A Comprehensive Review on Unsupervised Domain Adaptation for 3D Segmentation and Reconstruction in CT Urography Imaging †
Abstract
:1. Introduction
The Clinical Significance
2. Methodology
2.1. Objective of the Review
2.2. Research Questions
- RQ1
- What are the key methodologies developed for kidney segmentation in CTU imaging?
- RQ2
- How do different segmentation algorithms perform in terms of accuracy and computational efficiency?
- RQ3
- What are the applications and clinical significance of accurate kidney segmentation in CTU imaging?
2.3. Data Collection and Analysis
2.4. Data Synthesis
3. Background
3.1. Adversarial Networks
3.2. Unsupervised Domain Translation
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hameed, B.M.; Dhavileswarapu, A.V.; Raza, S.Z.; Karimi, H.; Khanuja, H.S.; Shetty, D.K.; Ibrahim, S.; Shah, M.J.; Naik, N.; Paul, R.; et al. Artificial Intelligence and its impact on urological diseases and management: A Comprehensive Review of the literature. J. Clin. Med. 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Saxena, J.; Vineetha, R.; Paul, R.; Shetty, D.K.; Sharma, S.; Smriti, K.; Singhal, D.K.; Naik, N. Age assessment through root lengths of mandibular second and third permanent molars using machine learning and Artificial Neural Networks. J. Imaging 2023, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.Z.; Shah, M.; Naik, N.; Singh Khanuja, H.; Paul, R.; Somani, B.K. Application of artificial intelligence-based classifiers to predict the outcome measures and stone-free status following percutaneous nephrolithotomy for staghorn calculi: Cross-validation of data and estimation of accuracy. Eur. Urol. 2021, 79, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the 18th International Conference on Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar] [CrossRef]
- Milletari, F.; Navab, N.; Ahmadi, S.-A. V-net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016. [Google Scholar]
- Zhu, J.-Y.; Park, T.; Isola, P.; Efros, A.A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017. [Google Scholar]
- Huo, Y.; Xu, Z.; Bao, S.; Assad, A.; Abramson, R.G.; Landman, B.A. Adversarial synthesis learning enables segmentation without target Modality Ground Truth. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018. [Google Scholar]
- Zeng, W.; Fan, W.; Chen, R.; Zheng, Z.; Zheng, S.; Chen, J.; Liu, R.; Zeng, Q.; Liu, Z.; Chen, Y.; et al. Accurate 3D kidney segmentation using unsupervised domain translation and adversarial networks. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), Nice, France, 13–16 April 2021. [Google Scholar]
- Yan, X.; Yuan, K.; Zhao, W.; Wang, S.; Li, Z.; Cui, S. An efficient hybrid model for kidney tumor segmentation in CT images. In Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), Iowa City, IA, USA, 3–7 April 2020. [Google Scholar]
- Bazgir, O.; Barck, K.; Carano, R.A.D.; Weimer, R.M.; Xie, L. Kidney segmentation using 3D U-net localized with expectation maximization. In Proceedings of the 2020 IEEE Southwest Symposium on Image Analysis and Interpretation (SSIAI), Albuquerque, NM, USA, 29–31 March 2020. [Google Scholar]
- Lv, Y.; Xu, Y.; Zhang, X.; Sun, Z.; Wang, J. Automatic Kidney CT segmentation and optimization based on self-learning. In Proceedings of the 2019 WRC Symposium on Advanced Robotics and Automation (WRC SARA), Beijing, China, 21–22 August 2019. [Google Scholar]
- Heller, N.; Isensee, F.; Maier-Hein, K.H.; Hou, X.; Xie, C.; Li, F.; Nan, Y.; Mu, G.; Lin, Z.; Han, M.; et al. The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the kits19 challenge. Med. Image Anal. 2021, 67, 101821. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, F.; Soliman, A.; Elmaghraby, A.; Gimel’farb, G.; El-Baz, A. 3D kidney segmentation from abdominal images using spatial-appearance models. Comput. Math. Methods Med. 2017, 2017, 9818506. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gu, J.; Chen, Y.; Liu, W.; Tang, L.; Shu, H.; Toumoulin, C. Automatic kidney segmentation in CT images based on multi-atlas image registration. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014. [Google Scholar]
- Zhao, W.; Jiang, D.; Peña Queralta, J.; Westerlund, T. MSS U-Net: 3D segmentation of kidneys and tumors from CT images with a multi-scale supervised U-Net. Inform. Med. Unlocked 2020, 19, 100357. [Google Scholar] [CrossRef]
- Li, D.; Xiao, C.; Liu, Y.; Chen, Z.; Hassan, H.; Su, L.; Liu, J.; Li, H.; Xie, W.; Zhong, W.; et al. Deep segmentation networks for segmenting kidneys and detecting kidney stones in unenhanced abdominal CT images. Diagnostics 2022, 12, 1788. [Google Scholar] [CrossRef]
- Xu, Y.; Lyu, J. The value of three-dimensional helical computed tomography for the retrograde flexible ureteronephroscopy in the treatment of lower pole calyx stones. Chronic Dis. Transl. Med. 2016, 2, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. Med. Image Comput. Comput. Assist. Interv. MICCAI 2016, 2016, 424–432. [Google Scholar]
- da Cruz, L.B.; Araújo, J.D.; Ferreira, J.L.; Diniz, J.O.; Silva, A.C.; de Almeida, J.D.; de Paiva, A.C.; Gattass, M. Kidney segmentation from computed tomography images using Deep Neural Network. Comput. Biol. Med. 2020, 123, 103906. [Google Scholar] [CrossRef]
- Cuingnet, R.; Prevost, R.; Lesage, D.; Cohen, L.D.; Mory, B.; Ardon, R. Automatic detection and segmentation of kidneys in 3D CT images using random forests. Med. Image Comput. Comput. Assist. Interv. MICCAI 2012, 2012, 66–74. [Google Scholar]
- Thong, W.; Kadoury, S.; Piché, N.; Pal, C.J. Convolutional networks for kidney segmentation in contrast-enhanced CT scans. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2016, 6, 277–282. [Google Scholar] [CrossRef]
- Yang, G.; Li, G.; Pan, T.; Kong, Y.; Wu, J.; Shu, H.; Luo, L.; Dillenseger, J.-L.; Coatrieux, J.-L.; Tang, L.; et al. Automatic segmentation of kidney and renal tumor in CT images based on 3D fully convolutional neural network with Pyramid Pooling Module. In Proceedings of the 2018 24th International Conference on Pattern Recognition (ICPR), Beijing, China, 20–24 August 2018. [Google Scholar]
- Khalifa, F.; Elnakib, A.; Beache, G.M.; Gimel’farb, G.; El-Ghar, M.A.; Ouseph, R.; Sokhadze, G.; Manning, S.; McClure, P.; El-Baz, A. 3D kidney segmentation from CT images using a level set approach guided by a novel stochastic speed function. In Proceedings of the 14th International Conference on Medical Image Computing and Computer-Assisted Intervention—MICCAI 2011, Toronto, ON, Canada, 18–22 September 2011; pp. 587–594. [Google Scholar]
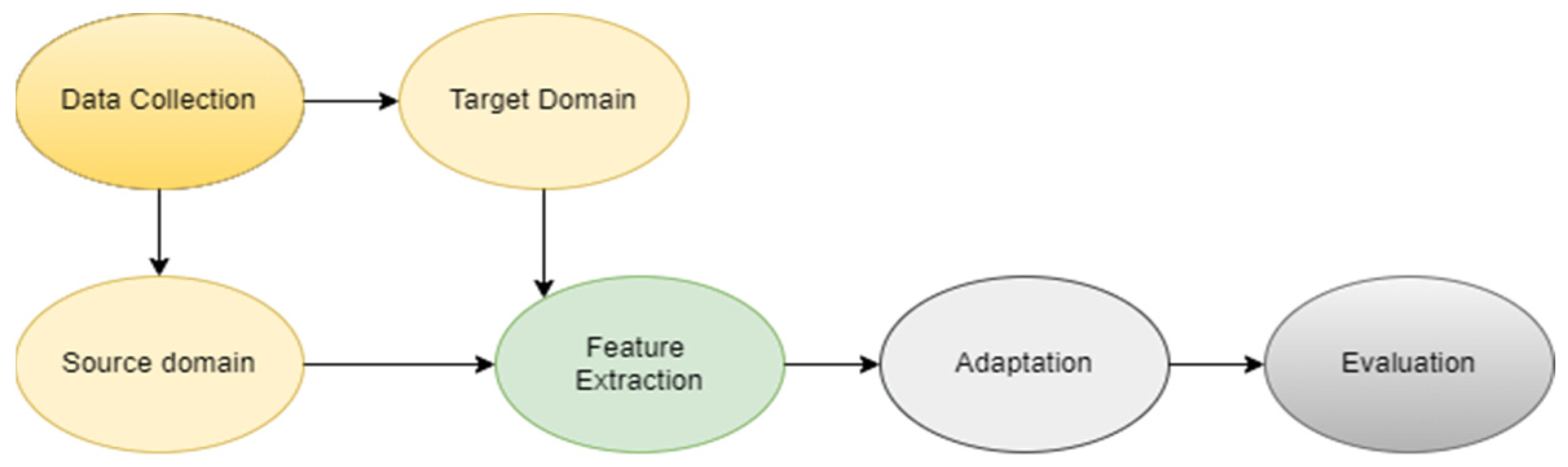
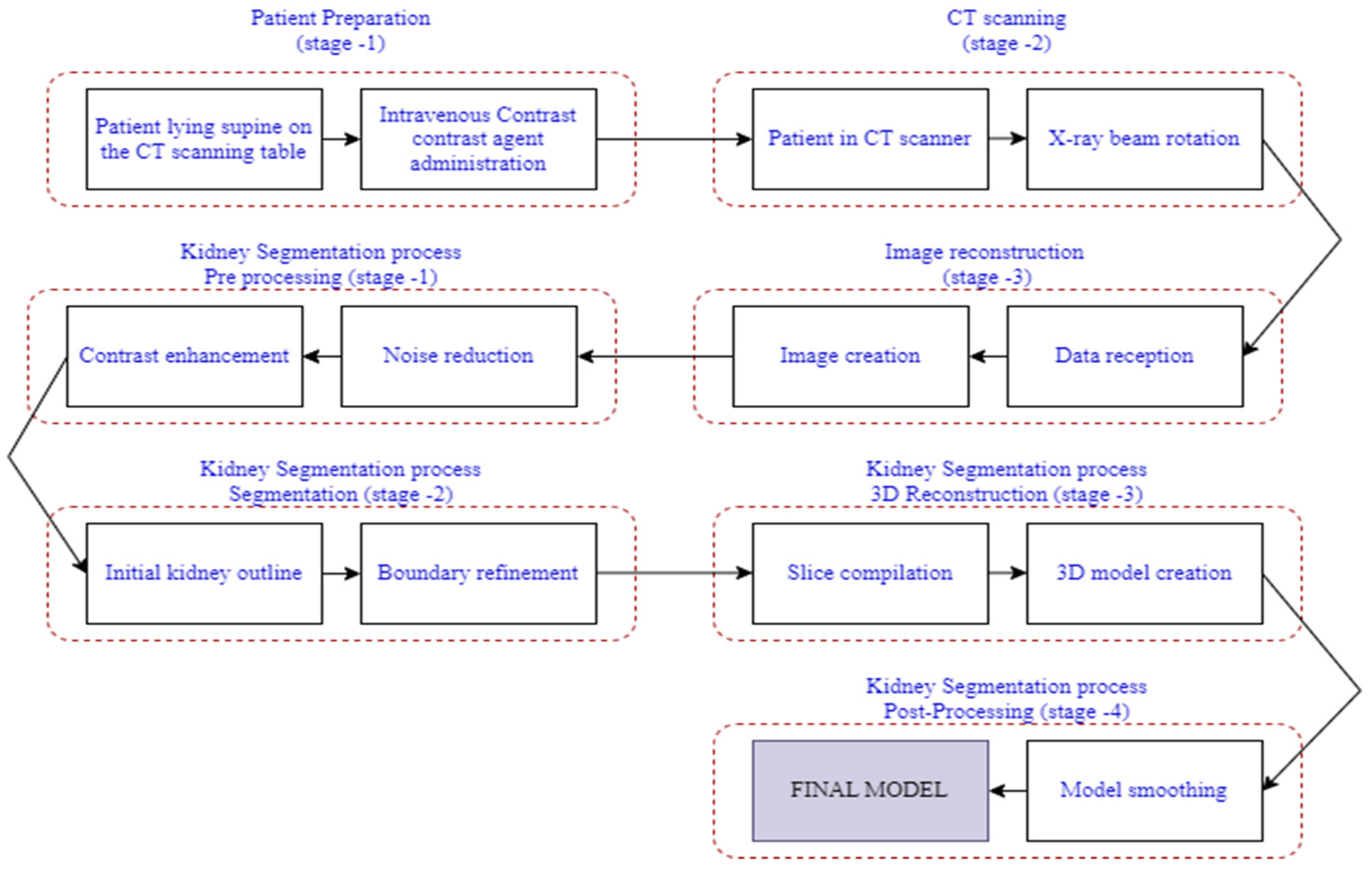
| Reference | Author/s | Domain Adaptation Strategy | Methodology Highlights | Key Contributions |
|---|---|---|---|---|
| [8] | (Zeng et al., 2021) | Unsupervised | Utilized 2D networks for kidney segmentation from urographic images | Comparable or better performance than supervised methods on clinical urography data |
| [14] | (Guanyu Yang et al., 2014) | Unsupervised | Implemented automatic kidney segmentation with multi-atlas image registration | Achieved high accuracy on CT urography and CT angiographic images |
| [15] | (Zhao et al., 2020) | Unsupervised | Proposed MSS 3D U-Net for segmenting kidneys and kidney tumors from CT images | Enhanced performance with a connected-component-based post-processing method |
| [16] | (Li et al., 2022) | Deep learning | Provided open-source, unenhanced abdominal CT dataset for training deep learning networks | Accomplished highly accurate 2D and 3D segmentation of kidneys and kidney stones |
| [17] | (Xu & Lyu, 2016) | Unsupervised | Employed 3D-HCT and IVU for treating lower pole calyx stones | Demonstrated advantages of 3D-HCT in kidney lower pole spatial anatomy analysis |
| [18] | (Çiçek et al., 2016) | Unsupervised | Applied volumetric segmentation using sparsely annotated images | Achieved effective results for complex 3D structures like Xenopus kidney |
| [19] | (da Cruz et al., 2020) | Deep learning | Utilized image-processing techniques and deep CNNs to delimit kidneys | Aided in the early diagnosis of kidney tumors |
| [20] | (Cuingnet et al., 2012) | Deep learning | Combined random forests and template deformation for segmentation | Achieved rapid and accurate detection and segmentation per volume |
| [21] | (Thong et al., 2016) | Deep learning | Implemented patch-wise approach with a ConvNet for voxel class prediction | Enabled efficient predictions for entire CT scan volume segmentation |
| Reference | Title of the Paper | Dice Similarity Coefficient | Jaccard Index | Surface-to-Surface Distance |
|---|---|---|---|---|
| [14] | “Automatic kidney segmentation in CT images based on multi-atlas image registration” | 95.2 | 0.913 mm | |
| [15] | “MSS U-Net: 3D segmentation of kidneys and tumors from CT images with a multi-scale supervised U-Net” | MSS U-Net: 96.9 Classic 3D U-Net: 96.2 | MSS U-Net: 94.1 Classic 3D U-Net: 93.0 | |
| [19] | “Kidney segmentation from computed tomography images using deep neural network” | 96.33 | 93.02 | |
| [21] | “Convolutional networks for kidney segmentation in contrast-enhanced CT scans” | ConvNet-Coarse Left: 94.53 91.72–95.04 Right: 93.07 89.99–94.28 ConvNet-Fine: Left: 93.62 91.99–94.98 Right: 92.52 88.83–94.47 | ||
| [22] | “Automatic Segmentation of Kidney and Renal Tumor in CT Images Based on 3D Fully Convolutional Neural Network with Pyramid Pooling Module” | 93.1 | 4.21 pixels | |
| [23] | “3D Kidney Segmentation from CT Images Using a Level Set Approach Guided by a Novel Stochastic Speed Function” | 97.0 |
| Ref. | Author/s | Applications | Clinical Implications |
|---|---|---|---|
| [8] | (Zeng et al., 2021) | Kidney Disease Diagnosis, Surgical Planning, Outcome Analysis, Intuitive Visualization, Automated Workflow | Enhanced Diagnostic Accuracy, Improved Surgical Outcomes, Reduced Variability, Efficient Workflow, Facilitating Research, Patient-Centered Care |
| [14] | (Guanyu Yang et al., 2014) | Computer-Aided Diagnosis, Treatment Planning, Disease Monitoring, Clinical Research, Education and Training | Improved Diagnostic Accuracy, Time Efficiency, Enhanced Treatment Decisions, Minimally Invasive Interventions, Tracking Treatment Response, Clinical Workflow Integration, Patient Care Standardization |
| [15] | (Zhao et al., 2020) | Radiomic Analysis, Surgical Planning, Automated Segmentation | Improved Diagnostic Accuracy, Efficient Training, Enhanced Segmentation Performance, Clinical Decision Making, Standardized Segmentation, Advancements in Medical Imaging, KiTS19 Challenge |
| [16] | (Li et al., 2022) | Automated Kidney Segmentation, Automated Kidney Stone Detection, Radiomics and Machine Learning Analyses, Open-Source Abdominal CT Dataset | Precise and Accurate Kidney Segmentation, AI-Driven Diagnostic Strategies, Personalized Patient Care, Decision-Making Support, Advancement in Kidney Disease Management |
| [17] | (Xu & Lyu, 2016) | Preoperative Planning, Anatomic Assessment | Advantages of 3D-HCT, Accuracy of 3D-HCT, Improved Preoperative Evaluation, Enhanced Surgical Outcomes, Patient Safety, Individualized Treatment, Clinical Decision Making |
| [18] | (Çiçek et al., 2016) | Semi-Automated Segmentation, Fully Automated Segmentation | Improved Efficiency in Segmentation, Potential for Standardization, Enhanced Diagnostic Accuracy, Data Augmentation for Robustness, No Pre-trained Network Required, Potential for Other Complex Structures |
| [19] | (da Cruz et al., 2020) | Improved Disease Diagnosis, Enhanced Treatment Planning, Time Savings and Efficiency, Consistency in Segmentation Results | Assistance in Early Detection of Kidney Tumors, Reduction of False Positives, Facilitating Medical Research |
| [20] | (Cuingnet et al., 2012) | Nephrology Information Extraction, Clinical Routine Integration, Automated Diagnosis and Assessment, Treatment Planning and Follow-Up | Improved Diagnosis Accuracy, Efficient Patient Care, Enhanced Research Opportunities, Standardization of Imaging Analysis, Reduced Workload for Radiologists and Nephrologists |
| [21] | (Thong et al., 2016) | Guiding Patient Diagnosis, Treatment Planning, Follow-ups and Monitoring | Time-Efficient Kidney Segmentation, Improved Diagnostic Accuracy, Standardization of Kidney Segmentation, Highly Variable Dataset |
| [22] | (Yang et al., 2018) | Improved Surgical Planning, Automated Segmentation for Efficiency, Enhanced Diagnostic and Prognostic Tools, Standardization and Consistency, Assisting Radiomics Studies | Enhanced Patient Outcomes, Potential for Personalized Treatment, Clinical Translation and Adoption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shreya; Sushanth; Shetty, D.K.; Bhatta, S.R.; Panwar, N. A Comprehensive Review on Unsupervised Domain Adaptation for 3D Segmentation and Reconstruction in CT Urography Imaging. Eng. Proc. 2023, 59, 13. https://doi.org/10.3390/engproc2023059013
Shreya, Sushanth, Shetty DK, Bhatta SR, Panwar N. A Comprehensive Review on Unsupervised Domain Adaptation for 3D Segmentation and Reconstruction in CT Urography Imaging. Engineering Proceedings. 2023; 59(1):13. https://doi.org/10.3390/engproc2023059013
Chicago/Turabian StyleShreya, Sushanth, Dasharathraj K. Shetty, Shreepathy Ranga Bhatta, and Nikita Panwar. 2023. "A Comprehensive Review on Unsupervised Domain Adaptation for 3D Segmentation and Reconstruction in CT Urography Imaging" Engineering Proceedings 59, no. 1: 13. https://doi.org/10.3390/engproc2023059013
APA StyleShreya, Sushanth, Shetty, D. K., Bhatta, S. R., & Panwar, N. (2023). A Comprehensive Review on Unsupervised Domain Adaptation for 3D Segmentation and Reconstruction in CT Urography Imaging. Engineering Proceedings, 59(1), 13. https://doi.org/10.3390/engproc2023059013







