Evaluation of Mediterranean Tree Leaves as Valuable Biomass of Digestive Enzymes and Bacterial Inhibitors in the Concept of Circular Bioeconomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Serial Extraction of Plant Material
2.4. Phenolic and Flavonoid Contents of Leaf Extracts
2.5. Inhibitory Effect of Leaf Extracts on Carbohydrate Digestive Enzymes
2.6. Bactericidal Effects of Leaf Extracts
2.7. Statistical Analysis
3. Results and Discussion
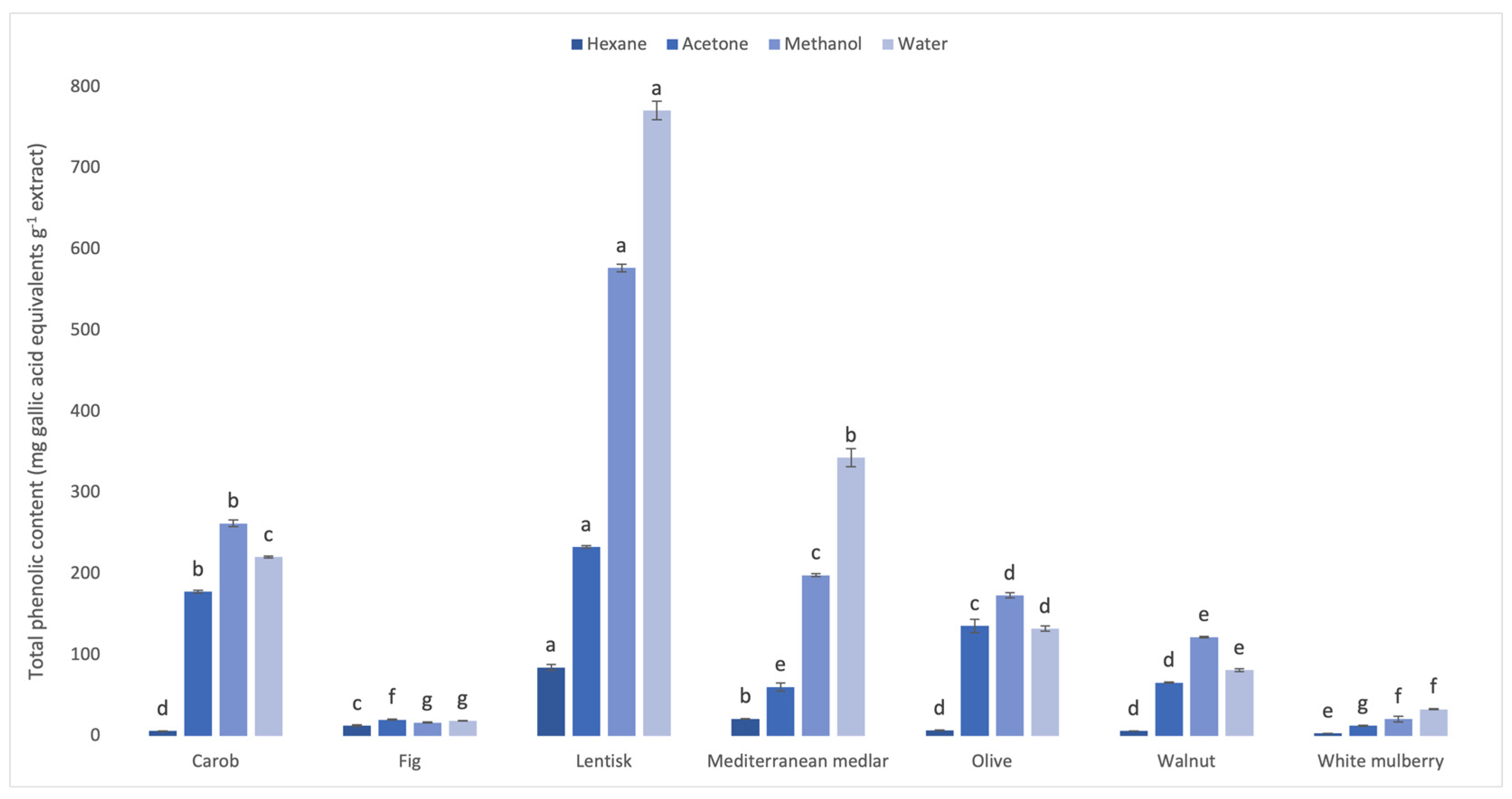
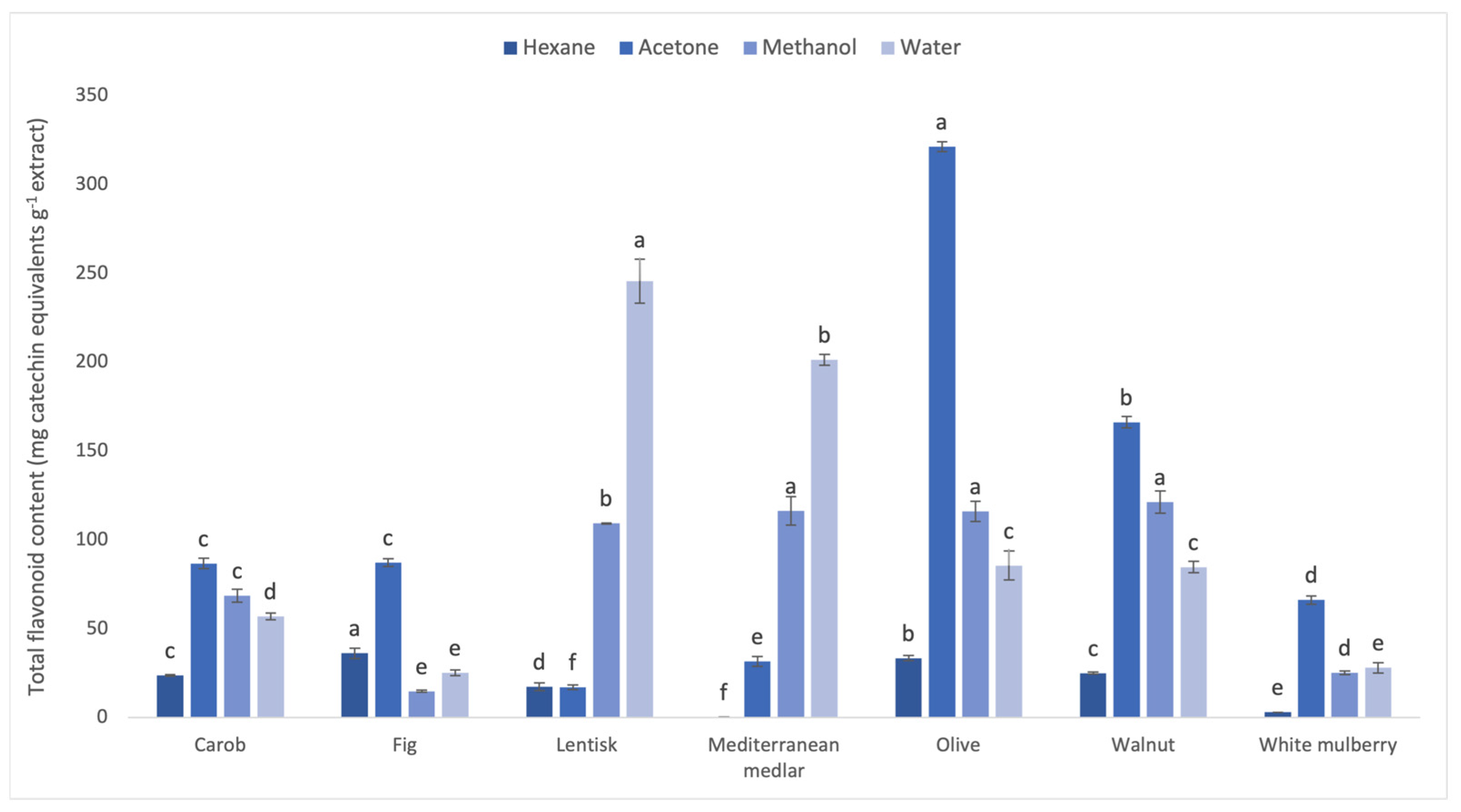
3.1. Phenolic and Flavonoid Contents of Leaf Extracts
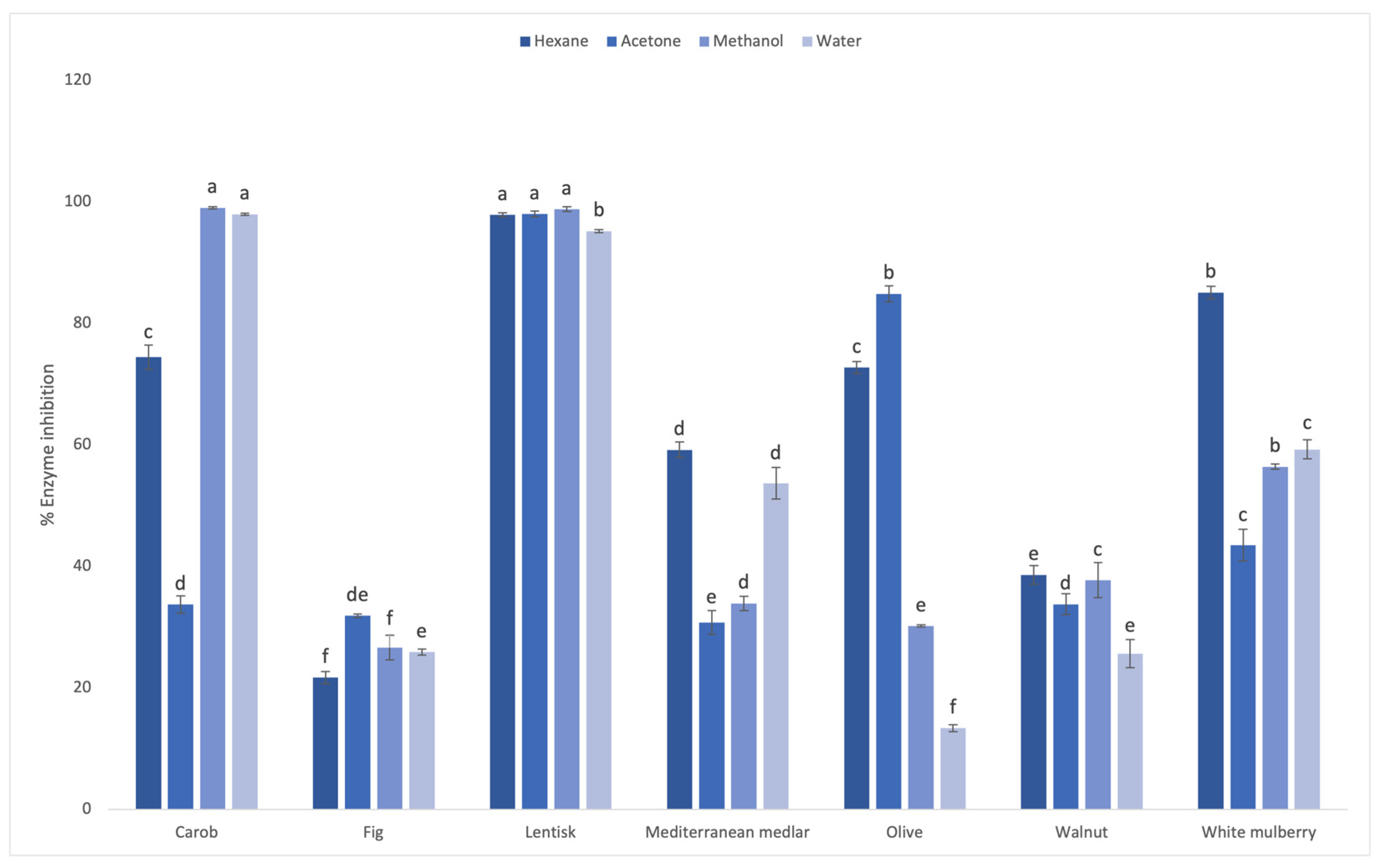
3.2. Inhibitory Effect of Leaf Extracts on Carbohydrate Digestive Enzymes
3.3. Anti-Bacterial Potential of Leaf Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food Waste Valorization Advocating Circular Bioeconomy—A Critical Review of Potentialities and Perspectives of Spent Coffee Grounds Biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Santagata, R.; Ripa, M.; Genovese, A.; Ulgiati, S. Food Waste Recovery Pathways: Challenges and Opportunities for an Emerging Bio-Based Circular Economy. A Systematic Review and an Assessment. J. Clean. Prod. 2021, 286, 125490. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.Y.; Bloomfield, S.F.; Courvalin, P.; Essack, S.Y.; Gandra, S.; Gerba, C.P.; Rubino, J.R.; Scott, E.A. Reducing Antibiotic Prescribing and Addressing the Global Problem of Antibiotic Resistance by Targeted Hygiene in the Home and Everyday Life Settings: A Position Paper. Am. J. Infect. Control 2020, 48, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Oh, M.S.; Hong, F.M.; Jia, Q. Natural Preservatives and Antimicrobial Agents, Including Compositions Thereof. U.S. Patent US 10,780,173 B2, 22 September 2020. [Google Scholar]
- Modak, S.M.; Dongre, S.; Caraos, L.; Baiju, N.; Ramachadran, H.K. Botanical Antimicrobial Compositions. U.S. Patent US009968101B2, 15 May 2018. [Google Scholar]
- Daigle, F.; Letellier, A.; Quessy, S. Aqueous Disinfectant Formulation Comprising a Phenolic Compound a Surfactant and a Solvent. European Patent Specification EP 2273875 B1, 7 March 2018. [Google Scholar]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of Plant Extracts to Meat and Meat Products to Extend Shelf-Life and Health-Promoting Attributes: An Overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of Plant Extracts and Essential Oils in Fighting against Postharvest Fruit Pathogens and Extending Fruit Shelf Life: A Review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic Phytoconstituents and Their Mode of Action on Metabolic Pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Oboh, G.; Temitope Isaac, A.; Jacobson Akinyemi, A.; Ajani, R.A. Inhibition of Key Enzymes Linked to Type 2 Diabetes and Sodium Nitroprusside Induced Lipid Peroxidation in Rats’ Pancreas by Phenolic Extracts of Avocado Pear Leaves. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef] [PubMed]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Badaoui, B.; Mourabit, Y.; Salhi, N.; Alshahrani, M.M.; Al Awadh, A.A.; Bouyahya, A.; Goh, K.W.; et al. Comparative Investigation of Chemical Constituents of Kernels, Leaves, Husk, and Bark of Juglans regia L., Using HPLC-DAD-ESI-MS/MS Analysis and Evaluation of Their Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. Molecules 2022, 27, 8989. [Google Scholar] [CrossRef] [PubMed]
- Foddai, M.; Kasabri, V.; Afifi, F.U.; Azara, E.; Petretto, G.L.; Pintore, G. In Vitro Inhibitory Effects of Sardinian Pistacia lentiscus L. and Pistacia terebinthus L. on Metabolic Enzymes: Pancreatic Lipase, α-Amylase, and α-Glucosidase. Starch/Staerke 2015, 67, 204–212. [Google Scholar] [CrossRef]
- Ergül, M.; Ergül, M.; Eruygur, N.; Ataş, M.; Uçar, E. In Vitro Evaluation of the Chemical Composition and Various Biological Activities of Ficus carica Leaf Extracts. Turk. J. Pharm. Sci. 2019, 16, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Samah, S.; Abdullah, K.; Ream, N. Phytochemical Screening of Alchemilla vulgaris, Sophora japonica, Crataegus azarolus, and Their Inhibitory Activity on Lipase and α-Amylase. Int. J. Acad. Scie Res. 2018, 6, 1–21. [Google Scholar]
- Custodio, L.; Patarra, J.; Albericio, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.R. Inhibition of A-Glucosidase and a-Amylase by Morus alba Linn. Leaf Extracts. J. Pharm. Res. 2012, 5, 285–289. [Google Scholar]
- Elbouzidi, A.; Taibi, M.; Ouassou, H.; Ouahhoud, S.; Ou-Yahia, D.; Loukili, E.H.; Aherkou, M.; Mansouri, F.; Bencheikh, N.; Laaraj, S.; et al. Exploring the Multi-Faceted Potential of Carob (Ceratonia siliqua Var. Rahma) Leaves from Morocco: A Comprehensive Analysis of Polyphenols Profile, Antimicrobial Activity, Cytotoxicity against Breast Cancer Cell Lines, and Genotoxicity. Pharmaceuticals 2023, 16, 840. [Google Scholar] [CrossRef]
- Alhadad, A.O.; Salem, G.S.; Elmhdwi, M.F.; Hussein, S.M.; Elshareef, S.M. Assessments of Antibacterial and Antioxidant Properties in the Methanolic and Aqueous Leaf Extracts of Pistacia lentiscus& against Different Antibiotic Resistance Pathogenic Bacteria. Adv. Biosci. Biotechnol. 2022, 13, 113–133. [Google Scholar]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Khali, M.; Benkhaled, A.; Benamirouche, K.; Baiti, I. Phenolic and Flavonoid Contents, Antioxidant and Antimicrobial Activities of Leaf Extracts from Ten Algerian Ficus carica L. Varieties. Asian Pac. J. Trop. Biomed. 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) Leaves: Phenolic Compounds, Antibacterial Activity and Antioxidant Potential of Different Cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Thabti, I.; Elfalleh, W.; Tlili, N.; Ziadi, M.; Campos, M.G.; Ferchichi, A. Phenols, Flavonoids, and Antioxidant and Antibacterial Activity of Leaves and Stem Bark of Morus Species. Int. J. Food Prop. 2014, 17, 842–854. [Google Scholar] [CrossRef]
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Sioud, B.; Amri, M.; Fattouch, S. Antioxidant and Antimicrobial Activities of Tunisian Azarole (Crataegus azarolus L.) Leaves and Fruit Pulp/Peel Polyphenolic Extracts. Int. J. Food Prop. 2013, 16, 1380–1393. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, C.; Michael, C.; Botsaris, G.; Goulas, V. Antibacterial and Carbohydrate Digestive Enzyme Inhibitory Effects of Native Plants Used for Medicinal and Culinary Purposes in Cyprus. Nat. Prod. Commun. 2024, 19. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; De Carvalho, I.S.; Končić, M.Z. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Wang, R.; Li, C.; Lin, X.; Wang, L. Screening and Identification of Natural α-Glucosidase and α-Amylase Inhibitors from Partridge Tea (Mallotus furetianus Muell-Arg) and in Silico Analysis. Food Chem. 2022, 388, 133004. [Google Scholar] [CrossRef]
- Park, M.; Horn, L.; Lappi, V.; Boxrud, D.; Hedberg, C.; Jeon, B. Antimicrobial Synergy between Aminoglycosides and Licorice Extract in Listeria monocytogenes. Pathogens 2022, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.D. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant Capacity and Phenolic Contents of Some Mediterranean Medicinal Plants and Their Potential Role in the Inhibition of Cyclooxygenase-1 and Acetylcholinesterase Activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Hayat, M.; Abbas, M.; Munir, F.; Hayat, M.Q.; Keyani, R.; Amir, R. Potential of Plant Flavonoids in Pharmaceutics and Nutraceutics. J. Biomol. Biochem. 2017, 1, 12–17. [Google Scholar]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A Review of Pistacia lentiscus Polyphenols: Chemical Diversity and Pharmacological Activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Hamahameen, B.A.; Jamal, B. Determination of Flavonoids in the Leaves of Hawthorn (Crataegus azarolus) of Iraqi Kurdistan Region by HPLC Analysis. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 67–70. [Google Scholar]
- Sehaki, C.; Molinie, R.; Mathiron, D.; Fontaine, J.X.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. Metabolomics-Based Profiling via a Chemometric Approach to Investigate the Antidiabetic Property of Different Parts and Origins of Pistacia lentiscus L. Metabolites 2023, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Luísa Escapa, A.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.; Manuel, J.; Nogueira, F.; Romano, A. In Vitro Cytotoxic Effects and Apoptosis Induction by a Methanol Leaf Extract of Carob Tree (Ceratonia siliqua L.). J. Med. Plant Res. 1987, 5, 1987–1996. [Google Scholar]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-Glucosidase and α-Amylase Enzyme Inhibitory of Hydroxytyrosol and Oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lu, T.; Wang, M.; Zou, X.; Zhang, Y.; Yang, X.; Dong, Y.; Zhou, H. Flavonoids from Morus alba L. Leaves: Optimization of Extraction by Response Surface Methodology and Comprehensive Evaluation of Their Antioxidant, Antimicrobial, and Inhibition of α-Amylase Activities through Analytical Hierarchy Process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Weng, Y.; Zhang, Y. Bioassay-Guided Screening and Isolation of α-Glucosidase and Tyrosinase Inhibitors from Leaves of Morus alba. Food Chem. 2012, 131, 617–625. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding Phenolic Acids Inhibition of α-Amylase and α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Mehenni, C.; Atmani-Kilani, D.; Dumarçay, S.; Perrin, D.; Gérardin, P.; Atmani, D. Hepatoprotective and Antidiabetic Effects of Pistacia lentiscus Leaf and Fruit Extracts. J. Food Drug Anal. 2016, 24, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayache, S.; Reis, F.S.; Inês Dias, M.; Pereira, C.; Glamočlija, J.; Soković, M.; Behija Saafi, E.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical Characterization of Carob Seeds (Ceratonia siliqua L.) and Use of Different Extraction Techniques to Promote Its Bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef] [PubMed]
- Djebari, S.; Wrona, M.; Boudria, A.; Salafranca, J.; Nerin, C.; Bedjaoui, K.; Madani, K. Study of Bioactive Volatile Compounds from Different Parts of Pistacia lentiscus L. Extracts and Their Antioxidant and Antibacterial Activities for New Active Packaging Application. Food Control 2021, 120, 107514. [Google Scholar] [CrossRef]
- Bakli, S.; Daoud, H.; Amina, Z.; Nouari, S.; Asma, B.; Soufiane, G.; Oumaima, N. Antimicrobial and Antioxidant Activities of Flavonoids Extracted from Pistacia lentiscus L., Leaves. J. Drug Deliv. Therap. 2020, 10, 83–89. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Cha, J.-D. Synergistic Antibacterial Activity of Fig (Ficus carica) Leaves Extract Against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Korean J. Microbiol. Biotechnol. 2010, 38, 405–413. [Google Scholar]
- Aelenei, P.; Luca, S.V.; Horhogea, C.E.; Rimbu, C.M.; Dimitriu, G.; Macovei, I.; Silion, M.; Aprotosoaie, A.C.; Miron, A. Morus alba Leaf Extract: Metabolite Profiling and Interactions with Antibiotics against Staphylococcus spp. Including MRSA. Phytochem. Lett. 2019, 31, 217–224. [Google Scholar] [CrossRef]
- Djenane, D.; Yangüela, J.; Montañés, L.; Djerbal, M.; Roncalés, P. Antimicrobial Activity of Pistacia lentiscus and Satureja montana Essential Oils against Listeria monocytogenes CECT 935 Using Laboratory Media: Efficacy and Synergistic Potential in Minced Beef. Food Control 2011, 22, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Tan, Y.; Shi, H. Anti-Initial Adhesion Activity and Mechanism of Mulberry (Morus alba L.) Leaf Polyphenols against Escherichia coli O157:H7 and Listeria monocytogenes to Fresh-Cut Lettuce. J. Food Meas. Charact. 2023, 17, 4616–4626. [Google Scholar] [CrossRef]
- Monte, J.; Abreu, A.C.; Borges, A.; Simões, L.C.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The Antibacterial Activity of Extracts of Nine Plant Species with Good Activity against Escherichia coli against Five Other Bacteria and Cytotoxicity of Extracts. BMC Complement. Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef]
- Kivcak, B.; Mert, T.; Öztürk, H.T. Antimicrobial and Cytotoxic Activities of Ceratonia siliqua L. Extracts. Turk. J. Biol. 2002, 4, 2. [Google Scholar]

| Plant | Solvent | Bacillus cereus | Listeria monocytogenes | Staphylococcus aureus | |||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| Carob | Hexane | 1000 | >2000 | >2000 | >2000 | 500 | 1000 |
| Acetone | 1000 | >2000 | >2000 | >2000 | 500 | 1000 | |
| Methanol | 250 | 500 | >2000 | >2000 | 250 | 500 | |
| Water | 250 | 500 | >2000 | >2000 | 250 | 500 | |
| Fig | Hexane | 1000 | >2000 | >2000 | >2000 | 500 | 1000 |
| Acetone | 1000 | 1000 | >2000 | >2000 | 500 | 1000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Lentisk | Hexane | 1000 | >2000 | >2000 | >2000 | 500 | 1000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 500 | 1000 | |
| Methanol | 250 | 500 | 500 | 1000 | 500 | 1000 | |
| Water | 250 | 500 | >2000 | >2000 | 500 | 1000 | |
| Mediterranean medlar | Hexane | >2000 | >2000 | >2000 | >2000 | 500 | 1000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | 500 | 1000 | |
| Methanol | 1000 | 1000 | >2000 | >2000 | 500 | 1000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Olive | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Walnut | Hexane | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 1000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| White mulberry | Hexane | 500 | 1000 | >2000 | >2000 | 250 | 500 |
| Acetone | 500 | 1000 | >2000 | >2000 | 250 | 500 | |
| Methanol | >2000 | >2000 | 500 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Plant | Solvent | Cronobacter sakazakii | Escherichia coli | Salmonella enterica | |||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| Carob | Hexane | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Fig | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Lentisk | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Mediterranean medlar | Hexane | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Olive | Hexane | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Walnut | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| White mulberry | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, A.; Stavrou, K.; Michael, C.; Botsaris, G.; Goulas, V. Evaluation of Mediterranean Tree Leaves as Valuable Biomass of Digestive Enzymes and Bacterial Inhibitors in the Concept of Circular Bioeconomy. Biomass 2024, 4, 442-454. https://doi.org/10.3390/biomass4020022
Christou A, Stavrou K, Michael C, Botsaris G, Goulas V. Evaluation of Mediterranean Tree Leaves as Valuable Biomass of Digestive Enzymes and Bacterial Inhibitors in the Concept of Circular Bioeconomy. Biomass. 2024; 4(2):442-454. https://doi.org/10.3390/biomass4020022
Chicago/Turabian StyleChristou, Atalanti, Konstantina Stavrou, Christodoulos Michael, George Botsaris, and Vlasios Goulas. 2024. "Evaluation of Mediterranean Tree Leaves as Valuable Biomass of Digestive Enzymes and Bacterial Inhibitors in the Concept of Circular Bioeconomy" Biomass 4, no. 2: 442-454. https://doi.org/10.3390/biomass4020022










