Recent Progress in Intestinal Toxicity of Microplastics and Nanoplastics: Systematic Review of Preclinical Evidence
Abstract
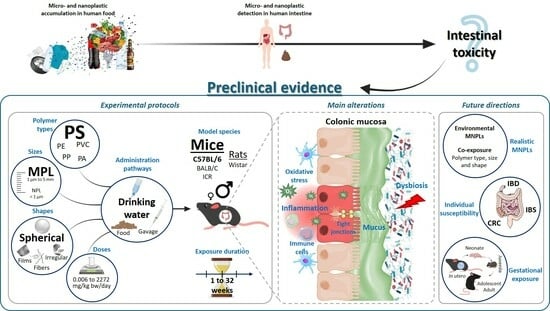
:1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics—The Fast Facts 2023. Plastics Europe. Plast. Eur. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 1 January 2024).
- Thacharodi, A.; Meenatchi, R.; Hassan, S.; Hussain, N.; Bhat, M.A.; Arockiaraj, J.; Ngo, H.H.; Le, Q.H.; Pugazhendhi, A. Microplastics in the Environment: A Critical Overview on Its Fate, Toxicity, Implications, Management, and Bioremediation Strategies. J. Environ. Manag. 2024, 349, 119433. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Sources, Fate and Effects of Microplastics in the Marine Environment (Part 1). Available online: http://www.gesamp.org/publications/reports-and-studies-no-90 (accessed on 14 December 2023).
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of Human Exposure to Microplastics, and Estimation of the Total Burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Kibria, G.; Nugegoda, D.; Haroon, A.K.Y. Microplastic Pollution and Contamination of Seafood (Including Fish, Sharks, Mussels, Oysters, Shrimps and Seaweeds): A Global Overview. In Microplastic Pollution: Environmental Occurrence and Treatment Technologies; Hashmi, M.Z., Ed.; Emerging Contaminants and Associated Treatment Technologies; Springer International Publishing: Cham, Switzerland, 2022; pp. 277–322. ISBN 978-3-030-89220-3. [Google Scholar]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and Translocation of Nano/Microplastics by Rice Seedlings: Evidence from a Hydroponic Experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, T.; Luo, J.; Bai, H.; Ma, S.; Qiang, H.; Guo, X. Internalization, Physiological Responses and Molecular Mechanisms of Lettuce to Polystyrene Microplastics of Different Sizes: Validation of Simulated Soilless Culture. J. Hazard. Mater. 2024, 462, 132710. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2022, 0, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Microplastics: Human Exposure Assessment through Air, Water, and Food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Liao, K.; Wu, P.; Jin, H. Microplastics in Dust from Different Indoor Environments. Sci. Total Environ. 2022, 833, 155256. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Zarei, F.; Keshavarzi, B.; Zarei, M.; Moore, F.; Busquets, R.; Kelly, F.J. Microplastic Occurrence in Settled Indoor Dust in Schools. Sci. Total Environ. 2022, 807, 150984. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You Are What You Eat: Microplastics in the Feces of Young Men Living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2022, 57, 17774–17785. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Anuar, S.T.; Azmi, A.A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of Microplastics in Human Colectomy Specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Cetin, M.; Demirkaya Miloglu, F.; Kilic Baygutalp, N.; Ceylan, O.; Yildirim, S.; Eser, G.; Gul, H.İ. Higher Number of Microplastics in Tumoral Colon Tissues from Patients with Colorectal Adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and Nanoplastics—Current State of Knowledge with the Focus on Oral Uptake and Toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory Properties and Lipid Disturbance of Polystyrene Microplastics in the Livers of Mice with Acute Colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Schwarzfischer, M.; Niechcial, A.; Lee, S.S.; Sinnet, B.; Wawrzyniak, M.; Laimbacher, A.; Atrott, K.; Manzini, R.; Morsy, Y.; Häfliger, J.; et al. Ingested Nano- and Microsized Polystyrene Particles Surpass the Intestinal Barrier and Accumulate in the Body. NanoImpact 2022, 25, 100374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jiang, X.; Zhou, Y.; Sumayyah, G.; Zhou, L.; Tu, B.; Qin, Q.; Qiu, J.; Qin, X.; Zou, Z.; et al. Results of a 30-Day Safety Assessment in Young Mice Orally Exposed to Polystyrene Nanoparticles. Environ. Pollut. 2022, 292, 118184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Wang, J.; Wu, B.; Guo, X. Polystyrene Microplastics Aggravate Inflammatory Damage in Mice with Intestinal Immune Imbalance. Sci. Total Environ. 2022, 833, 155198. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, Y.; Liu, S.; Chen, Y.; Yuan, H.; Xu, H. Polystyrene Microplastics Exacerbated Liver Injury from Cyclophosphamide in Mice: Insight into Gut Microbiota. Sci. Total Environ. 2022, 840, 156668. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene Microplastic Exposure Induces Insulin Resistance in Mice via Dysbacteriosis and Pro-Inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene Microplastics Exacerbate Experimental Colitis in Mice Tightly Associated with the Occurrence of Hepatic Inflammation. Sci. Total Environ. 2022, 844, 156884. [Google Scholar] [CrossRef]
- Chen, W.; Tu, P.; Ye, X.; Tang, Q.; Yu, T.; Zheng, X. Cyanidin-3-O-Glucoside Impacts Fecal Discharge of Polystyrene Microplastics in Mice: Potential Role of Microbiota-Derived Metabolites. Toxicol. Appl. Pharmacol. 2022, 453, 116212. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, H.; Huang, Y.; Zhang, Y.; Ren, H.; Fang, M.; Wang, Q.; Chen, W.; Hale, R.C.; Galloway, T.S.; et al. Long-Term Exposure to Environmentally Relevant Doses of Large Polystyrene Microplastics Disturbs Lipid Homeostasis via Bowel Function Interference. Environ. Sci. Technol. 2022, 56, 15805–15817. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, R.; Li, Z.; Liu, C.; Chen, Y.; Yu, Q. Microplastics Perturb Colonic Epithelial Homeostasis Associated with Intestinal Overproliferation, Exacerbating the Severity of Colitis. Environ. Res. 2023, 217, 114861. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bu, W.; Hu, W.; Zhao, Z.; Liu, L.; Luo, C.; Wang, R.; Fan, S.; Yu, S.; Wu, Q.; et al. Ferroptosis Is Involved in Sex-Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics during Pregnancy. ACS Nano 2023, 17, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Hasegawa, Y.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ. Health Perspect. 2023, 131, 027006. [Google Scholar] [CrossRef]
- Gao, B.; Shi, X.; Li, S.; Xu, W.; Gao, N.; Shan, J.; Shen, W. Size-Dependent Effects of Polystyrene Microplastics on Gut Metagenome and Antibiotic Resistance in C57BL/6 Mice. Ecotoxicol. Environ. Saf. 2023, 254, 114737. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Xue, J.; Niu, H.; Tang, Q.; Mo, Z.; Zheng, X.; Wu, L.; Chen, Z.; Cai, Y.; Wang, X. Deciphering Gut Microbiome Responses upon Microplastic Exposure via Integrating Metagenomics and Activity-Based Metabolomics. Metabolites 2023, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Peng, C.; Gan, Y.; Wang, Y.; Chen, Z.; Han, X.; Chen, Y. Differently Surface-Labeled Polystyrene Nanoplastics at an Environmentally Relevant Concentration Induced Crohn’s Ileitis-like Features via Triggering Intestinal Epithelial Cell Necroptosis. Environ. Int. 2023, 176, 107968. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Chen, Q.; Su, S.; Zhuang, J.; Qiao, D. Polystyrene Micro- and Nanoparticles Exposure Induced Anxiety-like Behaviors, Gut Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2023, 259, 115000. [Google Scholar] [CrossRef]
- Huang, H.; Wei, F.; Qiu, S.; Xing, B.; Hou, J. Polystyrene Microplastics Trigger Adiposity in Mice by Remodeling Gut Microbiota and Boosting Fatty Acid Synthesis. Sci. Total Environ. 2023, 890, 164297. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, N.; Dzhalilova, D.; Tsvetkov, I.; Makarova, O. Influence of Microplastics on Morphological Manifestations of Experimental Acute Colitis. Toxics 2023, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ge, L.; Zhang, J.; Xue, J.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Systemic Effects of Nanoplastics on Multi-Organ at the Environmentally Relevant Dose: The Insights in Physiological, Histological, and Oxidative Damages. Sci. Total Environ. 2023, 892, 164687. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Luo, T.; Xiao, Y.; Yang, G.; Jin, Y. Nano- and Micro-Polystyrene Plastics Interfered the Gut Barrier Function Mediated by Exosomal miRNAs in Rats. Environ. Pollut. 2023, 335, 122275. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Shen, Y.; Xu, S.; Wu, B.; Zhang, Z.; Liu, S. Underestimated Health Risks: Dietary Restriction Magnify the Intestinal Barrier Dysfunction and Liver Injury in Mice Induced by Polystyrene Microplastics. Sci. Total Environ. 2023, 898, 165502. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous Oral Exposure to Micro- and Nanoplastics Induced Gut Microbiota Dysbiosis, Intestinal Barrier and Immune Dysfunction in Adult Mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef]
- Li, L.; Lv, X.; He, J.; Zhang, L.; Li, B.; Zhang, X.; Liu, S.; Zhang, Y. Chronic Exposure to Polystyrene Nanoplastics Induces Intestinal Mechanical and Immune Barrier Dysfunction in Mice. Ecotoxicol. Environ. Saf. 2024, 269, 115749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene Microplastics Induce Size-Dependent Multi-Organ Damage in Mice: Insights into Gut Microbiota and Fecal Metabolites. J. Hazard. Mater. 2023, 461, 132503. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene Microplastic-Induced Oxidative Stress Triggers Intestinal Barrier Dysfunction via the NF-κB/NLRP3/IL-1β/MCLK Pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, X.; Zhou, L.; Yan, H.; Zhao, L.; Sun, J.; Li, Y.; Zhu, W.; Wu, F. An Integrated Analysis of the Fecal Metabolome and Metagenome Reveals the Distinct Effects of Differentially Charged Nanoplastics on the Gut Microbiota-Associated Metabolites in Mice. Sci. Total Environ. 2024, 906, 167287. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Gao, X.; Zhao, J.; Zhang, H. Polystyrene Nanoparticles Induced Mammalian Intestine Damage Caused by Blockage of BNIP3/NIX-Mediated Mitophagy and Gut Microbiota Alteration. Sci. Total Environ. 2024, 907, 168064. [Google Scholar] [CrossRef]
- Zha, H.; Tang, R.; Li, S.; Zhuge, A.; Xia, J.; Lv, J.; Wang, S.; Wang, K.; Zhang, H.; Li, L. Effects of Partial Reduction of Polystyrene Micro-Nanoplastics on the Immunity, Gut Microbiota and Metabolome of Mice. Chemosphere 2024, 349, 140940. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, S.; Ahn, J.; Kim, J.H.; Lee, E.; Lee, I.; Byun, S. Transcriptomic and Metabolomic Analysis Unveils Nanoplastic-Induced Gut Barrier Dysfunction via STAT1/6 and ERK Pathways. Environ. Res. 2024, 249, 118437. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lin, W.-Y.; Cheng, T.-J. Microbiota-Mediated Metabolic Perturbations in the Gut and Brain of Mice after Microplastic Exposure. Chemosphere 2024, 350, 141026. [Google Scholar] [CrossRef]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects Induced by Polyethylene Microplastics Oral Exposure on Colon Mucin Release, Inflammation, Gut Microflora Composition and Metabolism in Mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Li, B.; Li, J.; Li, L.; Zhang, R.; Du, Y.; Zhang, Y. Polyethylene Microplastics Cooperate with Helicobacter Pylori to Promote Gastric Injury and Inflammation in Mice. Chemosphere 2022, 288, 132579. [Google Scholar] [CrossRef]
- Djouina, M.; Vignal, C.; Dehaut, A.; Caboche, S.; Hirt, N.; Waxin, C.; Himber, C.; Beury, D.; Hot, D.; Dubuquoy, L.; et al. Oral Exposure to Polyethylene Microplastics Alters Gut Morphology, Immune Response, and Microbiota Composition in Mice. Environ. Res. 2022, 212, 113230. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, H.; Cheng, Y.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Xu, F.; Ma, Q.; Lin, F.; et al. Oral Feeding of Nanoplastics Affects Brain Function of Mice by Inducing Macrophage IL-1 Signal in the Intestine. Cell Rep. 2023, 42, 112346. [Google Scholar] [CrossRef]
- Wang, J.; Tian, H.; Shi, Y.; Yang, Y.; Yu, F.; Cao, H.; Gao, L.; Liu, M. The Enhancement in Toxic Potency of Oxidized Functionalized Polyethylene-Microplastics in Mice Gut and Caco-2 Cells. Sci. Total Environ. 2023, 903, 166057. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cao, J.; Lv, H.; Geng, Y.; Guo, M. Polyethylene Microplastics Induced Gut Microbiota Dysbiosis Leading to Liver Injury via the TLR2/NF-κB/NLRP3 Pathway in Mice. Sci. Total Environ. 2024, 917, 170518. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl Chloride Microplastics Induced Gut Barrier Dysfunction, Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Chronic Exposure to Polyvinyl Chloride Microplastics Induces Liver Injury and Gut Microbiota Dysbiosis Based on the Integration of Liver Transcriptome Profiles and Full-Length 16S rRNA Sequencing Data. Sci. Total Environ. 2022, 839, 155984. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xie, H.; Zhang, Y.; Tian, X.; Cui, L.; Shi, N.; Wang, L.; Zhao, J.; An, L.; Wang, J.; et al. The Toxicity of Nano Polyethylene Terephthalate to Mice: Intestinal Obstruction, Growth Retardant, Gut Microbiota Dysbiosis and Lipid Metabolism Disorders. Food Chem. Toxicol. 2023, 172, 113585. [Google Scholar] [CrossRef] [PubMed]
- Harusato, A.; Seo, W.; Abo, H.; Nakanishi, Y.; Nishikawa, H.; Itoh, Y. Impact of Particulate Microplastics Generated from Polyethylene Terephthalate on Gut Pathology and Immune Microenvironments. iScience 2023, 26, 106474. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, T.; Liu, J.; Hou, Y.; Tan, Q.; Zhang, X.; Li, Z.; Farooq, T.H.; Yan, W.; Li, Y. Intestinal Flora Variation Reflects the Short-Term Damage of Microplastic to the Intestinal Tract in Mice. Ecotoxicol. Environ. Saf. 2022, 246, 114194. [Google Scholar] [CrossRef] [PubMed]
- Toto, B.; Refosco, A.; O’Keeffe, M.; Barkhald, Ø.H.; Brønstad, A.; Lied, G.A.; Yadetie, F.; Goksøyr, A.; Kögel, T.; Dierkes, J. Intestinal Permeability and Gene Expression after Polyethylene and Polyamide Microplastic Ingestion in Wistar Rats. Toxicol. Lett. 2022, 370, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhao, M.; Jin, H. Microplastic-Induced Gut Microbiota and Serum Metabolic Disruption in Sprague-Dawley Rats. Environ. Pollut. 2023, 320, 121071. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, H.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Ma, Q.; Xu, F.; Cheng, H.; Sun, D.; et al. Nanoplastics Shape Adaptive Anticancer Immunity in the Colon in Mice. Nano Lett. 2023, 23, 3516–3523. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, Q.; Xu, L.; Chen, X. Combined Exposure to Polyvinyl Chloride and Polystyrene Microplastics Induces Liver Injury and Perturbs Gut Microbial and Serum Metabolic Homeostasis in Mice. Ecotoxicol. Environ. Saf. 2023, 267, 115637. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, R.; Wu, D.; Li, Y.; Chen, T.; Wang, A. Research Progress of the Effects of Bisphenol Analogues on the Intestine and Its Underlying Mechanisms: A Review. Environ. Res. 2023, 243, 117891. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.P.; Saravanan, C. An Insight into the Critical Role of Gut Microbiota in Triggering the Phthalate-Induced Toxicity and Its Mitigation Using Probiotics. Sci. Total Environ. 2023, 904, 166889. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, Y.; Xu, H. Trojan Horse in the Intestine: A Review on the Biotoxicity of Microplastics Combined Environmental Contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Shi, C.; Lv, J.; Xu, Y.; Yang, J.; Chua, S.L.; Jia, L.; Chen, H.; Liu, Q.; et al. Oligomer Nanoparticle Release from Polylactic Acid Plastics Catalysed by Gut Enzymes Triggers Acute Inflammation. Nat. Nanotechnol. 2023, 18, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Gałęcka, I.; Szyryńska, N.; Całka, J. Influence of Polyethylene Terephthalate (PET) Microplastic on Selected Active Substances in the Intramural Neurons of the Porcine Duodenum. Part. Fibre Toxicol. 2024, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Mao, X.; Xiang, X.; Ye, S.; Chen, J.; Zhu, A.; Meng, Y.; Yang, X.; Peng, S.; et al. Adverse Health Effects of Emerging Contaminants on Inflammatory Bowel Disease. Front. Public Health 2023, 11, 1140786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Williams, A.M.; Gordon, E.B.; Rudolph, S.E.; Longo, B.N.; Li, G.; Kaplan, D.L. Biological Effects of Polystyrene Micro- and Nano-Plastics on Human Intestinal Organoid-Derived Epithelial Tissue Models without and with M Cells. Nanomed. Nanotechnol. Biol. Med. 2023, 50, 102680. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Luo, J.; Qu, C.; Guo, P.; Yi, W.; Yang, J.; Yan, Y.; Guan, H.; Zhou, P.; Huang, R. Predictive Metabolomic Signatures for Safety Assessment of Three Plastic Nanoparticles Using Intestinal Organoids. Sci. Total Environ. 2024, 913, 169606. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.; Schins, R.P. Investigations of Acute Effects of Polystyrene and Polyvinyl Chloride Micro-and Nanoplastics in an Advanced in Vitro Triple Culture Model of the Healthy and Inflamed Intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; del Favero, G.; et al. Microplastics Role in Cell Migration and Distribution during Cancer Cell Division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Zhao, Y.; Zhao, J.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive Toxicity of Microplastics in Female Mice and Their Offspring from Induction of Oxidative Stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal Exposure to Polystyrene Nanoplastics during Gestation and Lactation Induces Hepatic and Testicular Toxicity in Male Mouse Offspring. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 160, 112803. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of Dysbiotic Human Gut Microbiome for Homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef]
| Sex Background Specie | Polymer Type, Shape, Mean Aerodynamic Diameter and Other Specificities of MPLs/NPLs | Exposure Conditions Concentration * Duration Administration Pathway | Main Alterations Observed | Reference |
|---|---|---|---|---|
| Polystyrene (PS) | ||||
| Male C57BL/6 mice | Spherical 5 µm PS | 500 µg/L so ≈0.11 mg/kg bw/day 4 weeks Drinking water | Increased intestinal permeability in mice with acute DSS 1-induced colitis | [26] |
| Male C57BL/6 mice | Spherical 70 nm NPL and 5 µm MPL PS, pristine PS, negatively charged carboxylated PS (PS-COOH) and positively charged aminated PS (PS-NH2) | 0.2 and 2 mg/kg bw/day 4 weeks Gavage | Gut toxicity PS-NH2 > PS-COOH > pristine PS Decreased expression of tight-junction proteins for PS-NH2 MPL and NPL Dysbiosis: MPL > NPL Chemically modified MNPL > pristine MNPL | [27] |
| Male C57BL/6 mice | Spherical 50, 500, 5000 nm individually and combined PS | 2,5, 50, 250, 500 mg/kg bw/day 4 weeks Gavage | Numerous significant effects on duodenum, jejunum, ileum, colon: dose-dependent and size-dependent, including Decreased mucus Increased ROS 2 generation (DHE) Increased apoptosis (TUNEL) Increased caspase-3 Increased intestinal permeability (4 and 70 kDa dextran) and decreased Ecad levels Combined toxicity of 50 and 500 nm NPLs | [28] |
| Female C57BL/6 mice | Spherical 50 nm and 1 µm PS | 0.2 mg/mouse so ≈9 mg/kg bw/day 12 and 23.7 weeks Drinking water | No effects under basal, acute colitis or chronic colitis conditions | [29] |
| Male C57BL/6 mice | Spherical 47 nm PS | 0.2, 1 and 10 mg/kg bw/day 4.2 weeks Gavage | Slight dysbiosis (impact on 3 to 8 taxa/group) Slight intestinal damage (only Tff3 and Klf3 mRNA decrease at highest dose) | [30] |
| Male C57BL/6 mice | Spherical 5 µm PS | 500 µg/L so ≈0.11 mg/kg bw/day 4 weeks Drinking water | In colon: Increased TNFα, IFNγ, IL1β, GPx Decreased Claudin1 and Occludin1 Worsened DSS 1-induced colitis (colon length) Decreased glycine and taurine production only under DSS 1-induced conditions | [31] |
| Male C57BL/6 mice | Spherical 5 µm PS | 18 and 180 µg/kg bw/day 12.8 weeks Drinking water | Dysbiosis | [32] |
| Male ICR mice | Spherical 5, 50, 100 and 200 µm PS | 80 mg/kg bw/day, including 20 mg/kg of 5, 50, 100 and 200 µm MPL 10 weeks Gavage | In normal-diet mice: induction of dysbiosis (including decreased Firmicutes/Bacteroides ratio) In high-fat-fed (HFD) mice: modification of HFD-induced dysbiosis (including worsening of Enterobacteriaceae abundance increase) | [33] |
| C57BL/6J mice (sex not provided) | Spherical 5 µm PS | 0.5 and 5 µg/mouse so ≈0.023 and 0.23 mg/kg bw/day 2 and 9 weeks Gavage | DSS 1-induced acute and chronic colitis exacerbation | [34] |
| Male C57BL/6 mice | Spherical 5 µm PS | 0.1 mg/day so ≈4.5 mg/kg bw/day 6 weeks Gavage | Alterations in gut microbiota and metabolites | [35] |
| Male C57BL/6 mice | Crushed 51 and 88 µm PS | 50 and 500 mg/kg bw/day 3 weeks Feed | Decreased SI 3 mucus layer thickness Increased SI 3 epithelial injury Fecal dysbiosis Host plasma lipid metabolism disturbance | [36] |
| Male C57BL/6 mice | Spherical 5 µm PS | 100 µg/L so ≈0.02 mg/kg bw/day 6 weeks Drinking water | In colon: Decreased goblet cell number Increased crypt depth and density Decreased Tff3 and Muc2 mRNA Increased Il1β, Il6, Dll1, Dll4, Jag1, Notch1, Hes1, Lgr5, Bmi1, Olfm4 and c-Myc mRNA levels Increased c-MYC and PCNA protein levels Worsened DSS 1-induced colitis (body weight, colon length, histological score, serum LPS, colon Il1β and Il6) | [37] |
| Male and female C57BL/6 mice | Spherical 71 nm PS | 50, 250 or 1250 µg/mouse/day So ≈2.3, 11.5 or 57.5 mg/kg bw/day 7.52 × 1011, 3.76 × 1012, and 1.88 × 1013 particles/day 3 times per week during gestation Oropharyngeal aspiration | In adult male and female offspring: Duodenum, jejunum and ileum histomorphological alterations in female and male mice Small intestine oxidative stress Small intestine ferroptosis | [38] |
| Male C57BL/6 mice | Spherical 0.45–0.53µm PS COOH | 1000 µg/L so ≈0.22 mg/kg bw/day 4 weeks Drinking water | On SI of high-fat-diet-fed mice: Decreased goblet cells Decreased mucus thickness Decreased villus height and higher crypt depth Increased innate lymphoid cells ILC1 and T bet+ ILC3 cells Increased M1/M2 macrophage ratio Decreased ILC3 cells Decreased palmitic acid, acetic acid, propionic acid and butanoic acid in feces Increased inflammatory cytokines and lipid metabolism gene expression | [39] |
| Male C57BL/6 mice | Spherical 0.05–0.1 µm and 9–10 µm PS | 1 ppm, 1 mg/L so ≈0.22 mg/kg bw/day 12 weeks Drinking water | Size-dependent Gut dysbiosis (bacteria and fungi) Disturbed microbial metabolic pathways Altered microbial antibiotic resistance genes and virulence factors | [40] |
| Male C57BL/6 mice | Spherical 5 µm PS | 0.1 mg/mouse so ≈4.5 mg/kg bw/day 6 weeks Gavage | Gut dysbiosis and variations in predicted functional pathways Modifications to metabolite profiles (bile acid metabolism enrichment) Increased bile acids, decreased purine and pyrimidine nucleosides Decreased fecal levels of acetic acid, propionic acid, butyric acid and isobutyric acid (SCFA) | [41] |
| Male BALB/c mice | Spherical 100 nm PS, PS-COOH and PS-NH2 | 1 mg/mouse/day so ≈45.5 mg/kg bw/day 4 weeks Gavage | Decreased villus length Increased crypt depth and lower ratio of villus length to crypt depth Increased the secretion of mucus in ileum Increased IL-17a-positive cells in the ileal lamina propria Increased ileal apoptosis (TUNEL) Increased necroptosis (RIPK3 and MLCK protein expression) Increased mitophagy (PINK1, PARKIN, SQSTM1/p62, LC3B, TOMM20 protein expression) | [42] |
| Male C57BL/6 mice | Spherical 100 nm NPL and 1 µm MPL PS | 0.5 mg/mouse so ≈22.7 mg/kg bw/day 8.5 weeks Gavage | Decreased colon mucus secretion Increased gut permeability (FITC dextran) Size-dependent dysbiosis Altered serum metabolites | [43] |
| Male C57BL/6 mice | Spherical 5 µm PS | 0.001, 0.1, 1 and 10 µg/mL so ≈0.002, 0.02, 0.2 and 2 mg/kg/day 10 weeks Drinking water | Dysbiosis from the 0.1 µg/mL concentration | [44] |
| Male C57BL/6 mice | Spherical 5 µm PS | 10 mg/L so ≈2.3 mg/kg bw/day 6 weeks Drinking water | In distal colon of healthy mice: Increase in the number of endocrine cells Increase in the content of highly sulfated mucins in goblet cells Increase in the number of cells in the lamina propria Decrease in the volume fraction of macrophages Worsening of DSS 1-induced colitis: greater prevalence of ulcers and inflammation; decrease in the content of neutral mucins in goblet cells | [45] |
| KN Mice (sex not provided) | Spherical 300 nm PS | 12 and 500 mg/kg bw/day 4 weeks Gavage | Dose-dependent Increased gut permeability Increased ileum Muc2 expression Decreased jejunum and ileum villus number and length Decreased colon mucus thickness and goblet cells number Ileum and jejunum oxidative stress | [46] |
| Male Wistar rat | Spherical 50 nm and 5 µm PS | 0.1 and 1 mg/kg bw/day 4 weeks Gavage | Higher for MPLs than NPLs: Decreased colon mucus secretion and MUC2 expression Decreased colon ZO-1 and occludin expression For both MPLs and NPLs: decreased colon exosomal miR-126a expression | [47] |
| Male C57/BL/6 mice | Spherical 5 µm PS | 200 µg/L so ≈0.04 mg/kg bw/day 5 weeks Drinking water | With normal diet: Increased serum Diamine oxidase (DAO), D-Lactate, D-Lactate dehydrogenase (D-LDH) and intestinal fatty acid-binding protein (IFABP) Decreased ileum secretory IgA Fecal dysbiosis Worsened with dietary restriction: Decreased ileum mucus secretion Increased serum DAO, D-Lactate, IFABP and TNF-α levels | [48] |
| Female BALB/C mice | Spherical 20, 500, 5000 nm PS | 6, 60, 600 µg/kg bw/day 2 or 4 weeks Gavage | Gut microbiota composition alterations Variation in short-chain fatty acid levels Modifications to intestinal permeability (decreased after 2 weeks, increased after 4 weeks) Increased leukocytes in SI (4 weeks) Decreased secretory IgA levels Decreased CD4+ and CD8+ T cells in MLN 4 | [49] |
| Female C57BL/6 mice | Spherical 50 nm PS | 0.1, 1 or 10 mg/L so ≈0.02, 0.23 or 2.3 mg/kg bw/day 32 weeks Drinking water | Increased caveolin and clathrin levels (endocytosis proteins) Histological damage in jejunum, ileum, colon Decreased Claudin-1, Occludin, ZO-1 levels Increased ROS2 and MDA levels Decreased SOD and GSH-Px levels Increased B lymphocytes in MLN4 Decreased γδ+CD8+ and CD3+ CD8+ T cells in intestine Increased intestinal mucosal IL1β, IL-6 and TNF-α. | [50] |
| Male C57BL/6 mice | Spherical 0.5 and 5 µm PS | 0.5 mg/mouse/day so ≈22.7 mg/kg bw/day 8 weeks Gavage | Decreased colon length (5 µm only) Increased colon IL6, TNFα, Il1β secretion (higher for 5 µm than 0.5 µm) Dysbiosis and variation in fecal metabolome (higher for 5 µm than 0.5 µm) | [51] |
| Male C57BL/6 mice | Spherical 0.2, 1 and 5 µm PS | 1 mg/kg bw/day 4 weeks Gavage | In colon: 5 µm PS: Oxidative stress Increased inflammatory cytokines Impaired tight junctions and mucins Lower impairments for 1 µm Fewest impairments for 0.2 µm | [52] |
| Male ICR mice | Spherical 44 nm nonfunctionalized PS, 51 nm PS-NH2, 50 nm PS-COOH | 80 µg/L 0.018 mg/kg bw/day 9 weeks Drinking water | Similar for the 3 NPLs: Increased colon histological score Decreased colon Ifnγ, Il6, Il10, Tff3 Increased Tlr3 expression NPL-specific variations in fecal microbiome and metabolome | [53] |
| Male BALB/C mice | Spherical 140 nm PS | 5 mg/kg bw/day 4 weeks Gavage | Jejunum and colon mitophagy (increased secretion of LC3B2/LC3B-1, BNIP3, NIX and p62) Dysbiosis | [54] |
| Male ICR mice | Spherical 99 nm and 5 µm PS | 200 or 500 µg/mouse so ≈9 or 22.5 mg/kg bw/day 5 weeks Gavage | Size- and concentration-dependent dysbiotic effects | [55] |
| Male C57BL/6 mice | Size and shape not provided PS NP | 0.5 and 1.5 mg/mouse/day so ≈22.7 and 68 mg/kg bw/day 4 or 6 weeks Gavage | Activation of pro-inflammatory gene expression (RNA sequencing) Activation JAK-STAT and ERK1/2 signaling pathways Depletion of taurine in colon Increased gut permeability (FITC-dextran and reduced Tight-junction proteins) DSS 1-induced colitis exacerbation | [56] |
| Male C57BL/6 mice | Spherical 200 and 800 nm Nile-red-labeled PS | 109/mouse 3 times per week for 4 weeks Gavage | Disruptions to cecal microbiome and metabolome | [57] |
| Polyethylene (PE) | ||||
| Female ICR mice | Spherical 1–10 µm PE | 0.002 and 0.2 µg/g bw/day 4 weeks Gavage | At 0.2 µg dosage: Decreased colon mucin Decreased Il1β, ERK1, NF-κB, and increased Il10 and Il8 mRNA Dysbiosis | [58] |
| Male BALB/C mice | Spherical 61 µm PE | 25 or 50 µg/mouse so ≈1.25 or 2.5 mg/kg bw/day 3 times in a week Gavage | Without H. pylori infection: Gastric pathological damage Increased gastric IL6 and TNF-α levels With H. pylori infection: Increased H. pylori gastric colonization Increased gastric injury Increased gastric inflammation (MPO, IL6, TNF-α) | [59] |
| Female C57BL/6 Mice | Spherical 36 and 116 µm PE | 100 µg/g feed so ≈16 mg/kg bw/day 6 weeks Feed | Colon hyperproliferation Increased colon mucus and Muc2 expression Colon inflammation Whole-gut immune population and epithelial cell disturbances Dysbiosis | [60] |
| BALB/c mice (sex not provided) | Polydisperse, grinded 530 and 2300 nm PE | 10 mg/kg bw/day in 0.5% CMC 1 week Gavage | Overt colitis Decreased colon length Increased colon Il1β, Th2, Treg, Th17 cells | [61] |
| Male C57BL/6 mice | Spherical 2.6 to 13 µm LDPE and oxidized LDPE | 5 mg/mouse/day so ≈227 mg/kg bw/day 4 weeks Gavage | Higher for oxidized LDPE than LDPE Decreased duodenum length Increased duodenum and colon crypt depth Oxidative stress Increase in Tnfα, Il1β, Il6 expression in duodenum and colon Same extent for both LDPEs: dysbiosis | [62] |
| Male C57BL/6 mice | Spherical 5 µm PE | 1 and 10 mg/L so ≈0.22 and 2.2 mg/kg bw/day 3 weeks Drinking water | Concentration-specific dysbiosis | [63] |
| Polyvinylchloride (PVC) | ||||
| Male C57BL/6 Mice | Spherical 2 µm PVC | 100 mg/kg bw/day 8.5 weeks Gavage | Increased intestinal permeability Decreased mucus secretion Decreased colon Muc1, Muc2, Muc3, Klf4, Retnlb mRNA expression Gut dysbiosis Modification to fecal metabolic profile | [64] |
| Male C57BL/6 Mice | Spherical 2 µm PVC | 0.5 mg/mouse So ≈22.7 mg/kg bw/day 8.5 weeks Gavage | Gut dysbiosis | [65] |
| Polypropylene (PP) | ||||
| Male C57BL/6 Mice | Irregular Grinded 8 and 70 µm PP | 0.1, 1, 10 mg/mL So ≈22, 227 and 2272 mg/kg bw/day 4 weeks Gavage | Mild colon submucosa edema Colon oxidative stress and inflammation Disruption of intestinal barrier TLR4/NF-κB signaling pathway Colon apoptosis | [66] |
| Polyethylene terephthalate (PET) | ||||
| Male and female KM mice | Spherical 200 nm and 700 nm PET | 200 mg/kg bw/day 4 weeks Gavage | LD50: 266 mg/kg bw for 200 nm-PET and 523 mg/kg bw for 700 nm-PET 200 nm PET only: Intestinal obstruction Perturbations to gut microbiome and metabolome | [67] |
| Female C57BL/6 mice | Pin made 1 µm PET | 3 × 104/mouse Gavage 8 weeks | In colon: No evidence of impaired histomorphology and mucus barrier No low-grade inflammation 139 differentially expressed genes In gut immune cells: Oxidative phosphorylation and reactive oxygen species pathways enrichment Dysbiosis | [68] |
| Several polymer types | ||||
| Kunming mice (sex not provided) | Spherical 150–130 µm PE, PET, PP, PS and PVC | 4 mg/mouse/day So, ≈182 mg/kg/day 7 days | All: colon damage (PS > PVC > PET > PE > PP) Polymer-specific oxidative stress Polymer-specific dysbiosis | [69] |
| Male and female Wistar rats | Spherical 15–20 µm polyamide (PA) 40–48 µm PE | 0.1%W/W (100 mg/kg) in feed So, ≈227 mg/kg/day 5 weeks Feed | Increased duodenum occludin and ZO1 expression | [70] |
| Male Sprague-Dawley rats | 100 µm PP, PET, PS, Rayon, PE, polyoxymethylene (POM), polycarbonate (PC), PA, PVC, polyurethane (PU) mixture | 12 mg/kg bw/day 6 weeks Gavage | Dysbiosis | [71] |
| BALB/C mice (sex not provided) | Spherical 500 nm PE 500 nm PS | 10 mg/kg bw/day 1 week Gavage | Both PE and PS NPL: In colon: Decreased length Pathological damage Increased ROS 2 production Increased proportion of pro-inflammatory macrophages, Th2, Th17 and Treg cells PE NPL: promotes the development of CT26-luc cell tumor | [72] |
| Male C57BL/6J mice | Spherical 2 µm PVC 1 µm PS | 0.5 mg/mouse day So ≈22.7 mg/kg bw/day 8.5 weeks Gavage | PS-PVC co-exposure: Decreased mucus secretion Increased gut permeability (FITC-dextran, serum LPS) Increased colon Il1β, Il6, Tnfα mRNA Decreased colon Muc2, Muc3, Klf4, Retnlb, Meprin-β, Claudin5, Claudin4, Tjp1 Dysbiosis | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djouina, M.; Loison, S.; Body-Malapel, M. Recent Progress in Intestinal Toxicity of Microplastics and Nanoplastics: Systematic Review of Preclinical Evidence. Microplastics 2024, 3, 217-233. https://doi.org/10.3390/microplastics3020013
Djouina M, Loison S, Body-Malapel M. Recent Progress in Intestinal Toxicity of Microplastics and Nanoplastics: Systematic Review of Preclinical Evidence. Microplastics. 2024; 3(2):217-233. https://doi.org/10.3390/microplastics3020013
Chicago/Turabian StyleDjouina, Madjid, Suzie Loison, and Mathilde Body-Malapel. 2024. "Recent Progress in Intestinal Toxicity of Microplastics and Nanoplastics: Systematic Review of Preclinical Evidence" Microplastics 3, no. 2: 217-233. https://doi.org/10.3390/microplastics3020013
APA StyleDjouina, M., Loison, S., & Body-Malapel, M. (2024). Recent Progress in Intestinal Toxicity of Microplastics and Nanoplastics: Systematic Review of Preclinical Evidence. Microplastics, 3(2), 217-233. https://doi.org/10.3390/microplastics3020013