Effect of Violet Laser Irradiation on the Optical Properties of Polyvinyl Alcohol/Methyl Orange Composite Thick Films: A Model for Medical Applications †
Abstract
:1. Introduction
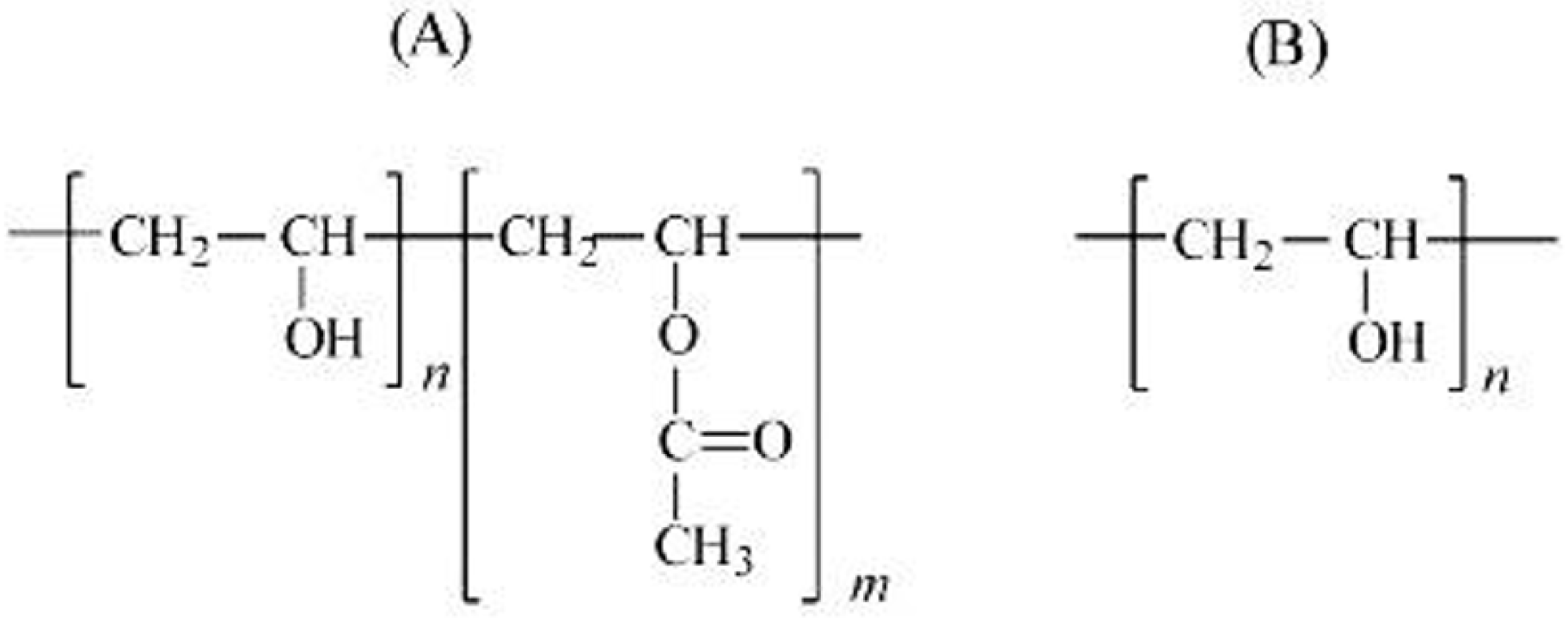
2. Materials and Methods
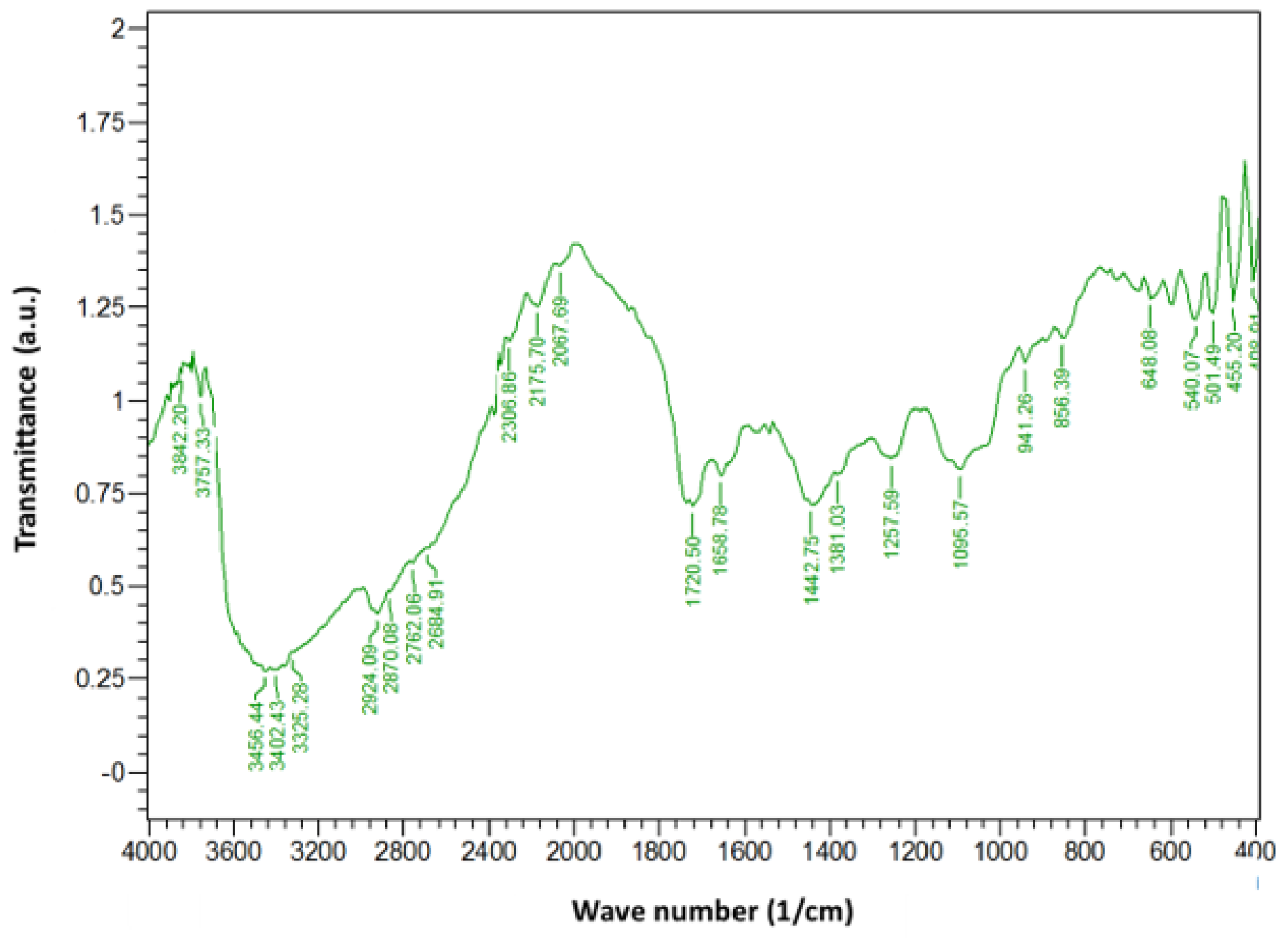
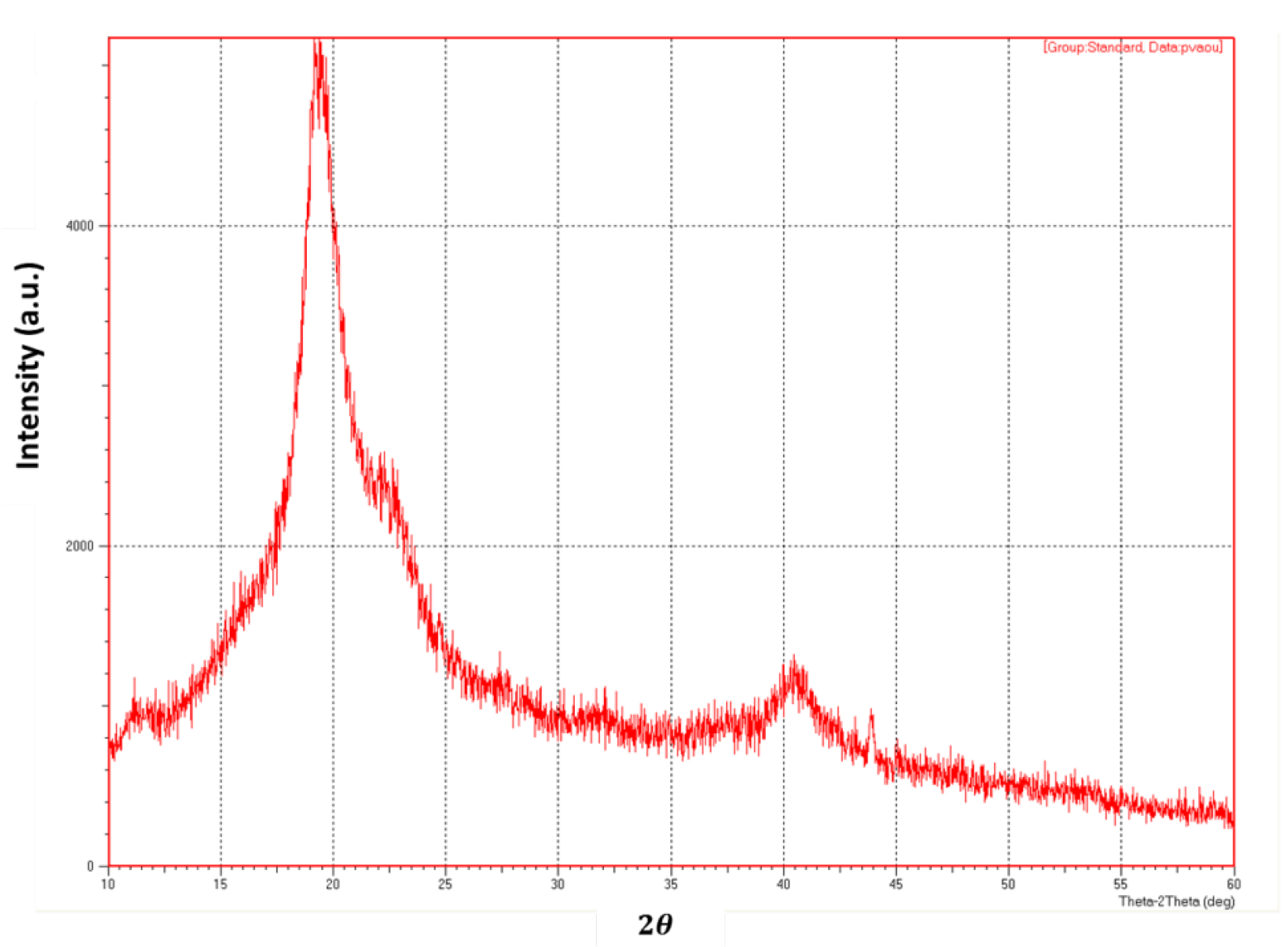
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebewele, R.O. Polymer Science and Technology; CRC Press: New York, NY, USA, 1995; Volume 16, p. 3. [Google Scholar]
- Nagarkar, R.; Patel, J. Polyvinyl Alcohol: A Comprehensive Study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- Patachia, A.P.; Valente, A.J.M. (Eds.) Poly (vinyl alcohol) [PVA]-Based Polymer Membranes; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Demerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Giri, M.; Singh, D.; Lal, J.; Jaggi, N.; Singh, N.; Jaiswal, R.M.P. Absorption and Fluorescence Spectra of Methyl Orange in Aqueous Solutions. Atti Fond. Giorgio Ronchi ANNO LXVII 2012, 2, 255. [Google Scholar]
- Al-kadhemy, M.F.H.; Saeed, A.A.; Kadhum, F.J.; Mazloum, S.A.; Aied, H.K. The effect of (He–Ne) laser irradiation on the optical properties of methyl orange doped PVA films. J. Radiat. Res. Appl. Sci. 2014, 7, 371–375. [Google Scholar] [CrossRef]
- Bujdák, J. Controversial Issues Related to Dye Adsorption on Clay Minerals: A Critical Review. Molecules 2023, 28, 6951. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Mohammed, H.S. Study the Effect of Immobilization on Electrostatic Potential for Methyl Orange Dye. Muthanna J. Pure Sci. 2018, 5, 38–43. [Google Scholar] [CrossRef]
- Bhat, R.; Mohan, N.; Sharma, S.; Rao, S. Influence of Seawater Absorption on the Hardness of Glass Fiber/Polyester Composite. J. Comput. Mech. Manag. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Singh, H.; Singh, K.; Vardhan, S. Enhancing Aluminum Matrix Composites with Hexagonal Boron Nitride (hBN) Particulates: A Comprehensive Review. J. Comput. Mech. Manag. 2023, 2, 5. [Google Scholar] [CrossRef]
- Srikanth, V.; Kowshik, S.; Narasimha, D.; Patil, S.; Samanth, K.; Rathee, U. Finite Element Modelling and Analysis of Fiber Reinforced Concrete under Tensile and Flexural Loading. J. Comput. Mech. Manag. 2022, 1, 11–17. [Google Scholar] [CrossRef]
- Chawla, K.K. Composite Materials; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Naik, N.; Suresh, P.; Yadav, S.; Nisha, M.P.; Arias-Gonzáles, J.L.; Cotrina-Aliaga, J.C.; Bhat, R.; Jalageri, M.D.; Kaushik, Y.; Kunjibettu, A.B. A Review on Composite Materials for Energy Harvesting in Electric Vehicles. Energies 2023, 16, 3348. [Google Scholar] [CrossRef]
- Hiremath, P.; Viswamurthy, S.R.; Shettar, M.; Naik, N.; Kowshik, S. Damage Tolerance of a Stiffened Composite Panel with an Access Cutout under Fatigue Loading and Validation Using FEM Analysis and Digital Image Correlation. Fibers 2022, 10, 105. [Google Scholar] [CrossRef]
- Mohan, N.; Sharma, S.; Bhat, R. A Comprehensive Study of Glass Fibre Reinforced Polymer (GFRP) Drilling. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 1–10. [Google Scholar]
- Nowakowski, K.A. Laser Beam Interaction with Materials for Microscale Applications. Doctoral Dissertation, Worcester Polytechnic Institute, Worcester, MA, USA, 2005. [Google Scholar]
- Hammood, A.; Al-Aarajiy, M. Preparation and Characterization of NiPc/Si Organic Solar Cell; University of Baghdad: Baghdad, Iraq, 2014. [Google Scholar]
- Burns, G. Solid State Physics; Cambridge University Press: New York, NY, USA, 1985. [Google Scholar]
- Hasan, M.; Khaleel, N. Study the Linear and Nonlinear Optical Properties for Methylene Blue Dye Doped SiO2 Nanoparticles; University of Babylon: Babylon, Iraq, 2022. [Google Scholar]
- Ejam, A.A.; Wahhab, N.A.A. Concentration effect on the optical properties of laser irradiation copper phthalocyanine (CuPc blue) solution. NeuroQuantology 2022, 20, 1–15. [Google Scholar] [CrossRef]
- AbdulWahhab, N.A. Optical properties of SnO2 thin films prepared by pulsed laser deposition technique. J. Opt. 2020, 49, 41–47. [Google Scholar] [CrossRef]
- Nasir, E.M.; Hussein, M.T.; Al-Aarajiy, A.H. Investigation of Nickel Phthalocyanine Thin Films for Solar Cell Applications. Adv. Mater. Phys. Chem. 2019, 9, 158–173. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Saber, D.R. Optical absorption of Polyvinyl alcohol films doped with Nickel Chloride. Appl. Mech. Mater. 2012, 110–116, 177–182. [Google Scholar] [CrossRef]
- Habubi, N.F.; Chiad, S.S. Optical Properties of Doped Polymers. Diyala J. Pure Sci. 2017, 7, 153–161. [Google Scholar]
- Sólyom, J. Fundamentals of the Physics of Solids, Volume 1 Structure and Dynamics; Springer: New York, NY, USA, 2007. [Google Scholar]
- Azim-Araghi, M.E.; Krier, A. Optical characterization of chloroaluminium phthalocyanine (ClAlPc) thin films. Pure Appl. Opt. J. Eur. Opt. Soc. 1997, 6, 443. [Google Scholar] [CrossRef]
- Thampraphaphon, B.; Phosri, C.; Pisutpaisal, N.; Thamvithayakorn, P.; Chotelersak, K.; Sarp, S.; Suwannasai, N. High Potential Decolourisation of Textile Dyes from Wastewater by Manganese Peroxidase Production of Newly Immobilised Trametes hirsuta PW17-41 and FTIR Analysis. Microorganisms. 2022, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Mahendia, S.; Tomar, A.K.; Chahal, R.P.; Goyal, P.; Kumar, S. Optical and structural properties of poly(vinyl alcohol) films embedded with citrate-stabilized gold nanoparticles. J. Phys. D Appl. Phys. 2011, 44, 20. [Google Scholar] [CrossRef]


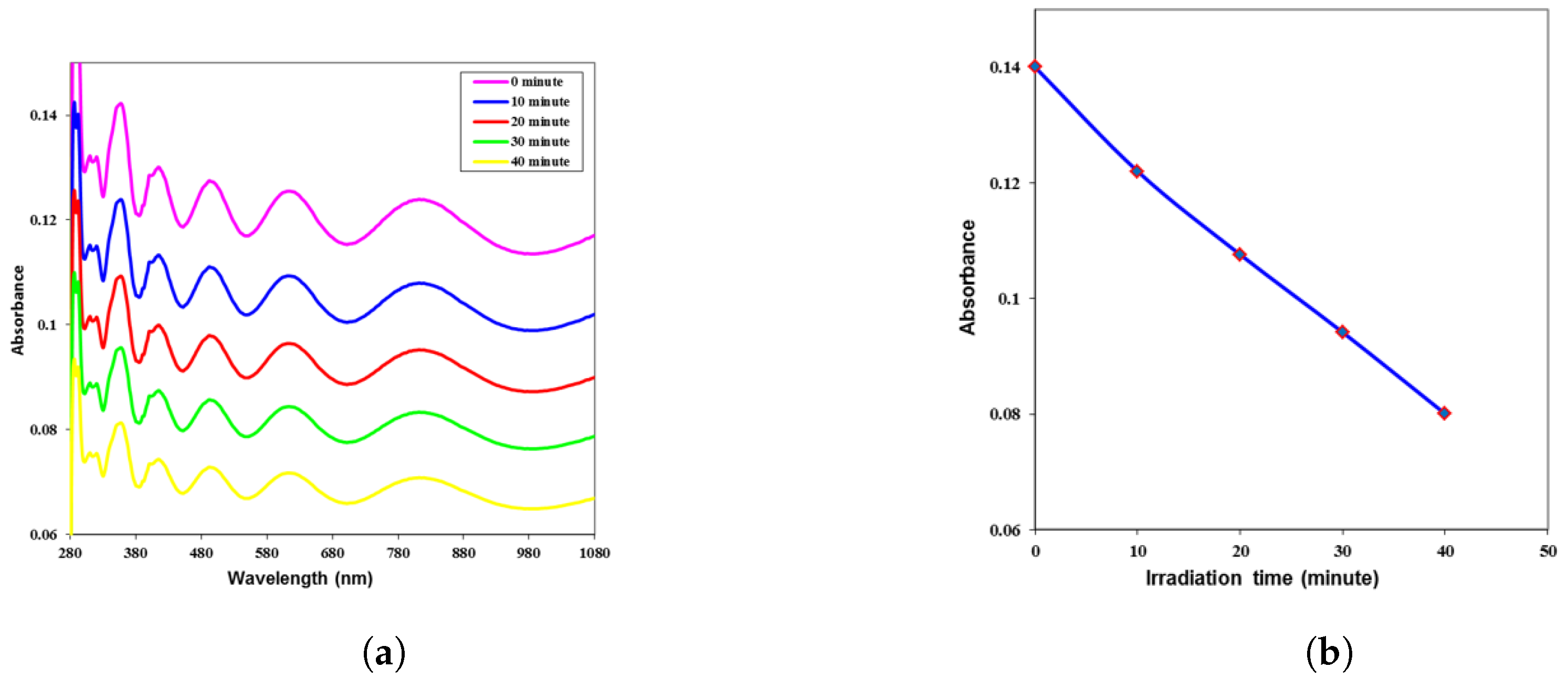
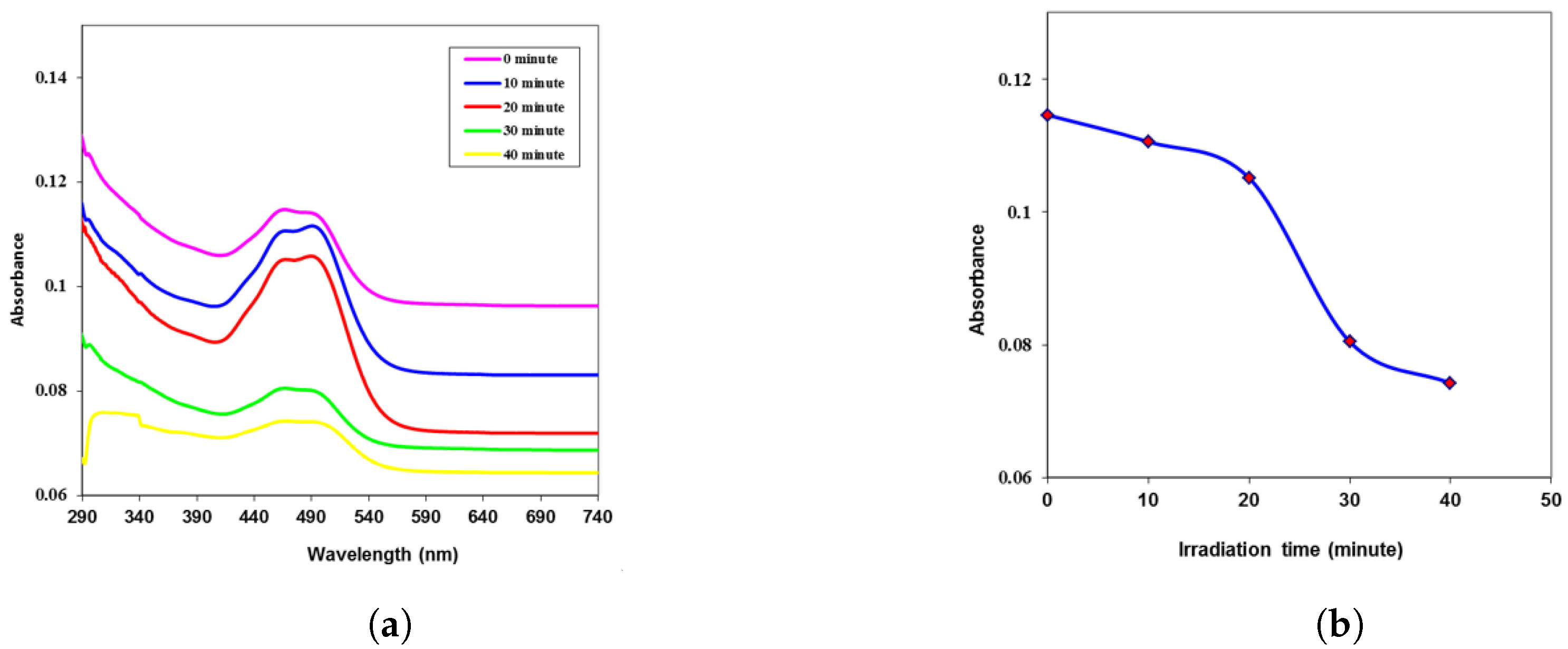
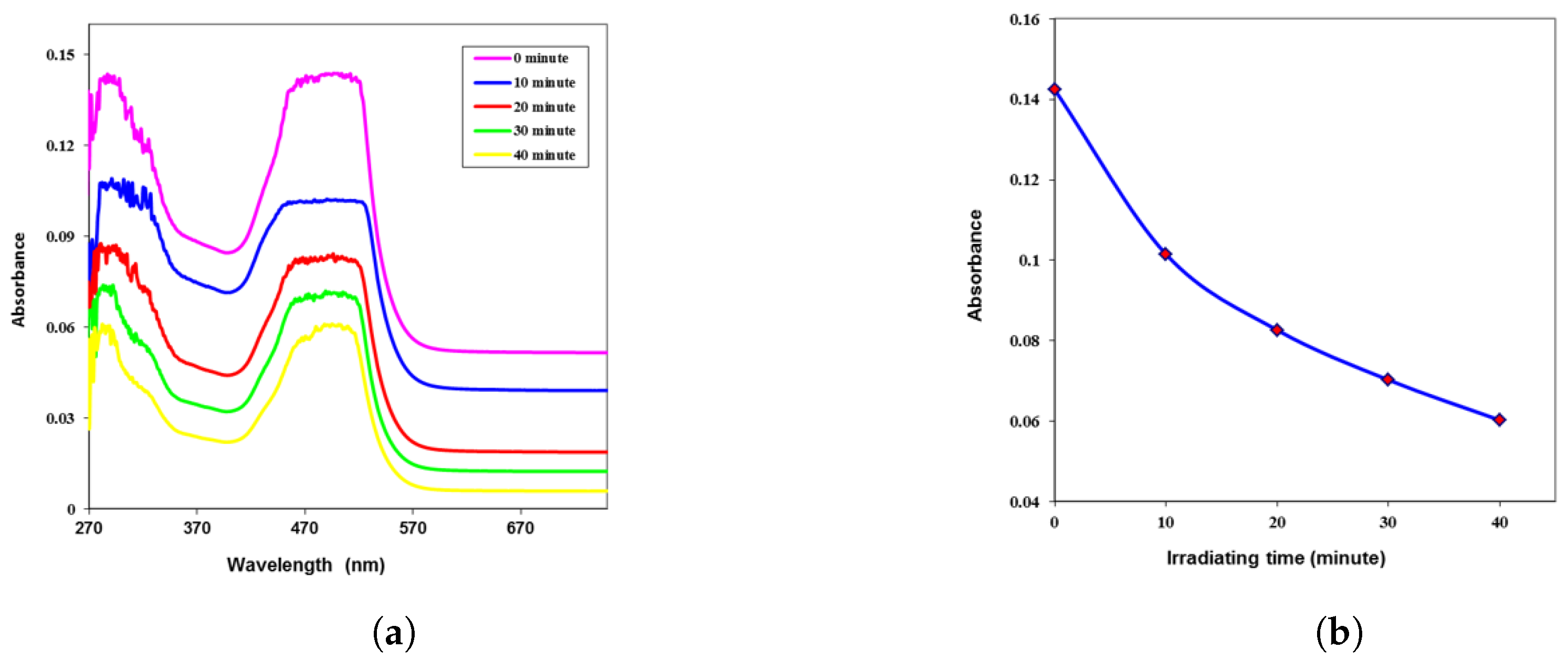
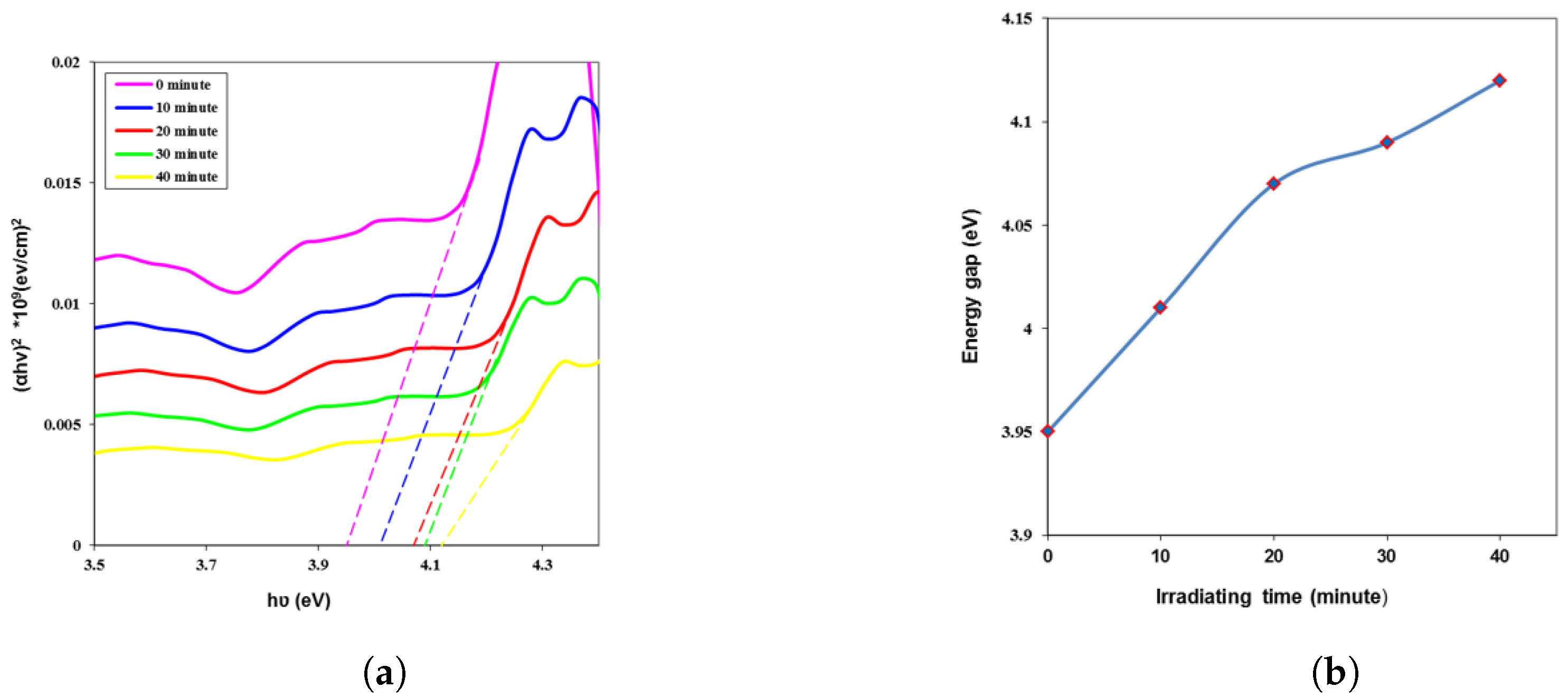
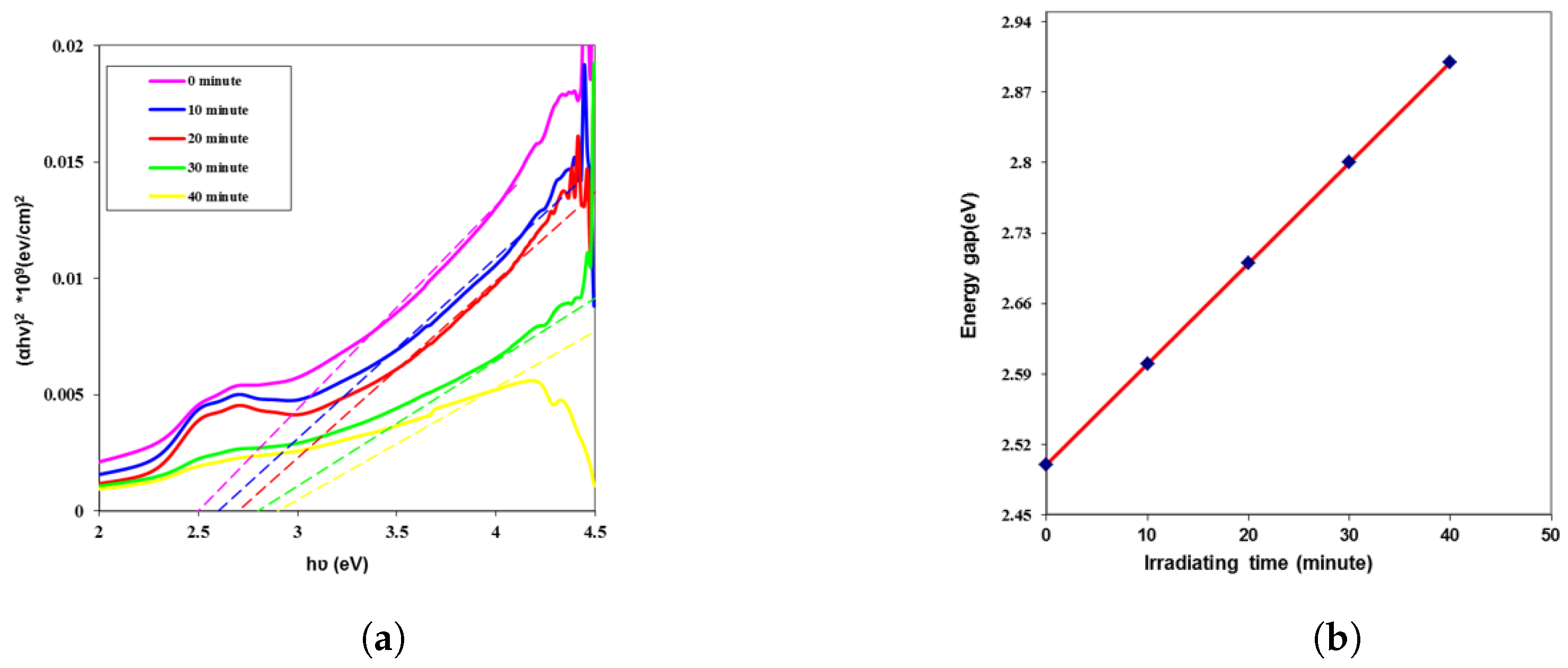

| Time (min) | k | n | (eV) | ||||
|---|---|---|---|---|---|---|---|
| (cm−1) | (s−1) | ||||||
| 0 | 0.107 | 0.0029 | 1.941 | 3.768 | 0.011 | 0.049 | 3.95 |
| 10 | 0.093 | 0.0025 | 1.840 | 3.388 | 0.009 | 0.041 | 4.01 |
| 20 | 0.082 | 0.0022 | 1.755 | 3.082 | 0.008 | 0.034 | 4.07 |
| 30 | 0.072 | 0.0020 | 1.672 | 2.796 | 0.006 | 0.028 | 4.09 |
| 40 | 0.061 | 0.0017 | 1.580 | 2.499 | 0.005 | 0.023 | 4.12 |
| Time (min) | k | n | (eV) | ||||
|---|---|---|---|---|---|---|---|
| (cm−1) | (s−1) | ||||||
| 0 | 0.087 | 0.0032 | 1.797 | 3.231 | 0.0118 | 0.0377 | 2.7 |
| 10 | 0.084 | 0.0031 | 1.773 | 3.145 | 0.0112 | 0.0359 | 2.9 |
| 20 | 0.080 | 0.0030 | 1.740 | 3.029 | 0.0105 | 0.0335 | 3.0 |
| 30 | 0.061 | 0.0023 | 1.583 | 2.507 | 0.0073 | 0.0233 | 3.1 |
| 40 | 0.056 | 0.0021 | 1.542 | 2.378 | 0.0065 | 0.0209 | 3.2 |
| Time (min) | k | n | (eV) | ||||
|---|---|---|---|---|---|---|---|
| (cm−1) | (s−1) | ||||||
| 0 | 0.054 | 0.0020 | 1.953 | 3.816 | 0.0081 | 0.0255 | 2.4 |
| 10 | 0.038 | 0.0014 | 1.718 | 2.951 | 0.0051 | 0.0159 | 2.5 |
| 20 | 0.031 | 0.0012 | 1.597 | 2.553 | 0.0038 | 0.0121 | 2.6 |
| 30 | 0.026 | 0.0010 | 1.515 | 2.295 | 0.0031 | 0.0097 | 2.7 |
| 40 | 0.023 | 0.0008 | 1.446 | 2.092 | 0.0025 | 0.0079 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tareq, S.M.; AbdulWahhab, N.A.; Al-araji, A.H. Effect of Violet Laser Irradiation on the Optical Properties of Polyvinyl Alcohol/Methyl Orange Composite Thick Films: A Model for Medical Applications. Eng. Proc. 2023, 59, 236. https://doi.org/10.3390/engproc2023059236
Tareq SM, AbdulWahhab NA, Al-araji AH. Effect of Violet Laser Irradiation on the Optical Properties of Polyvinyl Alcohol/Methyl Orange Composite Thick Films: A Model for Medical Applications. Engineering Proceedings. 2023; 59(1):236. https://doi.org/10.3390/engproc2023059236
Chicago/Turabian StyleTareq, Sarah Maysam, Nihal A. AbdulWahhab, and Addnan H. Al-araji. 2023. "Effect of Violet Laser Irradiation on the Optical Properties of Polyvinyl Alcohol/Methyl Orange Composite Thick Films: A Model for Medical Applications" Engineering Proceedings 59, no. 1: 236. https://doi.org/10.3390/engproc2023059236





