Hydrothermal Synthesis of Mesoporous FeTiO3 for Photo-Fenton Degradation of Organic Pollutants and Fluoride Adsorption †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
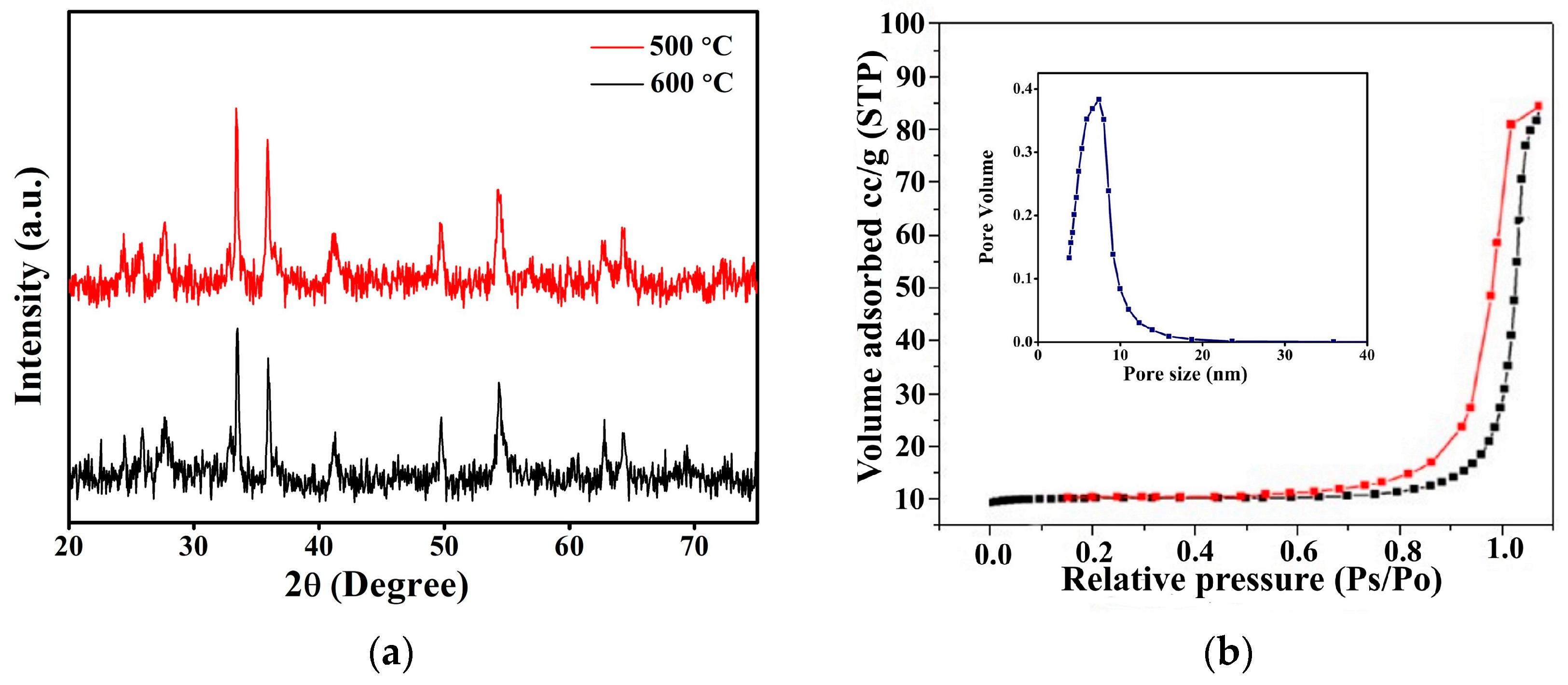
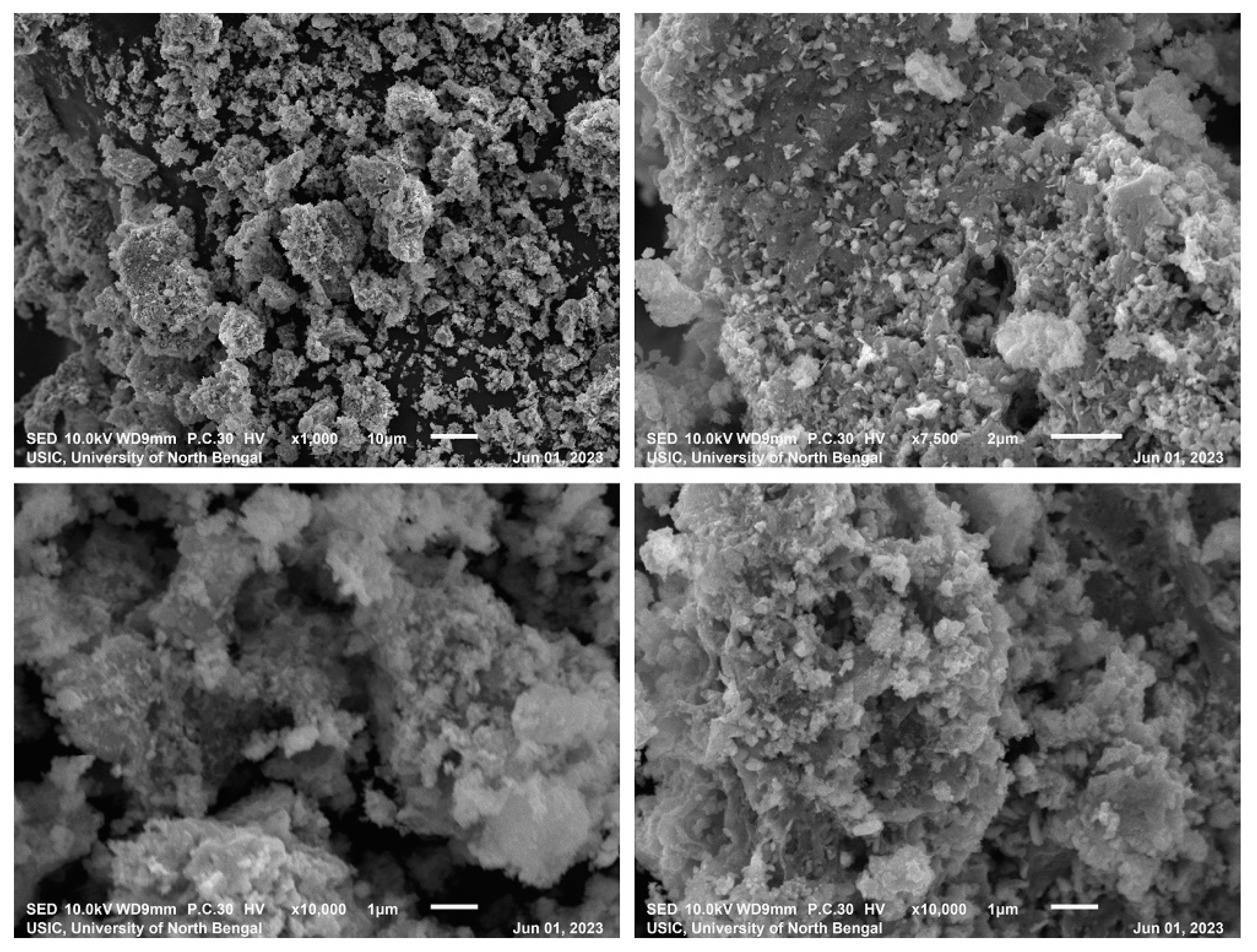
3.1. Characterization of FeTiO3 Photocatalyst
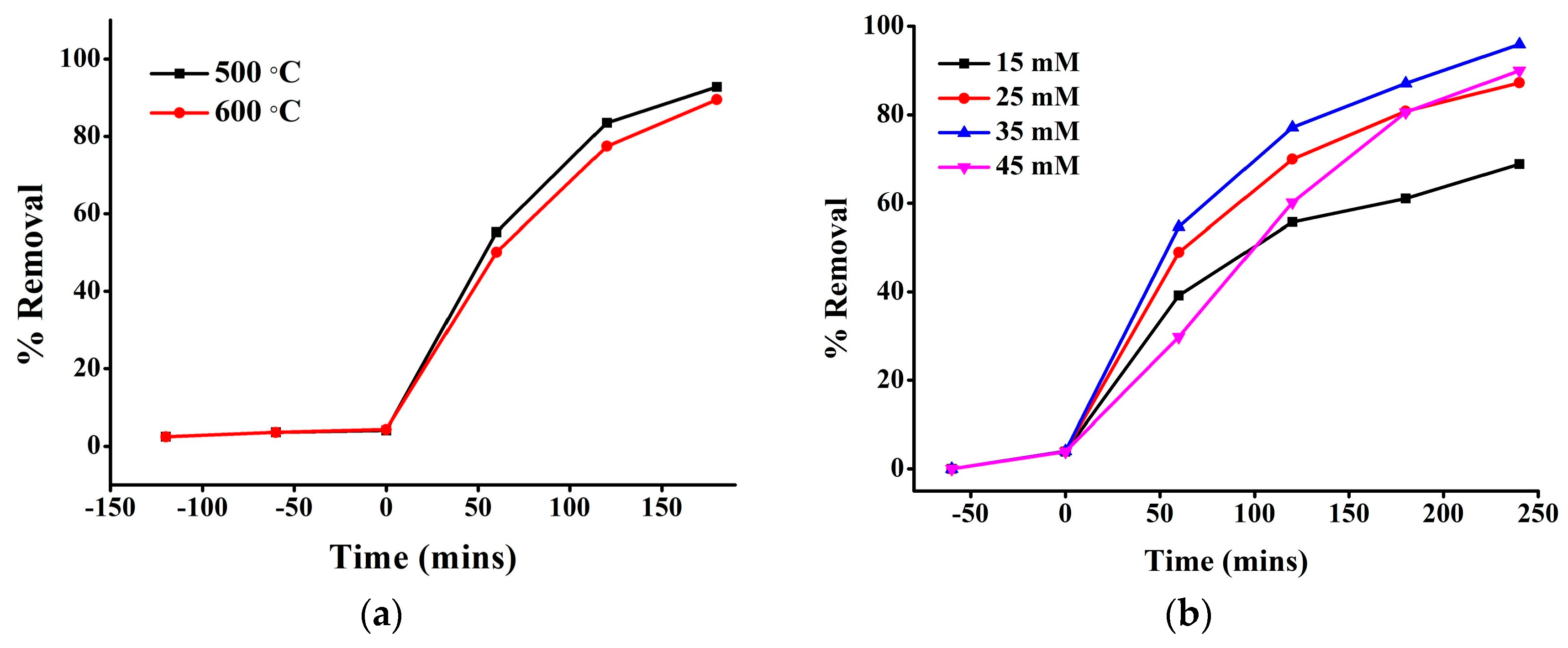
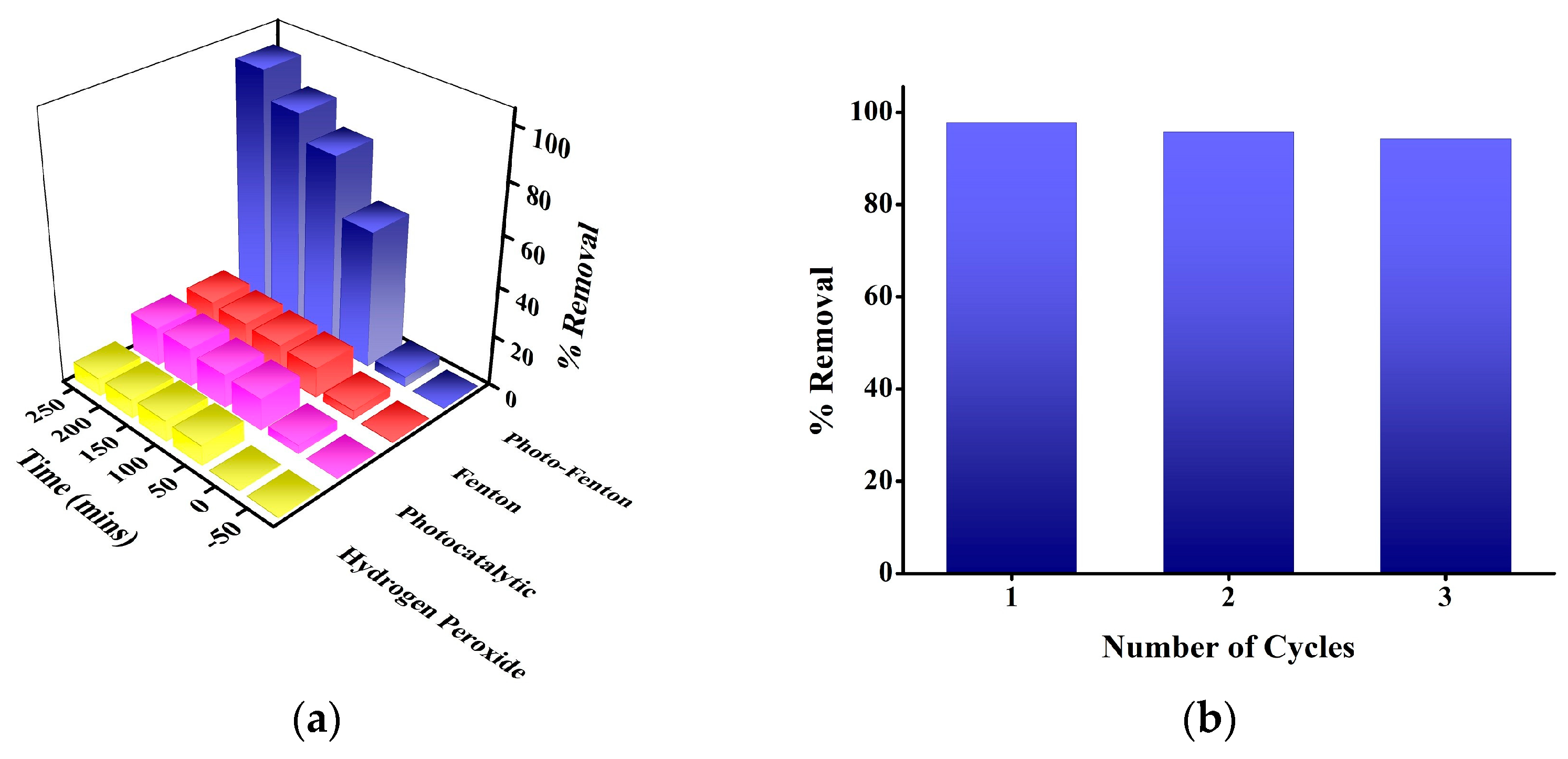
3.2. Catalytic Activity of FeTiO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamd, W.S.; Dutta, J. Heterogeneous photo-Fenton reaction and its enhancement upon addition of chelating agents. In Micro and Nano Technologies, Nanomaterials for the Detection and Removal of Wastewater Pollutants; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–330. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Y.; Li, G.; Wu, J.; Meng, G.; Guo, X.; Liu, Z. A novel strategy for preparation of an effective and stable heterogeneous photo-Fenton catalyst for the degradation of dye. Appl. Clay Sci. 2017, 136, 103–111. [Google Scholar] [CrossRef]
- Moradi, M.; Vasseghian, Y.; Khataee, A.; Harati, M.; Arfaeinia, H. Ultrasound-assisted synthesis of FeTiO3/GO nanocomposite for photocatalytic degradation of phenol under visible light irradiation. Sep. Purif. Technol. 2021, 261, 118274. [Google Scholar] [CrossRef]
- Zhang, S.T.; Ruan, Y.R.; Liu, C.; Wang, P.; Ma, Y.Q. The evolution of structure, chemical state and photocatalytic performance of α-Fe/FeTiO3/TiO2 with the nitridation at different temperatures. Mater. Res. Bull. 2017, 95, 503–508. [Google Scholar] [CrossRef]
- Truong, Q.D.; Liu, J.Y.; Chung, C.C.; Ling, Y.C. Photocatalytic reduction of CO2 on FeTiO3/TiO2 photocatalyst. Catal. Commun. 2012, 19, 85–89. [Google Scholar] [CrossRef]
- García-Muñoz, P.; Pliego, G.; Zazo, J.A.; Bahamonde, A.; Casas, J.A. Ilmenite (FeTiO3) as low cost catalyst for advanced oxidation processes. J. Environ. Chem. Eng. 2016, 4, 542–548. [Google Scholar] [CrossRef]
- Pataquiva-Mateus, A.Y.; Zea, H.R.; Ramirez, J.H. Degradation of orange II by Fenton reaction using ilmenite as catalyst. Environ. Sci. Pollut. Res. 2017, 24, 6187–6194. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Adak, M.K.; Upadhyay, S.; Khatun, J.; Dhak, P.; Khawas, S.; Ghorai, U.K.; Dhak, D. Efficient fluoride removal and dye degradation of contaminated water using Fe/Al/Ti oxide nanocomposite. ACS Omega 2019, 4, 9686–9696. [Google Scholar] [CrossRef] [PubMed]
- Kabir, H.; Gupta, A.K.; Tripathy, S. Fluoride and Human Health: Systematic appraisal of sources, exposures, metabolism, and toxicity. Crit. Rev. Environ. Sci. Technol. 2020, 5, 1116–1193. [Google Scholar] [CrossRef]
- Sen, S.; Choudhary, R.N.P.; Tarafdar, A.; Pramanik, P. Novel technique for synthesis and characterization of nanosized Ba1-XSrxSn0.15Ti0.85O3 ceramics. Phys. Status Solidi (a) 2004, 201, 937–943. [Google Scholar] [CrossRef]
- Poolakkandy, R.R.; Menamparambath, M.M. Soft-Template-Assisted Synthesis: A promising approach for the fabrication of transition metal oxides. Nanoscale Adv. 2020, 2, 5015–5045. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, W.; He, W.; Zhang, X.; Zhu, J. Multifunctional surfactants for synthesizing high-performance energy storage materials. Energy Storage Mater. 2021, 43, 1–19. [Google Scholar] [CrossRef]
- Dubey, N.; Arora, S. Surfactant assisted synthesis of pH responsive polyaniline-cellulose biocomposite for sensor applications. Polym. Plast. Technol. Mater. 2021, 60, 1135–1147. [Google Scholar] [CrossRef]
- Kumar, M.R.A.; Abebe, B.; Nagaswarupa, H.P.; Murthy, H.C.A.; Ravikumar, C.R.; Sabir, F.K. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: Its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Sarkar, A.; Gupta, N.; Biswas, S.K. Bimodal mesoporous α-Fe2O3/SiO2 composite: A highly efficient heterogeneous solar-driven photo-Fenton catalyst. J. Mol. Struct. 2023, 1284, 135373. [Google Scholar] [CrossRef]
- Zhou, L.; Lei, J.; Wang, L.; Liu, Y.; Zhang, J. Highly efficient photo-Fenton degradation of methyl orange facilitated by slow light effect and hierarchical porous structure of Fe2O3-SiO2 photonic crystals. Appl. Catal. B 2018, 237, 1160–1167. [Google Scholar] [CrossRef]
- Taher, T.; Yoshida, A.; Lesbani, A.; Kurnia, I.; Guan, G.; Abudula, A.; Ueda, W. Adsorptive removal and photocatalytic decomposition of cationic dyes on niobium oxide with deformed orthorhombic structure. J. Hazard. Mater. 2021, 41, 125635. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Sarkar, A.; Pradhan, B.; Biswas, S.K. Hydrothermal Synthesis of Mesoporous FeTiO3 for Photo-Fenton Degradation of Organic Pollutants and Fluoride Adsorption. Eng. Proc. 2023, 59, 134. https://doi.org/10.3390/engproc2023059134
Gupta N, Sarkar A, Pradhan B, Biswas SK. Hydrothermal Synthesis of Mesoporous FeTiO3 for Photo-Fenton Degradation of Organic Pollutants and Fluoride Adsorption. Engineering Proceedings. 2023; 59(1):134. https://doi.org/10.3390/engproc2023059134
Chicago/Turabian StyleGupta, Neha, Arpita Sarkar, Bivek Pradhan, and Soumya Kanti Biswas. 2023. "Hydrothermal Synthesis of Mesoporous FeTiO3 for Photo-Fenton Degradation of Organic Pollutants and Fluoride Adsorption" Engineering Proceedings 59, no. 1: 134. https://doi.org/10.3390/engproc2023059134





