Short-Term Microplastics Exposure to the Common Mysid Shrimp, Americamysis bahia: Effects on Mortality and DNA Methylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Quality Control
2.2. Experiment I Design
2.3. Experiment II Design
2.4. DNA Extraction and Methylation Detection Spectrophotometry
2.5. Fluorescent Microscopy
3. Results
3.1. Experiment I Mortality
3.2. Experiment II Mortality
3.3. Experiment I and II Control Group Comparison
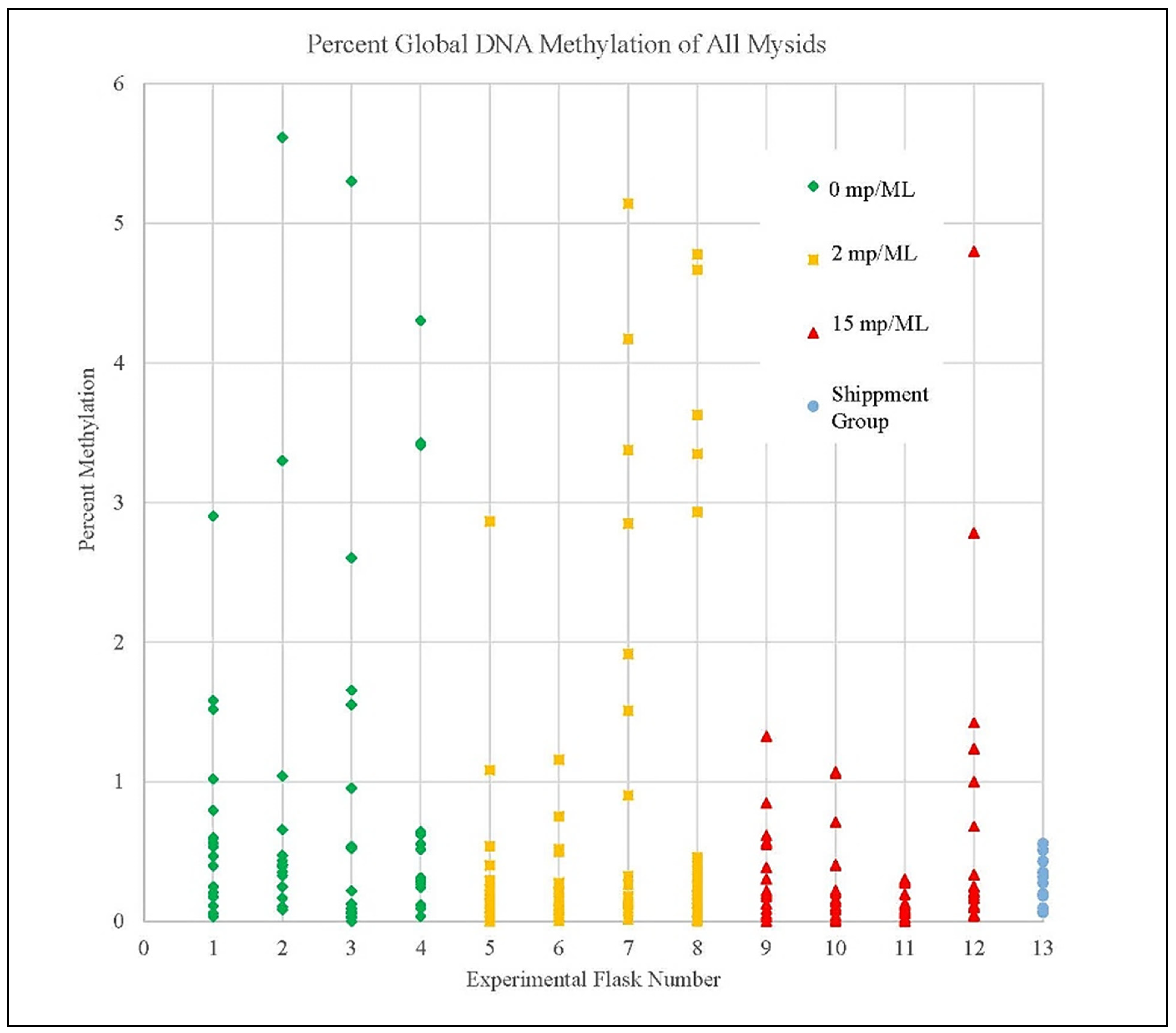
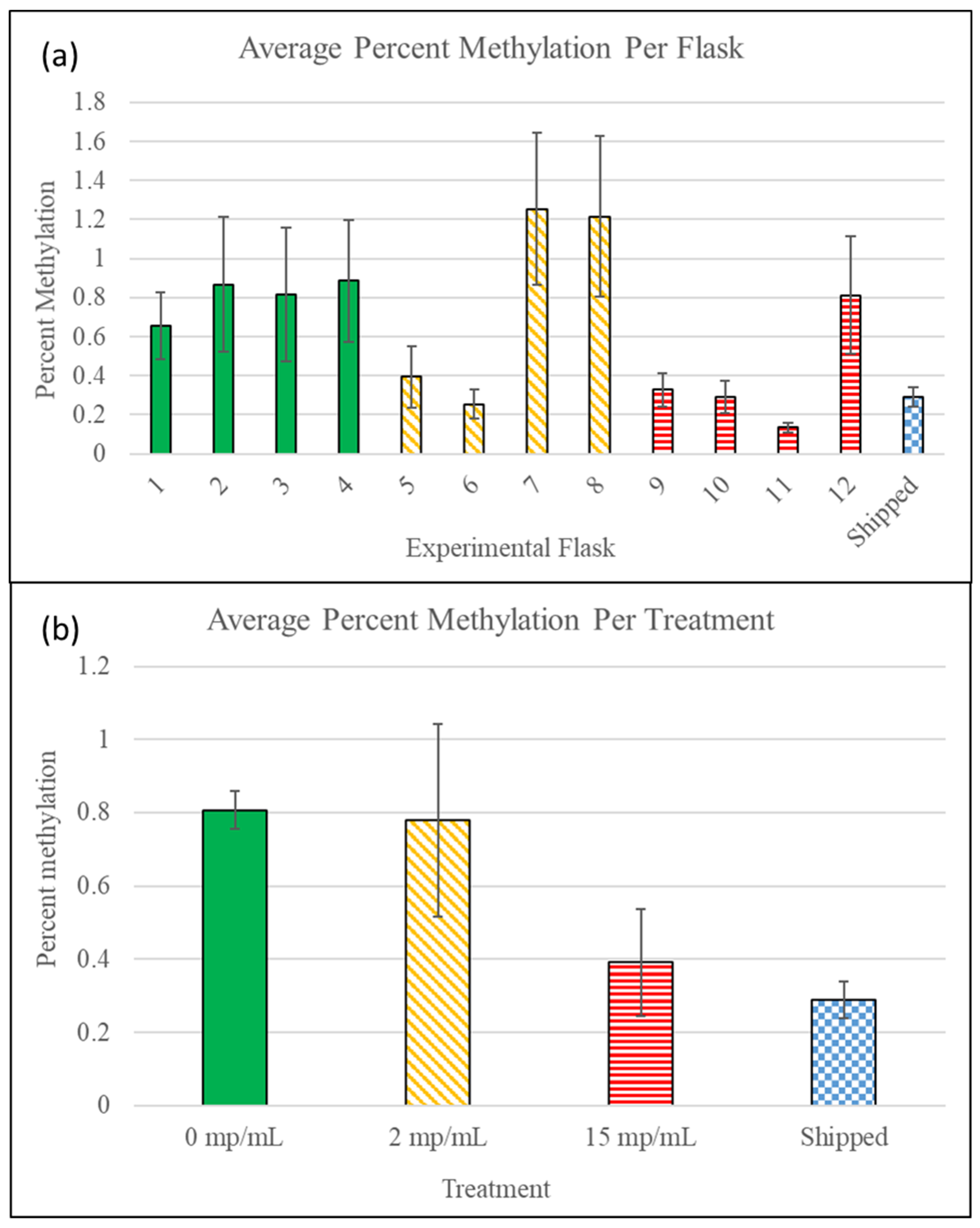
3.4. Methylation Detection Spectrophotometry of Experiment I Mysids
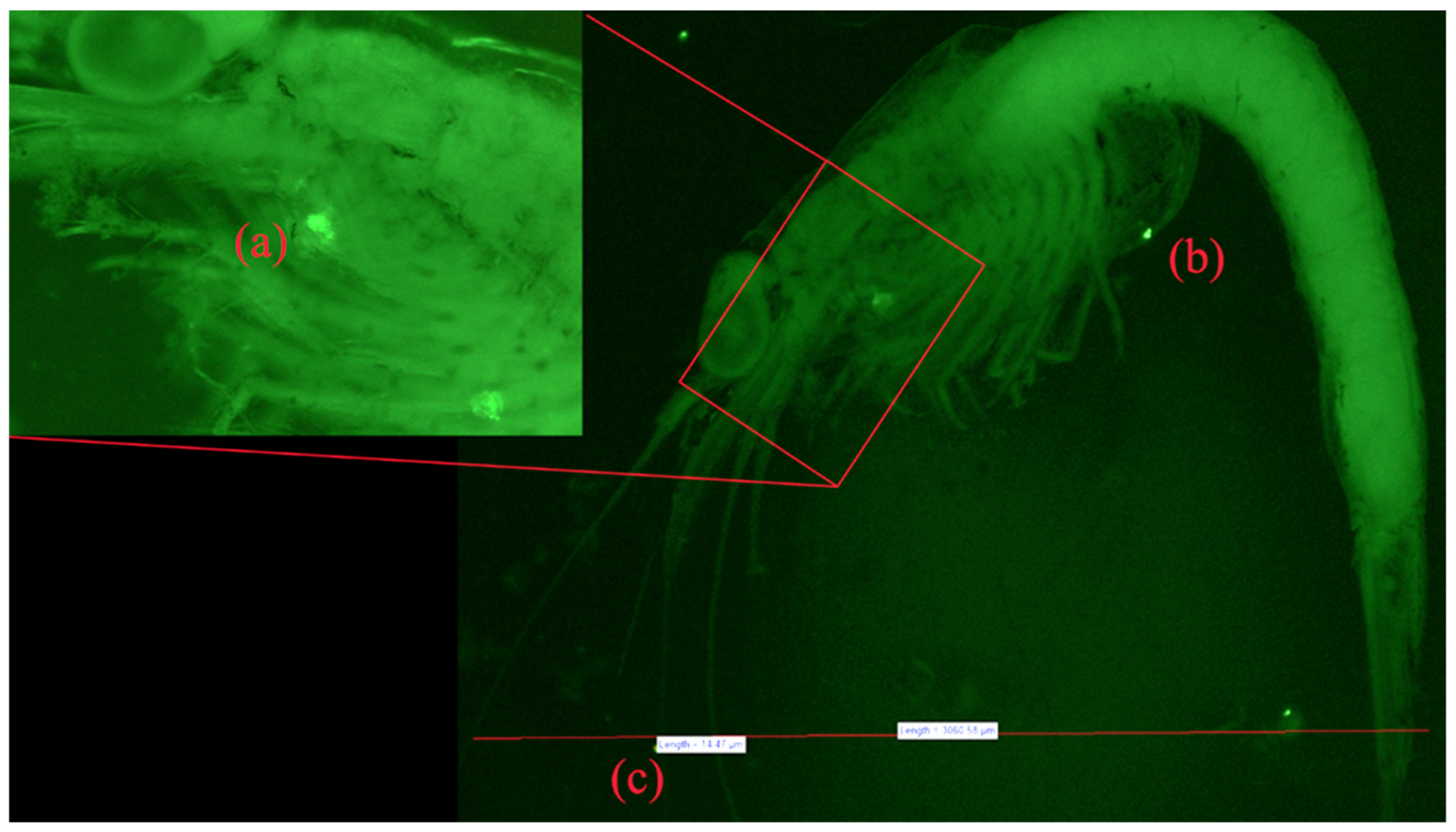
3.5. Fluorescent Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Short Communication: Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapu Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguhi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengston Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2015, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.K.; Filemann, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Panti, C.; Giannetti, M.; Baini, M.; Rubegni, F.; Minutoli, R.; Fossi, M.C. Occurrence, relative abundance and spatial distribution of microplastics and zooplankton NW of Sardinia in the Pelagos Sanctuary Protected Area, Mediterranean Sea. Environ. Chem. 2015, 12, 618–626. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Zhu, M.; Liang, J.; Zheng, S.; Zhao, Y. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017, 115, 217–224. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Luo, X.; Zheng, H. Short-term toxicity of polystyrene microplastics on mysid shrimps Neomysis japonica. IOP Conf. Ser. Earth. Environ. Sci. 2017, 61, 012136. [Google Scholar] [CrossRef]
- Lee, K.; Shim, W.J.; Kwon, O.Y.; Kang, J. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicas. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calamus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Brun, N.R.; Beenakker, M.M.T.; Hunting, E.R.; Ebert, D.; Vijver, M.G. Brood pouch-mediated polystyrene nanoparticle uptake during Daphnia magna embryogenesis. Nanotoxicology 2017, 11, 1059–1069. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci Total Environ. 2017, 584, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Contino, M.; Ferruggia, G.; Pecoraro, R.; Scalisi, E.M.; Cavallaro, G.; Bonaccorso, C.; Fortuna, C.G.; Salvaggio, A.; Capparucci, F.; Bottari, T.; et al. Uptake routes and biodistribution of polystyrene nanoplastics on zebrafish larvae and toxic effects on development. Fishes 2023, 8, 168. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Vandegehuchte, M.B.; Janssen, C.R. Epigenetics in an ecotoxicological context. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 764–765, 36–45. [Google Scholar] [CrossRef]
- Gombeau, K.; Pereira, S.; Ravanat, J.; Camilleri, V.; Cavalie, I.; Bourdineaud, J.; Adam- Guillermin, C. Depleted uranium induces sex- and tissue-specific methylation patterns in adult zebrafish. J. Environ. Radioact. 2016, 154, 25–33. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, L.; Yang, J. Epigenetic Flexibility Underlying Phenotypic Plasticity. In Progress in Botany; Lüttge, U., Beyschlag, W., Francis, D., Cushman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 74, pp. 153–163. [Google Scholar]
- Huang, X.; Li, S.; Ni, P.; Gao, Y.; Jiang, B.; Zhou, Z.; Zhan, A. Rapid response to changing environments during biological invasions: DNA methylation perspectives. Mol. Ecol. 2017, 26, 6621–6633. [Google Scholar] [CrossRef] [PubMed]
- Elmhiri, G.; Gloaguen, C.; Grison, S.; Kereselidze, D.; Elie, C.; Tack, K.; Benderitter, M.; Lestaevel, P.; Legendre, A.; Souidi, M. DNA methylation and potential multigenerational epigenetic effects linked to uranium chronic low-dose exposure in gonads of males and female rats. Toxicol. Lett. 2018, 282, 64–70. [Google Scholar] [CrossRef]
- Chen, H.; Hua, X.; Li, H.; Wang, C.; Dang, Y.; Ding, P.; Yu, Y. Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere 2021, 272, 129642. [Google Scholar] [CrossRef]
- Regev, A.; Lamb, M.J.; Jablonka, E. The role of DNA methylation in invertebrates: Developmental regulation or genome defense? Mol. Biol. Evol. 1998, 15, 880–891. [Google Scholar] [CrossRef]
- Barzotti, R.; Pelliccia, F.; Rocchi, A. DNA methylation, histone H3 methylation, and histone H4 acetylation in the genome of a crustacean. Genome 2006, 49, 87–90. [Google Scholar] [CrossRef]
- Norouzitallab, P.; Baruah, K.; Vandegehuchte, M.; Van Stappen, G.; Catania, F.; Vanden Bussche, J.; Vanhaecke, L.; Sorgeloos, P.; Bossier, P. Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. FASEB J. 2014, 28, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Sarda, S.; Zeng, J.; Hunt, B.G.; Yi, S.V. The evolution of invertebrate gene body methylation. Mol. Biol. Evol. 2012, 29, 1907–1916. [Google Scholar] [CrossRef]
- Wortham-Neal, J.; Price, W.W. Marsupial developmental stages in Americamysis bahia (Mysida: Mysidae). J. Crust. Biol. 2002, 22, 98–112. [Google Scholar] [CrossRef]
- Meland, K.; Mees, J.; Porter, M.; Wittmann, K.J. Taxonomic review of the orders Mysida and Stygiomysida (Crustacea, Peracarida). PLoS ONE 2015, 10, e0124656. [Google Scholar] [CrossRef]
- Di Mauro, R.; Kupchik, M.J.; Benfield, M.C. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut. 2017, 230, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.K.; Filemann, E.; Clark, J.; Lewis, C.; Halsband, C.; Galloway, T.S. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 2016, 50, 3239–3246. [Google Scholar] [CrossRef]
- Desforges, J.P.W.; Galbraith, M.; Ross, P.S. Ingestion of microplastics by zooplankton in the northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Eom, H.J.; Choi, J. Effect of Early-Life Exposure of Polystyrene Microplastics on Behavior and DNA Methylation in Later Life Stage of Zebrafish. Arch. Environ. Contam. Toxicol. 2022, 82, 558–568. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.S.; Park, J.W.; Kim, T.H.; Kwak, I.S. Bioaccumulation, microbiome composition and immunity, and epigenetic signatures associated with exposure to spherical, fibrous, and fragmented microplastics in the mussel Mytilus galloprovincialis. J. Hazard. Mater. 2024, 462, 132691. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Boughanem, H.; Dávalos, A. Untoward Effects of Micro- and Nanoplastics: An Expert Review of Their Biological Impact and Epigenetic Effects. Adv. Nutr. 2022, 13, 1310–1323. [Google Scholar] [CrossRef]
- Lussier, S.M.; Kuhn, A.; Chammas, M.J.; Sewall, J. Techniques for the laboratory culture of mysidopsis species (crustacea: Mysidacea). Environ. Toxicol. Chem. 1988, 7, 969–977. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Eviron. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Epigentek © 2024. MethylFlash™ Methylated DNA Quantification Kit (Colorimetric) User Guide. Farmingdale, NY. P-1034, 1–11. Available online: https://www.epigentek.com/docs/P-1034.pdf (accessed on 1 February 2024).
- Eberlain, G.A. Quantitation of proteins using HPLC-detector response rather than standard curve comparison. J. Pharm. Biomed. 2003, 13, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef] [PubMed]
- Grear, J.S.; Horowitz, D.B.; Gutjahr-Gobell, R. Mysid population responses to resource limitation differ from those predicted by cohort studies. Mar. Ecol. Prog. Ser. 2011, 432, 115–123. [Google Scholar] [CrossRef]
- Martins, A.; Guilhermino, L. Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci. Total Environ. 2018, 631–632, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Hong, S.H.; Park, J. Evaluation of microplastic toxicity in accordance with different sizes and exposure times in the marine copepod Tigriopus japonicus. Mar. Environ. Res. 2020, 153, 104838. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Huang, X.; Xiao, J.; Nong, S.; Li, C. MeDIP-seq reveals the features of mitochondrial genomic methylation in immature testis of the Chinese mitten crab Eriocheir sinensis. Mitochondrial DNA A Mapp. Seq. Anal. 2018, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Vandegehuchte, M.B.; Lemière, F.; Janssen, C.R. Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comp. Biochem. Phys. C 2009, 150, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Jiang, Z.; Xia, Y.; Lou, P.; Chen, L.; Wang, H.; Bai, L.; Xie, Y.; Liu, Y.; Li, W.; et al. Genome-wide DNA methylation changes in skeletal muscle between young and middle-aged pigs. BMC Genom. 2014, 15, 653. [Google Scholar] [CrossRef] [PubMed]
- Head, J.A. Patterns of DNA methylation in animals: An exotoxicological perspective. Integr. Comp. Biol. 2014, 54, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.P.; Pignatta, D.; Henikoff, S.; Gehring, M. Methylation-sensitive expression of a DNA demethylase gene serves as an epigenetic rheostat. PLoS Genet. 2015, 11, e1005142. [Google Scholar] [CrossRef] [PubMed]
- Gopisetty, G.; Ramachandran, K.; Singal, R. DNA methylation and apoptosis. Mol. Immunol. 2006, 43, 1729–1740. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Abelli, L.; Fossi, M.C.; Caliani, I.; Panti, C. Effects of microplastics on head kidney gene expression and enzymatic biomarkers in adult zebrafish. Comp. Biochem. Physiol. C. 2021, 245, 109037. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prior, J.H.; Whitaker, J.M.; Janosik, A.M. Short-Term Microplastics Exposure to the Common Mysid Shrimp, Americamysis bahia: Effects on Mortality and DNA Methylation. Microplastics 2024, 3, 234-249. https://doi.org/10.3390/microplastics3020014
Prior JH, Whitaker JM, Janosik AM. Short-Term Microplastics Exposure to the Common Mysid Shrimp, Americamysis bahia: Effects on Mortality and DNA Methylation. Microplastics. 2024; 3(2):234-249. https://doi.org/10.3390/microplastics3020014
Chicago/Turabian StylePrior, Jack H., Justine M. Whitaker, and Alexis M. Janosik. 2024. "Short-Term Microplastics Exposure to the Common Mysid Shrimp, Americamysis bahia: Effects on Mortality and DNA Methylation" Microplastics 3, no. 2: 234-249. https://doi.org/10.3390/microplastics3020014
APA StylePrior, J. H., Whitaker, J. M., & Janosik, A. M. (2024). Short-Term Microplastics Exposure to the Common Mysid Shrimp, Americamysis bahia: Effects on Mortality and DNA Methylation. Microplastics, 3(2), 234-249. https://doi.org/10.3390/microplastics3020014





