Molecular Discrimination and Phylogenetic Relationships of Physalis Species Based on ITS2 and rbcL DNA Barcode Sequence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Collection of Plant Samples
2.2. Genomic DNA Extraction
2.3. PCR Amplification and Sequencing
2.4. Sequence Alignment, Phylogenetic and Data Analysis
2.5. Analysis of Genetic Divergence
2.6. Determination of Intraspecific and Interspecific Genetic Distance
2.7. Nucleotide Polymorphism and Neutrality Tests
2.8. Barcoding Gap Analysis
3. Results
3.1. Success Rates of PCR Amplification and Sequencing
3.2. Species Discrimnation of Physalis Accessions Using BLASTn Analysis
3.3. Multiple Sequence Alignments
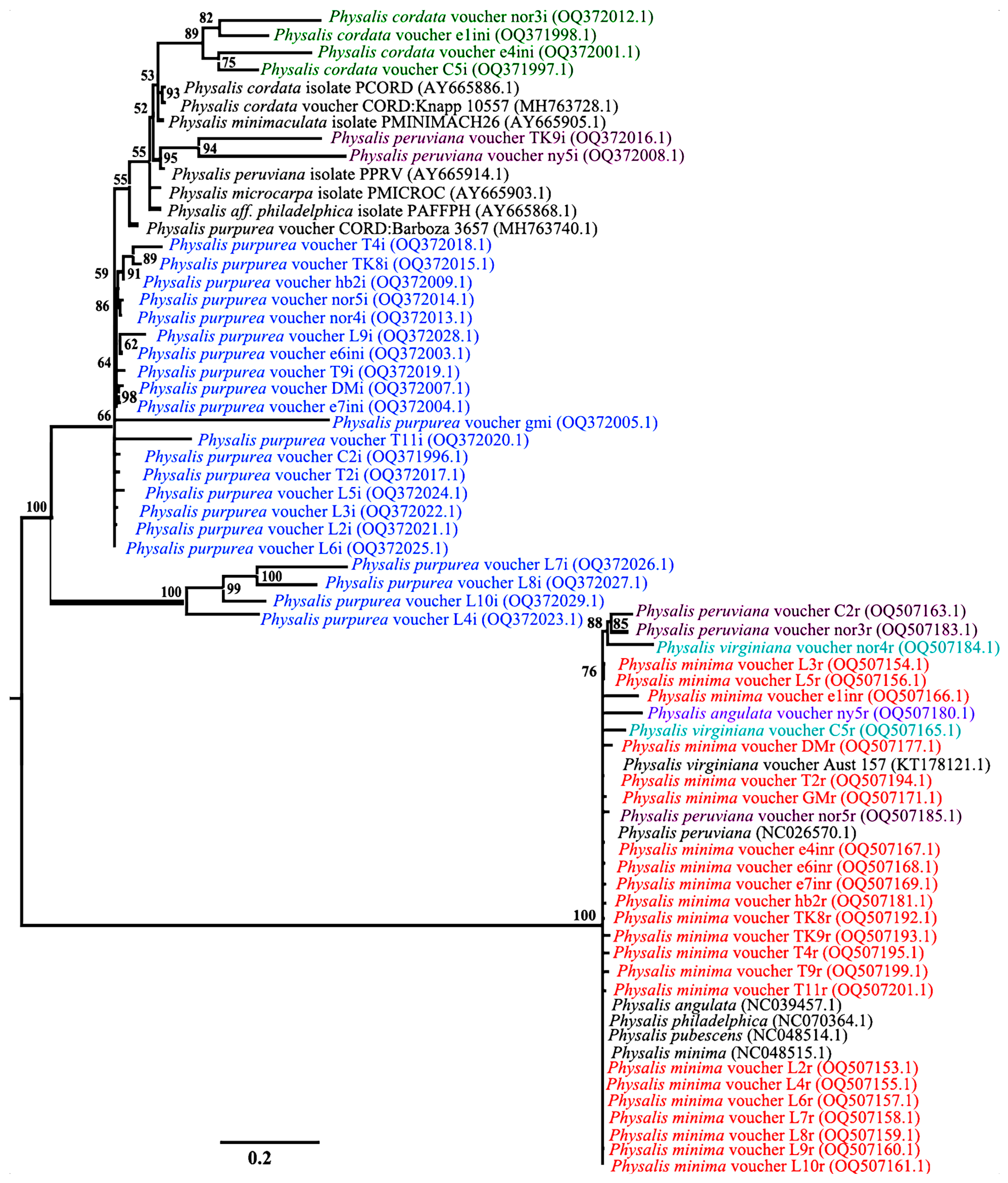
3.4. Species Discrimnation of Physalis Species Based on Phylogenetic Analysis
3.5. Genetic Divergence Analysis between and within Physalis Species Based on ITS2 Sequences
3.5.1. DNA Divergence between Populations Based on ITS2 Sequences
3.5.2. DNA Divergence within Populations Based on ITS2 Sequences
3.6. Genetic Distance between and within Physalis Species Based on ITS2 and rbcL Sequences
3.7. Nucleotide Polymorphism and Neutrality Tests
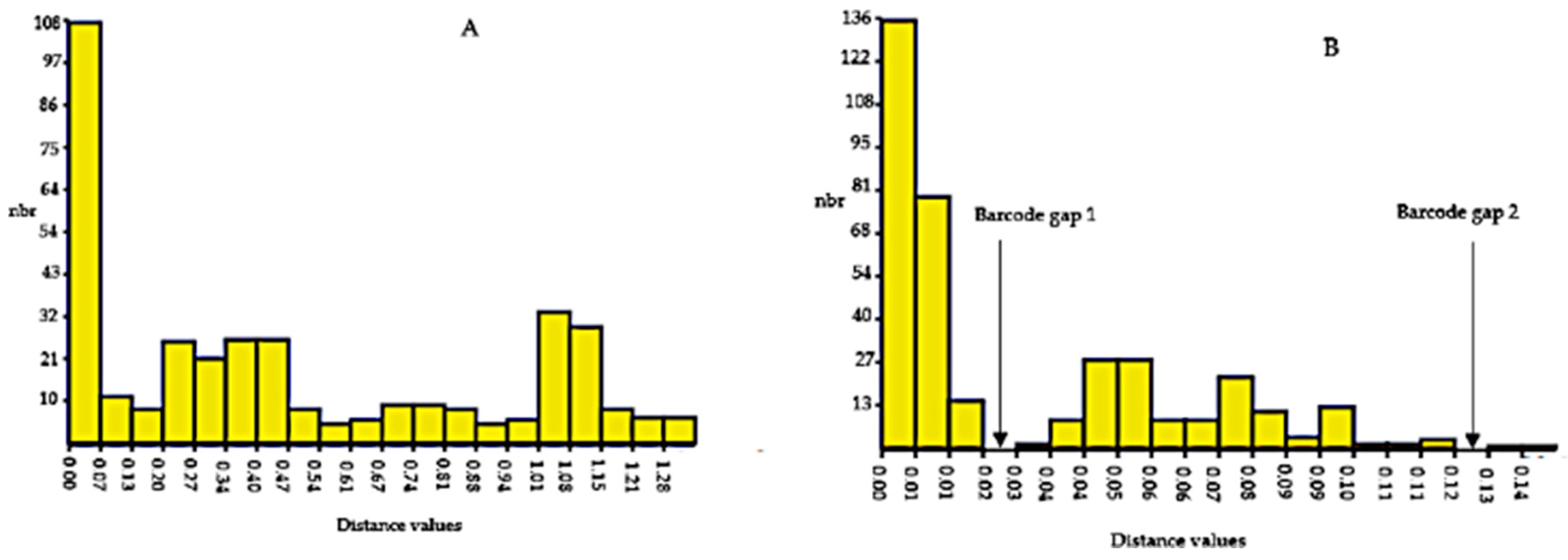
3.8. Barcoding Gap Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ralte, L.; Singh, Y.T. Use of rbcL and ITS2 for DNA barcoding and identification of Solanaceae plants in hilly state of Mizoram, India. Res. Crops 2021, 22, 616–623. [Google Scholar]
- Cháves-Gómez, J.L.; Becerra-Mutis, L.M.; Chávez-Arias, C.C.; Restrepo-Díaz, H.; Gómez-Caro, S. Screening of different Physalis genotypes as potential rootstocks or parents against vascular wilt using physiological markers. Front. Plant Sci. 2020, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Flórez, F.; López-Cristoffanini, C.; Jáuregui, O.; Melgarejo, L.M.; López-Carbonell, M. Changes in ABA, IAA and JA levels during calyx, fruit and leaves development in cape gooseberry plants (Physalis peruviana L.). Plant Physiol. Biochem. 2017, 115, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, C.; Ana, Y.; Estrada Mesa, E.M.; Cortés Rodríguez, M. The influence of drying on the physiological quality of cape gooseberry (Physalis peruviana L.) fruits added with active components. Acta Agron. 2017, 66, 512–518. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.T. Oil goldenberry (Physalis peruviana L.). J. Agric. Food Chem. 2003, 51, 969–974. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Li, H.B. Chemical components and bioactivities of Cape gooseberry (Physalis peruviana). Int. J. Food Nutr. Saf. 2013, 3, 15–24. [Google Scholar]
- Barirega, A. Potential for value chain improvement and commercialization of cape gooseberry (Physalis peruviana L.) for livelihood improvement in Uganda. Ethnobot. Res. Appl. 2014, 12, 131–140. [Google Scholar]
- Afroz, M.; Akter, S.; Ahmed, A.; Rouf, R.; Shilpi, J.A.; Tiralongo, E.; Sarker, S.D.; Göransson, U.; Uddin, S.J. Ethnobotany and antimicrobial peptides from plants of the solanaceae family: An update and future prospects. Front. Pharmacol. 2020, 11, 565. [Google Scholar] [CrossRef]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Reddy, C.V.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int. 2010, 4, 285–288. [Google Scholar] [CrossRef]
- Arun, M.; Asha, V.V. Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J. Ethnopharmacol. 2007, 111, 110–114. [Google Scholar] [CrossRef]
- Zhang, W.N.; Tong, W.Y. Chemical constituents and biological activities of plants from the genus Physalis. Chem. Biodivers. 2016, 13, 48–65. [Google Scholar] [CrossRef]
- Abdul-Nasir-Deen, A.Y.; Boakye, Y.D.; Osafo, N.; Agyare, C.; Boamah, D.; Boamah, V.E.; Agyei, E.K. Anti-inflammatory and wound healing properties of methanol leaf extract of Physalis angulata L. S. Afr. J. Bot. 2020, 133, 124–131. [Google Scholar] [CrossRef]
- Franco, L.A.; Matiz, G.E.; Calle, J.; Pinzón, R.; Ospina, L.F. Antiinflammatory activity of extracts and fractions obtained from Physalis peruviana L. calyces. Biomedica 2007, 27, 110–115. [Google Scholar] [CrossRef]
- Wu, S.J.; Tsai, J.Y.; Chang, S.P.; Lin, D.L.; Wang, S.S.; Huang, S.N.; Ng, L.T. Supercritical carbon dioxide extract exhibits enhanced antioxidant and anti-inflammatory activities of Physalis peruviana. J. Ethnopharmacol. 2006, 108, 407–413. [Google Scholar] [CrossRef]
- Pinto, M.D.; Ranilla, L.G.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of antihyperglycemia and antihypertension potential of native Peruvian fruits using in vitro models. J. Med. Food. 2009, 12, 278–291. [Google Scholar] [CrossRef]
- Lan, Y.H.; Chang, F.R.; Pan, M.J.; Wu, C.C.; Wu, S.J.; Chen, S.L.; Wang, S.S.; Wu, M.J.; Wu, Y.C. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009, 116, 462–469. [Google Scholar] [CrossRef]
- Shenstone, E.; Lippman, Z.; Van Eck, J. A review of nutritional properties and health benefits of Physalis species. Plant Foods Hum. Nutr. 2020, 75, 316–325. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, M.; Shi, Y.; Jiao, K.; Shen, C.; Lu, J.; Ying, Q.; Wang, H. Application of the ribosomal DNA ITS2 region of Physalis (Solanaceae): DNA barcoding and phylogenetic study. Front. Plant Sci. 2016, 7, 1047. [Google Scholar] [CrossRef]
- Menzel, M.Y. The cytotaxonomy and genetics of Physalis. Proc. Am. Philos. Soc. 1951, 95, 132–183. [Google Scholar]
- Vargas-Ponce, O.; Pérez-Álvarez, L.F.; Zamora-Tavares, P.; Rodríguez, A. Assessing genetic diversity in Mexican husk tomato species. Plant Mol. Biol. Rep. 2011, 29, 733–738. [Google Scholar] [CrossRef]
- Feng, S.; Jiao, K.; Zhu, Y.; Wang, H.; Jiang, M.; Wang, H. Molecular identification of species of Physalis (Solanaceae) using a candidate DNA barcode: The chloroplast psbA–trnH intergenic region. Genome 2018, 61, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, X.I.; Liu, C.; Newmaster, S.; Ragupathy, S.; Kress, W.J. Progress in the use of DNA barcodes in the identification and classification of medicinal plants. Ecotoxicol. Environ. Saf. 2021, 208, 111691. [Google Scholar] [CrossRef] [PubMed]
- Schindel, D.E.; Miller, S.E. DNA barcoding a useful tool for taxonomists. Nature 2005, 435, 17. [Google Scholar] [CrossRef]
- Qian, Z.H.; Munywoki, J.M.; Wang, Q.F.; Malombe, I.; Li, Z.Z.; Chen, J.M. Molecular Identification of African Nymphaea Species (Water Lily) Based on ITS, trnT-trnF and rpl16. Plants 2022, 11, 2431. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Kumar, K. DNA barcoding of plants: Selection of core markers for taxonomic groups. Plant Sci. Today 2018, 5, 9–13. [Google Scholar] [CrossRef]
- Kress, W.J. Plant DNA barcodes: Applications today and in the future. J. Syst. Evol. 2017, 55, 291–307. [Google Scholar] [CrossRef]
- Dormontt, E.E.; Van Dijk, K.J.; Bell, K.L.; Biffin, E.; Breed, M.F.; Byrne, M.; Caddy-Retalic, S.; Encinas-Viso, F.; Nevill, P.G.; Shapcott, A.; et al. Advancing DNA barcoding and metabarcoding applications for plants requires systematic analysis of herbarium collections—An Australian perspective. Front. Ecol. Evol. 2018, 6, 134. [Google Scholar] [CrossRef]
- Li, H.; Xiao, W.; Tong, T.; Li, Y.; Zhang, M.; Lin, X.; Zou, X.; Wu, Q.; Guo, X. The specific DNA barcodes based on chloroplast genes for species identification of Orchidaceae plants. Sci. Rep. 2021, 11, 1424. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, Z.; Zang, R.; Long, W. DNA barcoding analysis and phylogenetic relationships of tree species in tropical cloud forests. Sci. Rep. 2017, 7, 12564. [Google Scholar] [CrossRef]
- Nurhasanah; Sundari; Papuangan, N. Amplification and analysis of Rbcl gene (Ribulose-1, 5-Bisphosphate Carboxylase) of clove in Ternate Island. IOP Conf. Ser. Earth Environ. 2019, 276, 12061. [Google Scholar] [CrossRef]
- Manzara, T.; Gruissem, W. Organization and expression of the genes encoding ribulose-1, 5-bisphosphate carboxylase in higher plants. Mol. Biol. Photosyn. 1988, 621–643. [Google Scholar] [CrossRef]
- CBOL Plant Working Group 1; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. PNAS 2009, 106, 12794–12797. [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA mini-preparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed]
- Lledo, M.D.; Crespo, M.B.; Cameron, K.M.; Fay, M.F.; Chase, M.W. Systematics of Plumbaginaceae based upon cladistic analysis of rbcL sequence data. Syst. Bot. 1998, 23, 21–29. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 20–24. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.F.; dos Reis, M.; Yang, Z. A biologist’s guide to Bayesian phylogenetic analysis. Nat. Ecol. Evol. 2017, 1, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Kartavtsev, Y.P. Divergence at Cyt-b and Co-1 mtDNA genes on different taxonomic levels and genetics of speciation in animals. Mitochondrial DNA 2011, 22, 55–65. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Tajima, F. The effect of change in population size on DNA polymorphism. Genetics 1989, 123, 597–601. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G.J. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Almaraz-Abarca, N.; González-Elizondo, M.S.; Uribe-Soto, J.N.; González-Valdez, L.S.; Herrera-Arrieta, Y. Phenolic constituents and antioxidant properties of five wild species of Physalis (Solanaceae). Bot. Stud. 2015, 56, 24. [Google Scholar] [CrossRef]
- Huang, X.C.; Ci, X.Q.; Conran, J.G.; Li, J. Application of DNA barcodes in Asian tropical trees–a case study from Xishuangbanna Nature Reserve, Southwest China. PLoS ONE 2015, 10, e129295. [Google Scholar] [CrossRef]
- Simeone, M.C.; Piredda, R.; Papini, A.; Vessella, F.; Schirone, B. Application of plastid and nuclear markers to DNA barcoding of Euro-Mediterranean oaks (Quercus, Fagaceae): Problems, prospects and phylogenetic implications. Bot. J. Linn. Soc. 2013, 172, 478–499. [Google Scholar] [CrossRef]
- Denk, T.; Grimm, G.W. The oaks of western Eurasia: Traditional classifications and evidence from two nuclear markers. Taxon 2010, 59, 351–366. [Google Scholar] [CrossRef]
- Abeysinghe, P.D.; Wijesinghe, K.G.; Tachida, H.; Yoshda, T.; Thihagoda, M. Molecular characterization of Cinnamon (Cinnamomum verum Presl) accessions and evaluation of genetic relatedness of Cinnamon species in Sri Lanka based on trnL intron region, intergenic spacers between trnT-trnL, trnL-trnF, trnH-psbA and nuclear ITS. J. Agric. Biol. Sci. 2009, 5, 1079–1088. [Google Scholar]
- Ross, H.A.; Murugan, S.; Sibon Li, W.L. Testing the reliability of genetic methods of species identification via simulation. Syst. Biol. 2008, 57, 216–230. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Tyagi, A.; Kumar, A.; Singh, A.; Singh, S.; Chaudhary, L.B.; Roy, S. The internal transcribed spacer (ITS) region and trnhH-psbA are suitable candidate loci for DNA barcoding of tropical tree species of India. PLoS ONE 2013, 8, e57934. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Fazekas, A.J.; Ragupathy, S.D. DNA barcoding in land plants: Evaluation of rbcL in a multigene tiered approach. Botany 2006, 84, 335–341. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Binet, M.; Gascuel, O.; Scornavacca, C.; P Douzery, E.J.; Pardi, F. Fast and accurate branch lengths estimation for phylogenomic trees. BMC Bioinform. 2016, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rach, J.; DeSalle, R.; Sarkar, I.N.; Schierwater, B.; Hadrys, H. Character-based DNA barcoding allows discrimination of genera, species and populations in Odonata. Proc. Royal Soc. B-Biol. Sci. 2008, 275, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.A.; Ibrahim, K.M.; Boursot, P.; Bruford, M.W. Measuring genetic distance. In Molecular Tools for Screening Biodiversity: Plants and Animals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; pp. 315–325. [Google Scholar]
- Carlson, C.S.; Thomas, D.J.; Eberle, M.A.; Swanson, J.E.; Livingston, R.J.; Rieder, M.J.; Nickerson, D.A. Genomic regions exhibiting positive selection identified from dense genotype data. Genome Res. 2005, 15, 1553–1565. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xia, C.; Wang, J.; Zhang, X.; Ma, X.; Zhou, Q. The efficacy of DNA barcoding in the classification, genetic differentiation, and biodiversity assessment of benthic macro-invertebrates. Ecol. Evol. 2021, 11, 5669–5681. [Google Scholar] [CrossRef]
- Chandrasekara, C.B.; Naranpanawa, D.N.; Bandusekara, B.S.; Pushpakumara, D.K.; Wijesundera, D.S.; Bandaranayake, P.C. Universal barcoding regions, rbc L, mat K and trn H-psb A do not discriminate Cinnamomum species in Sri Lanka. PLoS ONE 2021, 16, e245592. [Google Scholar] [CrossRef]
- Kipkiror, N.; Muge, E.K.; Ochieno, D.M.; Nyaboga, E.N. DNA barcoding markers provide insight into species discrimination, genetic diversity and phylogenetic relationships of yam (Dioscorea spp.). Biologia 2023, 78, 689–705. [Google Scholar] [CrossRef]



| Barcode Region | Samples Tested (n) | Number of Amplicons Produced | Number of Sequences Produced | Percentage of Amplification Efficiency | Percentage of Sequencing Efficiency | Alignment Length (bp) | Mean Sequence Length (bp) | Mean GC Content (%) |
|---|---|---|---|---|---|---|---|---|
| ITS2 | 64 | 49 | 32 | 77 | 65 | 841 | 525 | 61.00 |
| rbcL | 64 | 54 | 48 | 84 | 89 | 841 | 690 | 43.40 |
| Barcode Locus | Base Contents (%) | |||||
|---|---|---|---|---|---|---|
| A | T | G | C | AT | GC | |
| ITS2 | 19.42 | 19.39 | 29.78 | 31.41 | 39.00 | 61.00 |
| rbcL | 28.22 | 28.40 | 23.10 | 20.28 | 56.58 | 43.42 |
| Population | P. peruviana (P1) | P. cordata (P2) | P. peruviana (P1) | P. purpurea (P2) | P. purpurea (P1) | P. cordata (P2) |
|---|---|---|---|---|---|---|
| Polymorphic sites in each population | 14 | 21 | 12 | 18 | 2 | 4 |
| Total number of polymorphic sites | 35 | 23 | 4 | |||
| Average number of nucleotide differences | 17.600 | 6.351 | 0.889 | |||
| Nucleotide diversity Pi (t) | 0.33208 | 0.18147 | 0.14821 | |||
| Number of fixed differences | 6 | 1 | 0 | |||
| Polymorphic mutations in population 1 (P1) but monomorphic ones in population 2 (P2) | 13 | 10 | 2 | |||
| Polymorphic mutations in P2 but monomorphic ones in P1 | 28 | 22 | 1 | |||
| Shared mutations | 1 | 2 | 2 | |||
| Average number of nucleotide differences between populations | 20.625 | 10.158 | 1.477 | |||
| Average nucleotide substitution per site between populations (Dxy) | 0.38915 | 0.29026 | 0.24621 | |||
| Number of net nucleotide substitutions per site between populations (Da) | 0.12343 | 0.03881 | 0.01299 | |||
| Physalis Species | P. peruviana | P. cordata | P. purpurea |
|---|---|---|---|
| Total number of sequences | 2 | 4 | 22 |
| Number of polymorphic (segregating) sites (S) | 70 | 83 | 20 |
| Nucleotide diversity Pi (Total) | 0.31250 | 0.18095 | 0.14898 |
| Nucleotide diversity Pi (JC-Total) | 0.40425 | 0.20708 | 0.16609 |
| Theta (Total) | 0.31250 | 0.19675 | 0.17396 |
| Total number of substitutions | 70 | 101 | 26 |
| Groups | P. purpurea | P. peruviana | P. cordata |
|---|---|---|---|
| P. purpurea | 198.92 | 1589.41 | |
| P. peruviana | 9.58 | 357.92 | |
| P. cordata | 21.99 | 9.53 |
| ITS2 | rbcL | |||||
|---|---|---|---|---|---|---|
| Polymorphic Sites/Segregation Sites (S) | 4 | Position in the Gene | Variants | 59 | Positions in the Gene | Variants |
| Singleton | 1 | 177 | 2 | 48 | 141,272,273,276,280,283,284,293,298, 301,308,309,310,322,325,327,331,334, 335,337,339,340,345,346,347,348,350, 353,357,365,366,373,375,376,386,395, 396,398,413,414,416,419,436,441,447, 457 344,359 | 2 3 |
| Parsimony informative sites | 3 | 179 176 178 | 2 3 4 | 11 | 302,336,341,355,358,362,401,430,444 282,363 | 2 3 |
| Nucleotide diversity (Pi) | 0.15917 | 0.01632 | ||||
| Average number of nucleotide differences (k) | 0.955 | 5.844 | ||||
| Sequence length (base pairs) | 532 | 716 | ||||
| Number of sequences | 28 | 28 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pere, K.; Mburu, K.; Muge, E.K.; Wagacha, J.M.; Nyaboga, E.N. Molecular Discrimination and Phylogenetic Relationships of Physalis Species Based on ITS2 and rbcL DNA Barcode Sequence. Crops 2023, 3, 302-319. https://doi.org/10.3390/crops3040027
Pere K, Mburu K, Muge EK, Wagacha JM, Nyaboga EN. Molecular Discrimination and Phylogenetic Relationships of Physalis Species Based on ITS2 and rbcL DNA Barcode Sequence. Crops. 2023; 3(4):302-319. https://doi.org/10.3390/crops3040027
Chicago/Turabian StylePere, Katherine, Kenneth Mburu, Edward K. Muge, John Maina Wagacha, and Evans N. Nyaboga. 2023. "Molecular Discrimination and Phylogenetic Relationships of Physalis Species Based on ITS2 and rbcL DNA Barcode Sequence" Crops 3, no. 4: 302-319. https://doi.org/10.3390/crops3040027
APA StylePere, K., Mburu, K., Muge, E. K., Wagacha, J. M., & Nyaboga, E. N. (2023). Molecular Discrimination and Phylogenetic Relationships of Physalis Species Based on ITS2 and rbcL DNA Barcode Sequence. Crops, 3(4), 302-319. https://doi.org/10.3390/crops3040027





