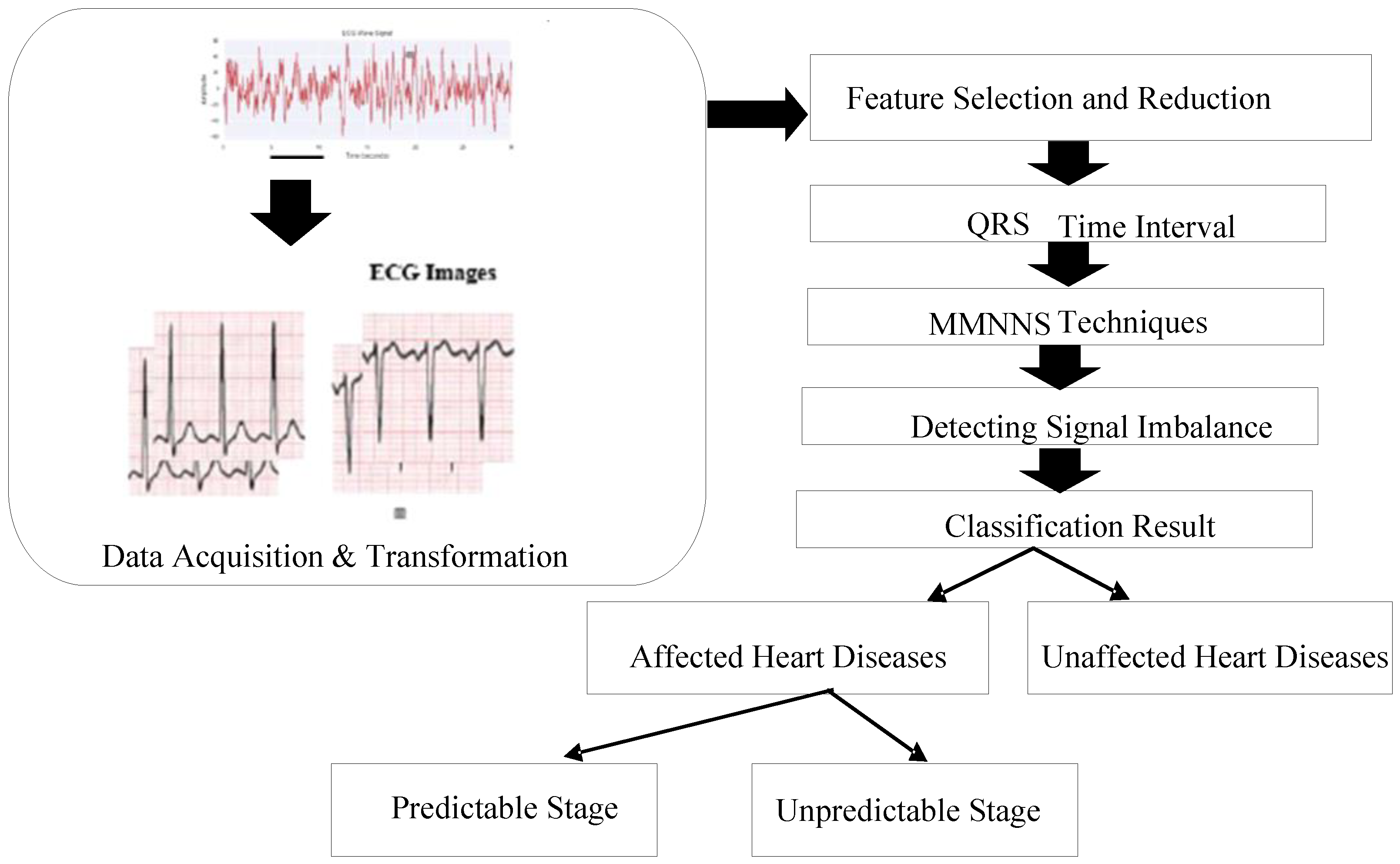
3.2. Acquisition of Data & Transformation
- Data Preprocessing: Numerous different types of artefacts and sounds may commonly contaminate the signal of an ECG recording, which will have an impact on the categorization of the findings of any future experiments. It is crucial to put into practice an appropriate pre-processing method that preserves all of the relevant information from the original raw ECG signal. There are only a few number of noises where the spectrum of the signal matches with the pertinent data, even if ECG signals were obtained from public datasets that do not have as much noises as those collected directly from patients. The user should adopt a feasible preparation technique that preserves valuable dataset information while working with the original ECG signal.
- Denoising ECG Signal: The process of eliminating noise from the ECG signal is referred to as denoising. The precise identification of various anomalies and the removal of noise data from the ECG signal has been a primary area of focus for researchers. Applying a sample entropy band pass filters (0.05–45 Hz) to ECG signals as part of neural network denoise techniques verifies the accuracy of the data. Noise may cause vital health alerts. After collecting data from data sources for ECG analysis, noise is often eliminated.
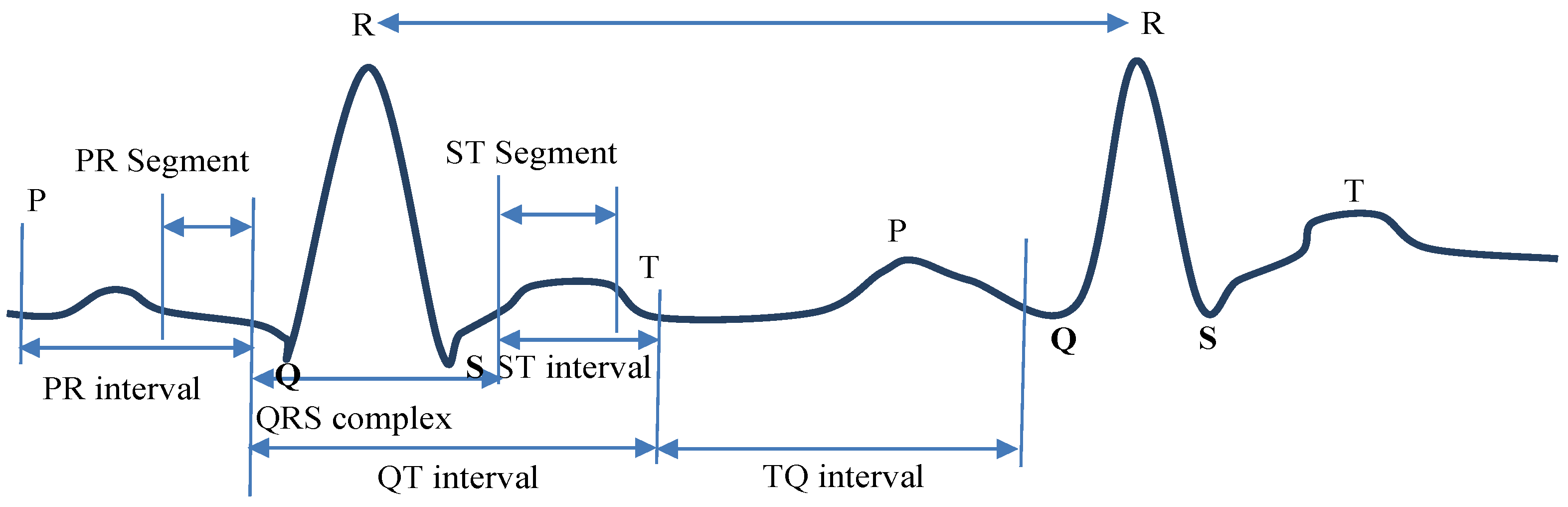
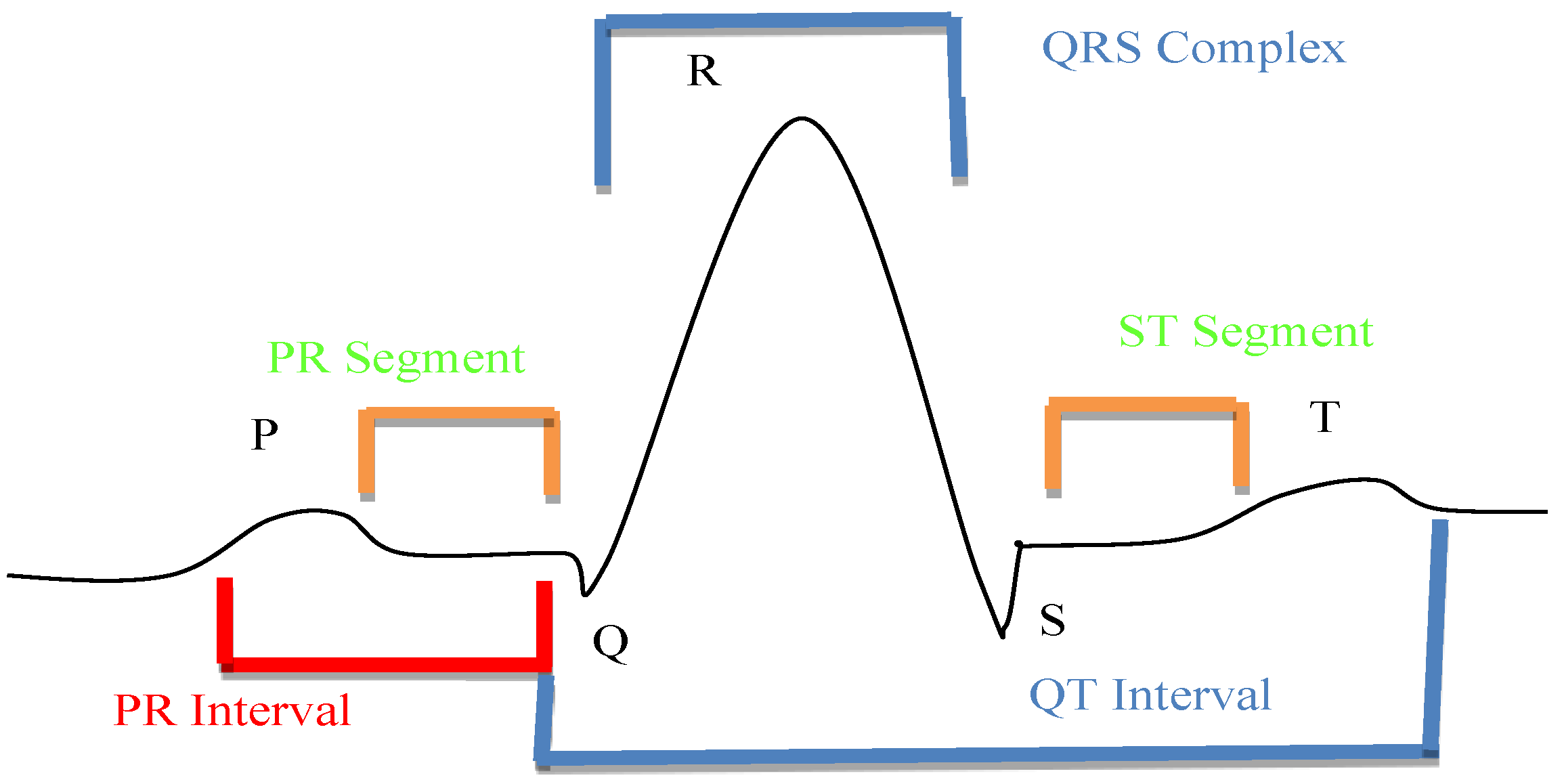
- QRS Regions’ processing of Intervals: ECG waves are called P, QRS, and T-U in alphabetical sequences. Significant information on health and the state of cardiac issues may be obtained from the amplitude, shape, and time. Atrial depolarization is represented by the P wave is [19]. The QRS complex is used to characterize the reflections of ventricular depolarization. Depolarization of the ventricles is represented by the T-U wave. As a depolarization current moves towards the positive pole of an ECG line, an electrocardiograph may show a positive wave. Figure 3 shows the ECG curves classification by QRS interval.
3.3. Hybrid Feature Extraction
3.3.1. Cuckoo Search Algorithm (CSA)
3.3.2. MCSO
- Several groups may be seen in the chicken swarm. The dominating rooster, a few hens, and chicks are present in each group.
- The chicken fitness ratings determine how to organize and identify each bird in the swarm. Each of the chickens acting as roosters would be the one with highest overall fitness score. Chick status would be assigned to the hens with the lowest multiple fitness levels. Which group to dwell in, the hens decide at random. Additionally, at random, the hens and chicks are placed in a mother–child connection.
- In a group, the mother–child connection, dominance relationship, and hierarchical order all stay the same. Only sometimes, (G) time steps, do these statuses change.
- The chickens may stop the others from eating their own food as they follow the rooster in the group in quest of nourishment. Assume that birds will haphazardly take the tasty food that has already been discovered by others. Around their mother (a hen), the chicks go in quest of nourishment. In struggles for food, dominant species hold an edge.
- i
- Movement of the Chickens
3.4. MMNNS Machine Learning Algorithm
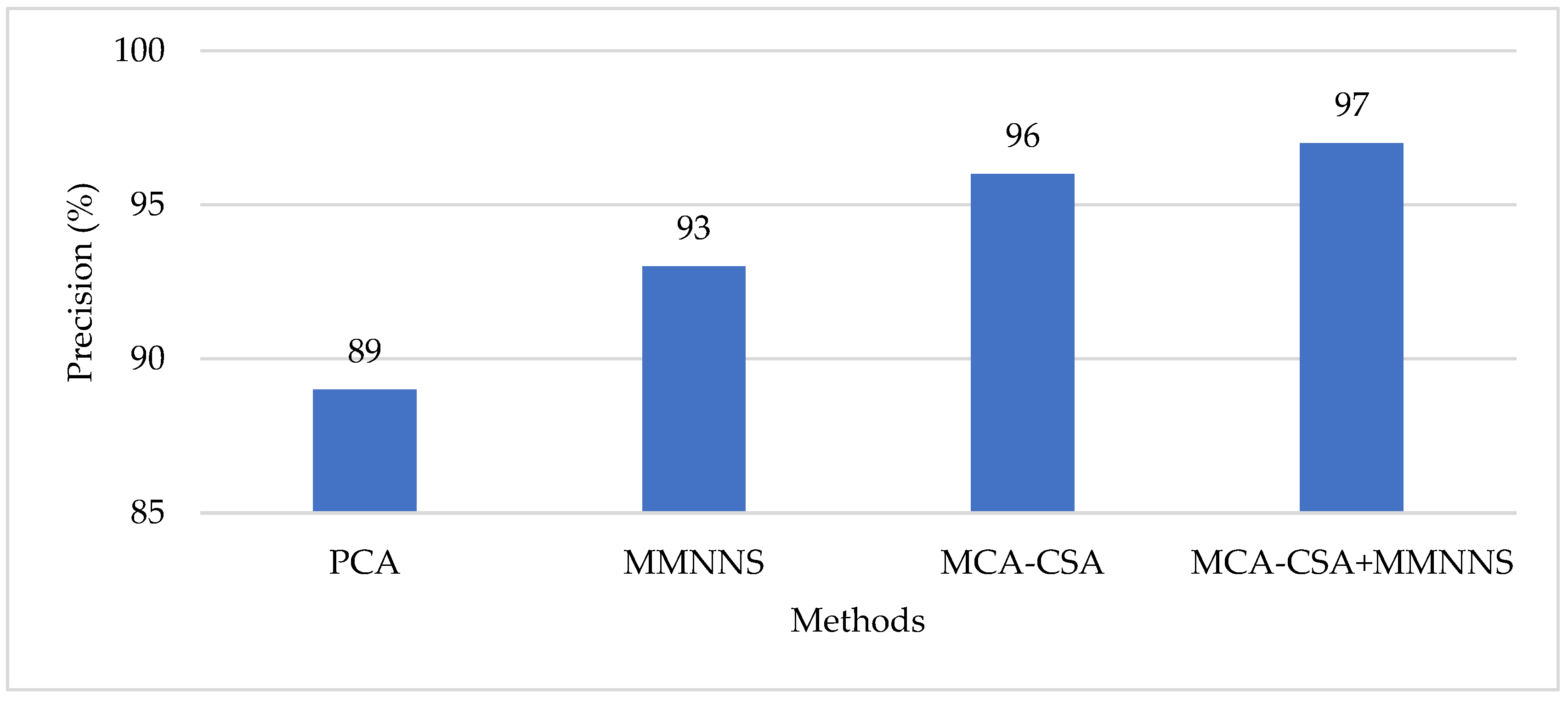
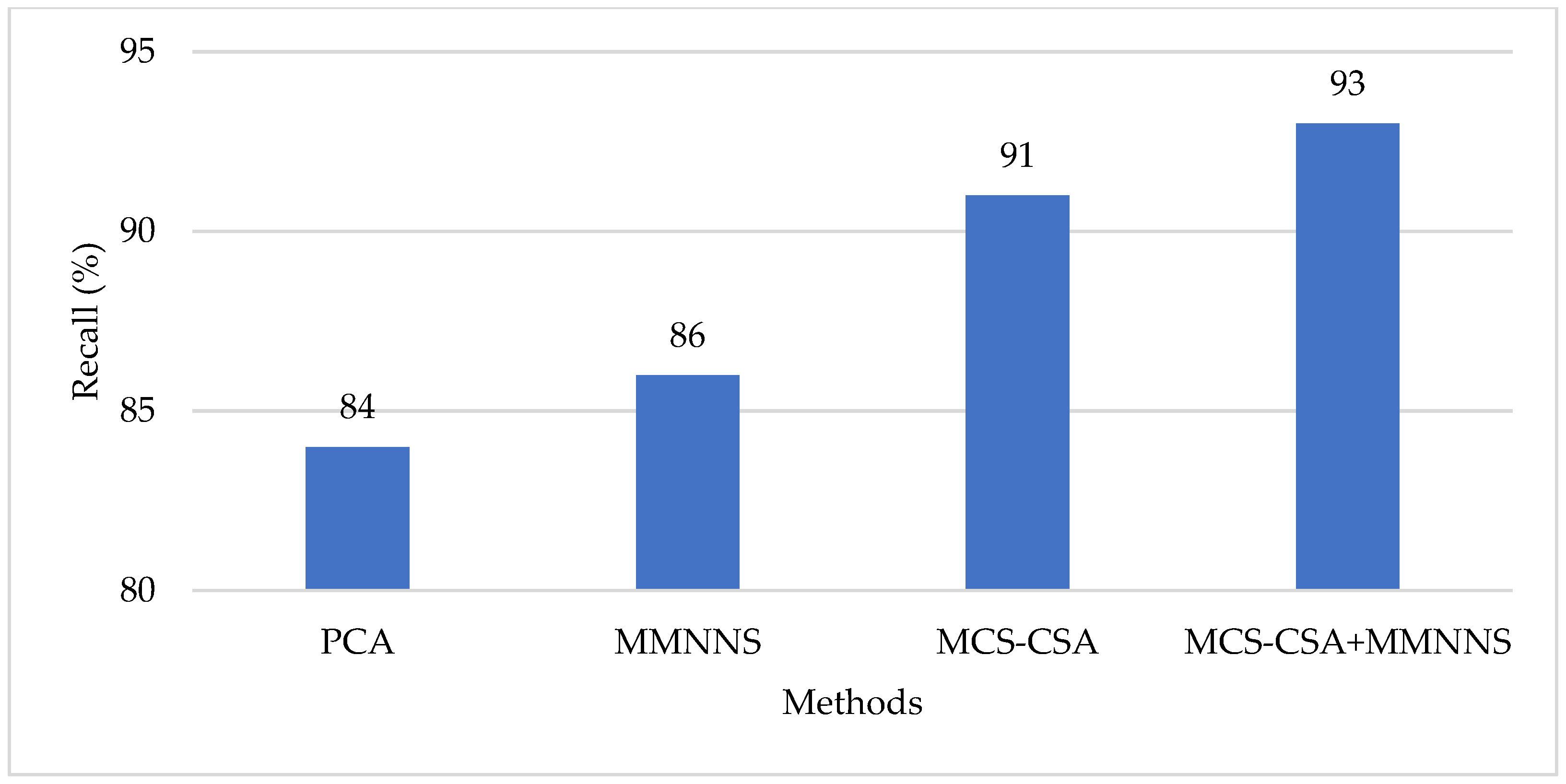
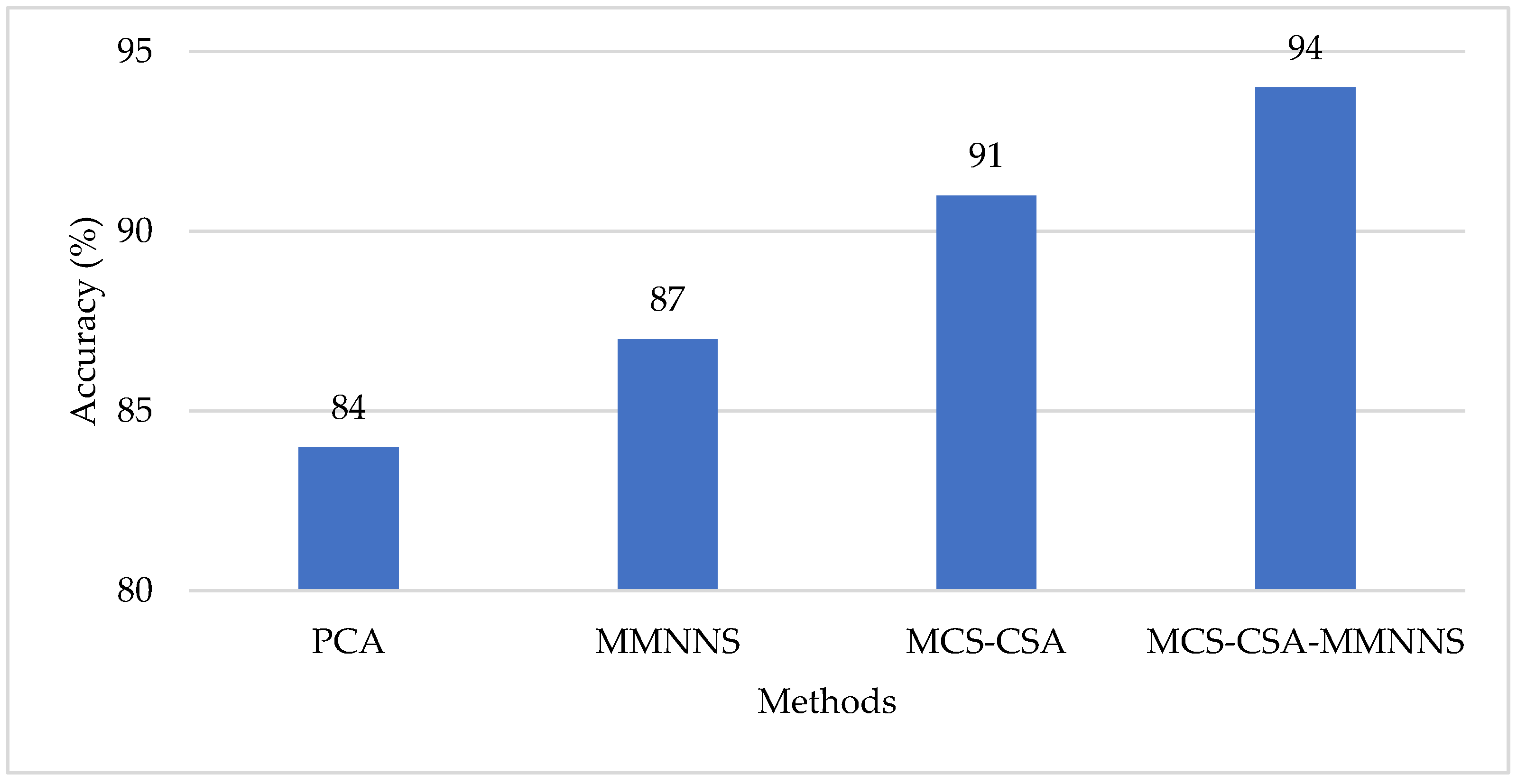
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamaruddin, N.H.; Murugappan, M.; Omar, M.I. Early prediction of Cardiovascular Diseases using ECG signal. In Proceedings of the IEEE Student Conference on Research and Development, Pulau Pinang, Malaysia, 5–6 December 2012. [Google Scholar]
- Tabassum, T.; Islam, M. An approach of cardiac disease prediction by analyzing ECG signal. In Proceedings of the 3rd International Conference on Electrical Engineering and Information Communication Technology, Dhaka, Bangladesh, 22–24 September 2016. [Google Scholar]
- Zhang, W.; Yu, L.; Ye, L.; Zhuang, W.; Ma, F. ECG signal classification with deep learning for heart disease identification. In Proceedings of the International Conference on Big Data and Artificial Intelligence, Beijing, China, 22–24 June 2018. [Google Scholar]
- Murukesan, L.; Murugappan, M.; Iqbal, M. Sudden cardiac death prediction using ECG signal derivative (heart rate variability): A review. In Proceedings of the IEEE 9th international colloquium on signal processing and its applications, Kuala Lumpur, Malaysia, 8–10 March 2013. [Google Scholar]
- Sakhare, P.B.; Ghongade, R. An approach for ECG beats classification using adaptive neuro fuzzy inference system. In Proceedings of the Annual IEEE India Conference, New Delhi, India, 17–20 December 2015. [Google Scholar]
- Anwar, T.; Zakir, S. Effect of image augmentation on ECG image classification using deep learning. In Proceedings of the International Conference on ArtifIntell, Islamabad, Pakistan, 5–7 April 2021. [Google Scholar]
- Nazmy, T.M.; El-Messiry, H.; Al-Bokhity, B. Adaptive neuro-fuzzy inference system for classification of ECG signals. In Proceedings of the 7th International Conference on Informatics and Systems (INFOS), Cairo, Egypt, 28–30 March 2010. [Google Scholar]
- Nagavelli, U.; Samanta, D.; Chakraborty, P. Machine learning technology-based heart disease detection models. J. Healthc. Eng. 2022, 2022, 7351061. [Google Scholar] [CrossRef] [PubMed]
- Muthuvel, K.; Suresh, L.P. Adaptive neuro-fuzzy inference system for classification of ECG signal. In Proceedings of the International Conference on Circuits, Power and Computing Technologies (ICCPCT), Nagercoil, India, 20–21 March 2013. [Google Scholar]
- Agarwal, S.; Krishnamoorthy, V.; Pratiher, S. ECG signal analysis using wavelet coherence and s-transform for classification of cardiovascular diseases. In Proceedings of the International Conference on Advances in Computing, Communications and Informatics (ICACCI), Jaipur, India, 21–24 September 2016. [Google Scholar]
- Ishaq, A.; Sadiq, S.; Umer, M.; Ullah, S.; Mirjalili, S.; Rupapara, V.; Nappi, M. Improving the prediction of heart failure patients’ survival using SMOTE and effective data mining techniques. IEEE Access 2021, 9, 39707–39716. [Google Scholar] [CrossRef]
- Thiyagarajan, C. A survey on diabetes mellitus prediction using machine learning techniques. Int. J. Appl. Eng. 2016, 11, 1810–1814. [Google Scholar]
- Acharya, U.R.; Fujita, H.; Lih, O.S.; Adam, M.; Tan, J.H.; Chua, C.K. Automated detection of coronary artery disease using different durations of ECG segments with convolutional neural network. Knowl. Based Syst. 2017, 132, 62–71. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, S.; Tang, Q.; Zheng, Z.; Elgendi, M.; Chen, Z. Deep learning algorithm classifies heartbeat events based on electrocardiogram signals. Front. Physiol. 2020, 11, 569050. [Google Scholar] [CrossRef] [PubMed]
- Vafaie, M.H.; Ataei, M.; Koofigar, H.R. Heart diseases prediction based on ECG signals’ classification using a genetic-fuzzy system and dynamical model of ECG signals. Biomed. Signal Process. Control 2014, 14, 291–296. [Google Scholar] [CrossRef]
- Chen, J.; Valehi, A.; Razi, A. Smart heart monitoring: Early prediction of heart problems through predictive analysis of ECG signals. IEEE Access 2019, 7, 120831–120839. [Google Scholar] [CrossRef]
- Alarsan, F.I.; Younes, M. Analysis and classification of heart diseases using heartbeat features and machine learning algorithms. J. Big Data 2019, 6, 81. [Google Scholar] [CrossRef]
- Jahmunah, V.; Ng, E.Y.K.; San, T.R.; Acharya, U.R. Automated detection of coronary artery disease, myocardial infarction and congestive heart failure using GaborCNN model with ECG signals. Comput. Biol. Med. 2021, 134, 104–457. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.; Marikkannu, P. Machine Learning Techniques Applied for Exploring Heart Disease and Classifying Stages through ECG Signal. Neuro Quantology 2022, 20, 473–482. [Google Scholar] [CrossRef]
- Rajabioun, R. Cuckoo optimization algorithm. Appl. Soft Comput. 2021, 11, 5508–5518. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Gao, X.; Zhang, H. A new bio-inspired algorithm: Chicken swarm optimization. In Proceedings of the Advances in Swarm Intelligence: 5th International Conference, ICSI 2014, Hefei, China, 17–20 October 2014. [Google Scholar]
- Hafez, A.I.; Zawbaa, H.M.; Emary, E.; Mahmoud, H.A.; Hassanien, A.E. An innovative approach for feature selection based on chicken swarm optimization. In Proceedings of the 7th International Conference of Soft Computing and Pattern Recognition (SoCPaR), Fukuoka, Japan, 13–15 November 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, B.; Soundararajan, R.; Natesan, K.; Santhi, R.M. Hybrid Feature Selection and Classifying Stages through Electrocardiogram (ECG) Signal for Heart Disease Prediction. Eng. Proc. 2023, 59, 126. https://doi.org/10.3390/engproc2023059126
Kumar B, Soundararajan R, Natesan K, Santhi RM. Hybrid Feature Selection and Classifying Stages through Electrocardiogram (ECG) Signal for Heart Disease Prediction. Engineering Proceedings. 2023; 59(1):126. https://doi.org/10.3390/engproc2023059126
Chicago/Turabian StyleKumar, Babu, Radhakrishnan Soundararajan, Kanimozhi Natesan, and Roobini Maridhas Santhi. 2023. "Hybrid Feature Selection and Classifying Stages through Electrocardiogram (ECG) Signal for Heart Disease Prediction" Engineering Proceedings 59, no. 1: 126. https://doi.org/10.3390/engproc2023059126
APA StyleKumar, B., Soundararajan, R., Natesan, K., & Santhi, R. M. (2023). Hybrid Feature Selection and Classifying Stages through Electrocardiogram (ECG) Signal for Heart Disease Prediction. Engineering Proceedings, 59(1), 126. https://doi.org/10.3390/engproc2023059126




