A Comparative Study on Meat Quality Characteristics of Murrah Buffalo and Nellore Cattle Commercialized in Southeastern Brazil
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Quality Characteristics
3.2. Volatile Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; 163p. [Google Scholar]
- Naveena, B.M.; Kiran, M. Buffalo meat quality, composition, and processing characteristics: Contribution to the global economy and nutritional security. Anim. Front. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Vilella, G.F.; Gomes, C.L.; Battaglia, C.T.; Pacheco, M.T.B.; da Silva, V.S.N.; Rodas-González, A.; Pflanzer, S.B. Effects of combined wet- and dry-aging techniques on the physicochemical and sensory attributes of beef ribeye steaks from grain-fed crossbred Zebu steers. Can. J. Anim. Sci. 2019, 99, 497–504. [Google Scholar] [CrossRef]
- Andrade, B.F.; Castro, M.M.; Rodrigues, L.M.; Torres Filho, R.A.; Fontes, P.R.; Ramos, E.M.; Ramos, A.L. Effects of delayed chilling on rigor development and meat quality of Murrah buffalo from different production systems. Res. Soc. Dev. 2021, 10, 6. [Google Scholar] [CrossRef]
- Marques, C.S.S.; Oaigen, R.P.; Moraes, C.M.; Santos, M.A.S.; Lourenço Júnior, J.d.B.; Abel, I. Segmentation of the buffalo meat consumer market in Belém, Pará, Brazil. R. Bras. Zootec. 2016, 45, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Abdel-Naeem, H.H.S.; Mohamed, H.M.H.; Yassien, N.A. Comparing the physico-chemical characteristics and sensory attributes of imported Brazilian beef meat and imported Indian buffalo meat. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Lapitan, R.M.; Del Barrio, A.N.; Katsube, O.; Ban-Tokuda, T.; Orden, E.A.; Robles, A.Y.; Cruz, L.C.; Kanai, Y.; Fujihara, T. Comparison of fattening performance in Brahman grade cattle (Bos indicus) and crossbred water buffalo (Bubalus bubalis) fed on high roughage diet. Anim. Sci. J. 2008, 79, 76–82. [Google Scholar] [CrossRef]
- Mello, J.L.M.; Rodrigues, A.B.B.; Giampietro-Ganeco, A.; Ferrari, F.B.; Souza, R.A.; Souza, P.A.; Borba, H. Characteristics of carcasses and meat from feedlot-finished buffalo and Bos indicus (Nellore) bulls. Anim. Prod. Sci. 2018, 58, 1366–1374. [Google Scholar] [CrossRef]
- Valin, C.; Pinkas, A.; Dragnev, H.; Boikovski, S.; Polikronov, D. Comparative study of buffalo meat and beef. Meat. Sci. 1984, 10, 69–84. [Google Scholar] [CrossRef]
- Ijaz, M.; Jaspal, M.H.; Hayat, Z.; Yar, M.K.; Badar, I.H.; Ullah, S.; Hussain, S.; Ali, S.; Farid, M.U.; Farooq, M.Z.; et al. Effect of animal age, postmortem chilling rate, and aging time on meat quality attributes of water buffalo and humped cattle bulls. Anim. Sci. J. 2020, 91, e13354. [Google Scholar] [CrossRef]
- Irurueta, M.; Cadoppi, A.; Langman, L.; Grigioni, G.; Carduza, F. Effect of aging on the characteristics of meat from water buffalo grown in the Delta del Paraná region of Argentina. Meat. Sci. 2008, 79, 529–533. [Google Scholar] [CrossRef]
- Luz, P.A.C.; Jorge, A.M.; Francisco, C.D.L.; Mello, J.L.M.d.; Santos, C.T.; Andrighetto, C. Chemical-physical characteristics of buffalo (Bubalus bubalis) meat subjected to different aging times. Acta Sci. Anim. Sci. 2017, 39, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, K.; Oommen, G.T. Myofibril fragmentation index as an immediate postmortem predictor of buffalo meat tenderness. J. Food Process. Preserv. 2015, 39, 1166–1171. [Google Scholar] [CrossRef]
- Aroeira, C.N.; Torres Filho, R.A.; Fontes, P.R.; Gomide, L.A.M.; Ramos, A.L.S.; Ladeira, M.M.; Ramos, E.M. Freezing, thawing and aging effects on beef tenderness from Bos indicus and Bos taurus cattle. Meat. Sci. 2016, 116, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hill, F. The solubility of intramuscular collagen in meat animals of various ages. J. Food Sci. 1966, 31, 161–166. [Google Scholar] [CrossRef]
- Ramos, E.M.; Gomide, L.A.M. Meat Quality Assessment: Fundamentals and Methodologies, 2nd ed.; Editora UFV: Viçosa, Brazil, 2017. (In Portuguese) [Google Scholar]
- Krzywicki, K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci 1979, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Torres Filho, R.A.; Cazedey, H.P.; Fontes, P.R.; Ramos, A.L.; Ramos, E.M. Comparison of Warner–Bratzler shear force values between round and square cross-section cores from cooked beef and pork. Meat. Sci. 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Spanghero, M.; Gracco, L.; Valusso, R.; Piasentier, E. In vivo performance, slaughtering traits and meat quality of bovine (Italian Simmental) and buffalo (Italian Mediterranean) bulls. Livest. Prod. Sci. 2004, 91, 129–141. [Google Scholar] [CrossRef]
- Neath, K.E.; Del Barrio, A.N.; Lapitan, R.M.; Herrera, J.R.; Cruz, L.C.; Fujihara, T.; Muroya, S.; Chikuni, K.; Hirabayashi, M.; Kanai, Y. Difference in tenderness and pH decline between water buffalo meat and beef during postmortem aging. Meat. Sci. 2007, 75, 499–505. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat. Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat. Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Dosi, R.; Di Maro, A.; Chambery, A.; Colonna, G.; Costantini, S.; Geraci, G.; Parente, A. Characterization and kinetics studies of water buffalo (Bubalus bubalis) myoglobin. Comp. Biochem. Physiol. B 2006, 145, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.F.; Smith, D.M. Use of volatiles as indicators of lipid oxidation in muscle foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, H.; Huang, M.; Wang, T.; Hu, Y.; Wang, L.; Zhou, G. Influence of proteolytic enzyme treatment on the changes in volatile compounds and odors of beef longissimus dorsi. Food Chem. 2020, 333, 127549. [Google Scholar] [CrossRef]
- Resconi, V.C.; Escudero, A.; Beltrán, J.A.; Olleta, J.L.; Sañudo, C.; Mar Campo, M.D. Color, lipid oxidation, sensory quality, and aroma compounds of beef steaks displayed under different levels of oxygen in a modified atmosphere package. J. Food Sci. 2012, 77, S10–S18. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mottram, D.S.; Edwards, R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983, 34, 517–522. [Google Scholar] [CrossRef]
- Giuffrida-Mendoza, M.; de Moreno, L.A.; Huerta-Leidenz, N.; Uzcátegui-Bracho, S.; Valero-Leal, K.; Romero, S.; Rodas-González, A. Cholesterol and fatty acid composition of longissimus thoracis from water buffalo (Bubalus bubalis) and Brahman-influenced cattle raised under savannah conditions. Meat. Sci. 2015, 106, 44–49. [Google Scholar] [CrossRef]

| Specie | Aging (Days) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics (1) | Buffalo | Bovine | 0 | 7 | 14 | 21 | SEM | S | A | S × A |
| pH | 5.58 B | 5.68 A | 5.62 | 5.62 | 5.64 | 5.64 | 0.01 | <0.001 | 0.615 | 0.984 |
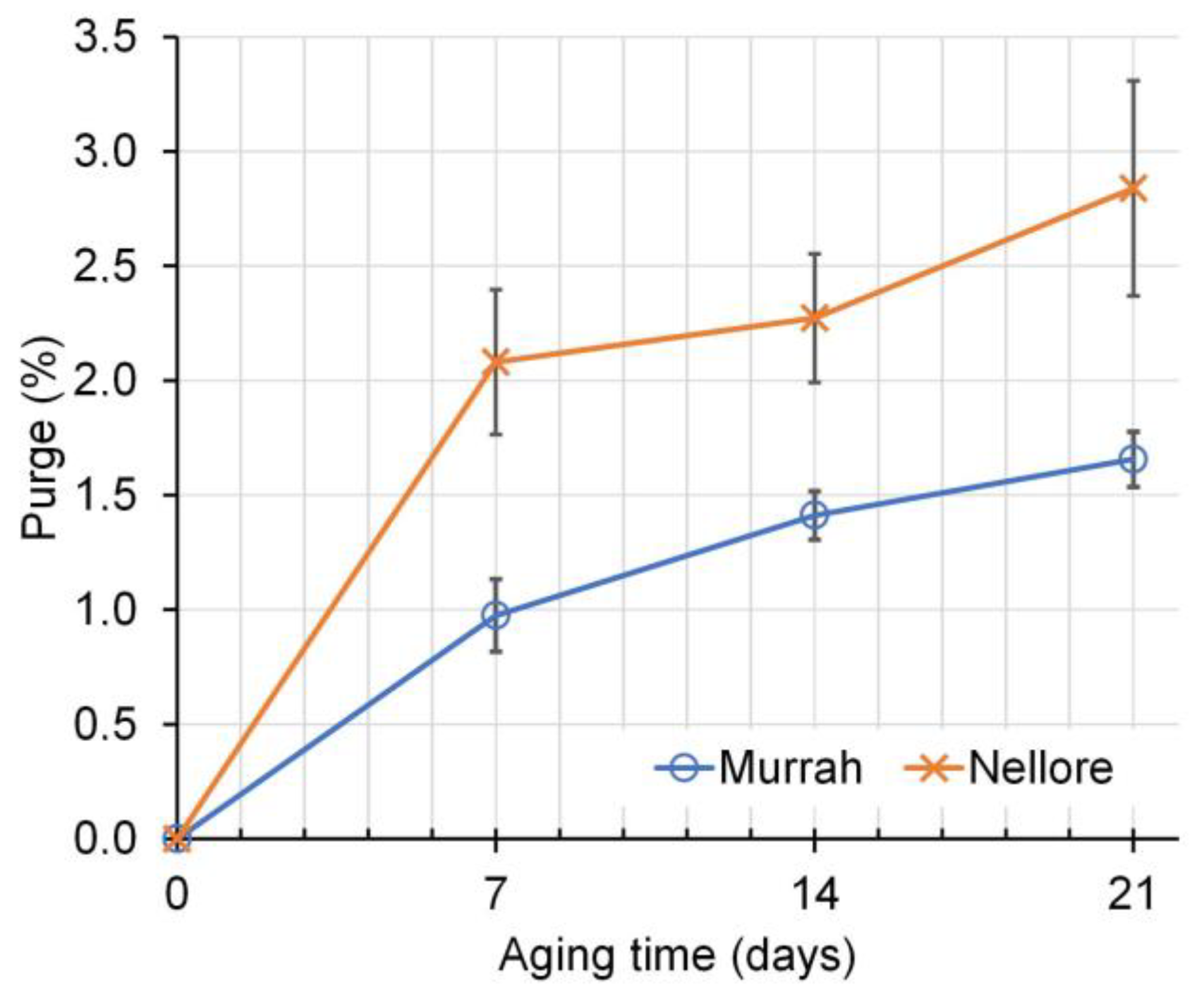
| Purge (%) | 1.01 | 1.59 | 0.00 | 1.53 | 1.89 | 2.17 | 0.13 | <0.001 | <0.001 | 0.022 |
| Water holding capacity, WHC | 0.37 A | 0.30 B | 0.41 a | 0.32 b | 0.32 b | 0.28 b | 0.01 | <0.001 | <0.001 | 0.253 |
| Cooking loss (%) | 21.22 B | 23.21 A | 21.80 | 21.11 | 22.52 | 23.06 | 0.45 | 0.010 | 0.463 | 0.101 |
| Collagen content | ||||||||||
| Total collagen, TC (mg/g) | 1.49 B | 1.99 A | 1.53 | 1.73 | 1.89 | 1.69 | 0.07 | <0.001 | 0.079 | 0.073 |
| Insoluble collagen (mg/g) | 1.27 B | 1.64 A | 1.29 | 1.51 | 1.60 | 1.32 | 0.06 | <0.001 | 0.115 | 0.056 |
| Soluble collagen (mg/g) | 0.22 B | 0.34 A | 0.24 b | 0.22 b | 0.29 ab | 0.37 a | 0.02 | <0.001 | 0.005 | 0.309 |
| Soluble collagen (% TC) | 14.57 | 17.33 | 16.15 b | 12.31 b | 14.35 b | 21.12 a | 0.94 | 0.111 | 0.016 | 0.539 |
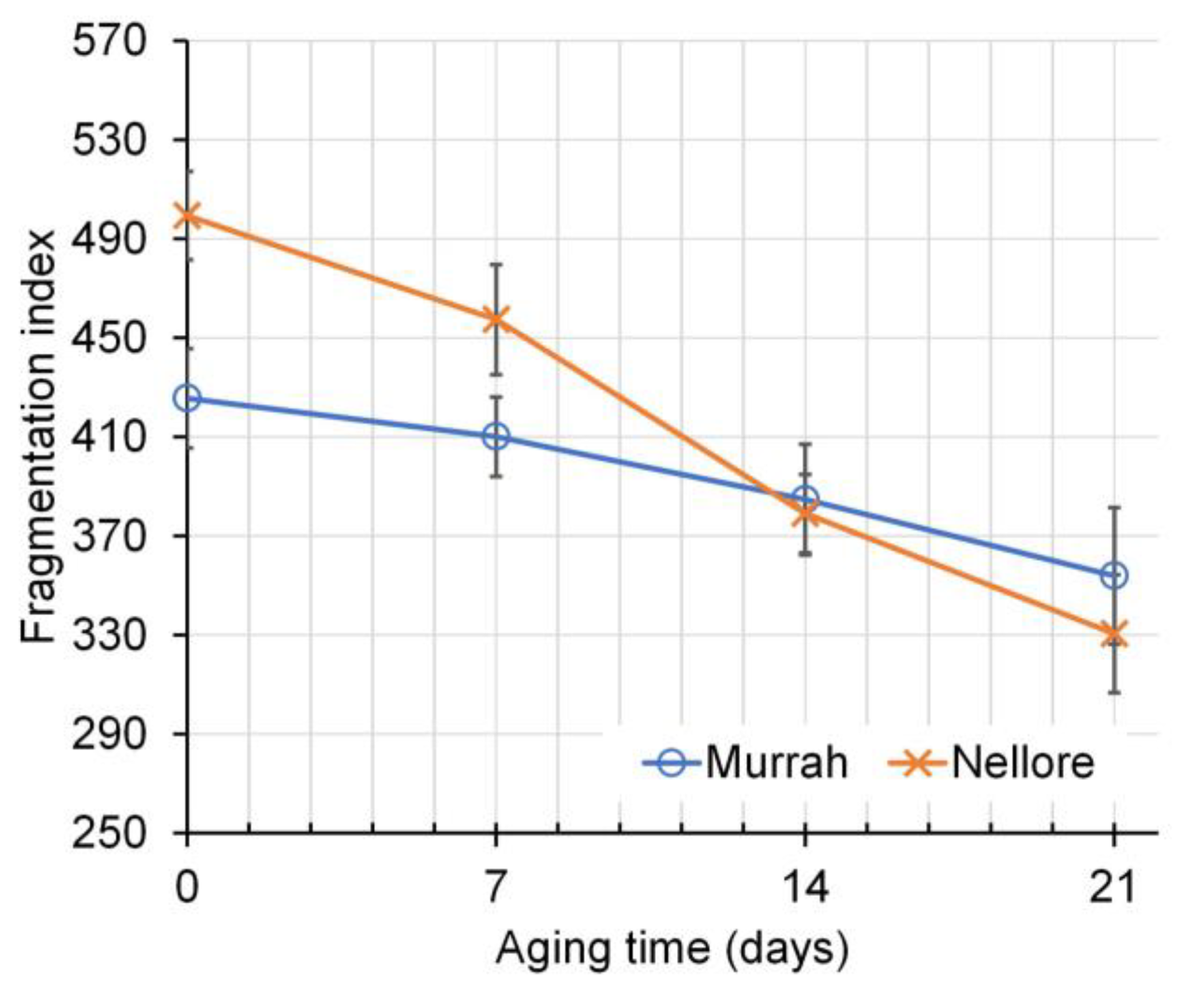
| Fragmentation index, FI | 394 | 441 | 447 | 427 | 390 | 367 | 10 | 0.194 | 0.003 | 0.006 |
| Shear force, WBsSF (N) | 35.82 B | 56.40 A | 58.31 a | 51.21 b | 41.39 c | 33.53 d | 2.37 | <0.001 | <0.001 | 0.186 |
| Myoglobin redox forms | ||||||||||
| Oxymyoglobin, OMb (%) | 62.77 A | 67.11 B | 62.31 | 66.44 | 67.02 | 64.80 | 1.02 | 0.036 | 0.236 | 0.711 |
| Deoxymyoglobin, DMb (%) | 7.82 A | 18.37 B | 18.91 a | 12.08 b | 11.17 b | 9.96 b | 1.01 | <0.001 | 0.002 | 0.855 |
| Metmyoglobin, MMb (%) | 29.41 A | 14.52 B | 18.77 | 21.48 | 21.81 | 25.24 | 1.19 | <0.001 | 0.365 | 0.296 |
| CIE color | ||||||||||
| Lightness, L* | 41.38 B | 43.67 A | 41.64 b | 43.12 ab | 43.59 a | 42.03 ab | 0.32 | <0.001 | 0.026 | 0.307 |
| Redness, a* | 25.18 A | 21.50 B | 22.22 | 24.29 | 23.67 | 22.94 | 0.37 | <0.001 | 0.093 | 0.506 |
| Yellowness, b* | 16.84 A | 14.57 B | 14.32 b | 16.38 a | 16.27 a | 15.87 a | 0.25 | <0.001 | 0.001 | 0.487 |
| Chrome, C* | 30.30 A | 26.00 B | 26.44 b | 29.30 a | 28.74 a | 27.91 ab | 0.44 | <0.001 | 0.030 | 0.494 |
| Hue, h (°) | 33.76 | 34.19 | 32.72 b | 33.99 b | 34.67 ab | 34.86 a | 0.25 | 0.200 | 0.004 | 0.686 |
| Murrah | Nellore | ||||||
|---|---|---|---|---|---|---|---|
| No. | Compound | Unaged | Aged | Unaged | Aged | Mean | SEM |
| ALCOHOLS | |||||||
| 1 | 1-Heptanol | nd | nd | nd | 26 | 26 | 3 |
| 2 | 1-Nonanol | nd | nd | nd | 36 | 36 | 4 |
| 3 | 1-Octanol | 151 | 23 | 285 | 126 | 146 | 21 |
| 4 | 1-Octan-3-ol | nd | nd | 3 | 51 | 27 | 4 |
| 5 | 2-Phenylethanol | nd | nd | nd | 354 | 534 | 46 |
| ALDEHYDES | |||||||
| 6 | Benzaldehyde | 287 | 175 | 105 | 207 | 194 | 46 |
| 7 | Phenylacetaldehyde | 32 | 1334 | nd | nd | 683 | 177 |
| 8 | Decanal | 30 | nd | 37 | nd | 34 | 12 |
| 9 | Heptanal | 95 | nd | 84 | nd | 90 | 19 |
| 10 | Hexanal | 620 | 1051 | 119 | nd | 597 | 129 |
| 11 | Nonanal | 2336 | 709 | 1806 | 520 | 1343 | 241 |
| 12 | Octanal | 474 | 52 | 234 | 60 | 205 | 58 |
| 13 | Tetradecanal | 253 | 276 | 79 | 439 | 262 | 32 |
| KETONES | |||||||
| 14 | 2-Heptanone | 7175 | 3008 | nd | nd | 5092 | 687 |
| 15 | Octan-3-one | 23 | 72 | nd | nd | 48 | 17 |
| ΣALCOHOLS | 151 | 23 | 288 | 773 | |||
| ΣALDEHYDES | 4127 | 3597 | 2464 | 1226 | |||
| ΣKETONES | 7198 | 3080 | nd | nd | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, B.F.; Rodrigues, L.M.; De Paula, L.M.A.F.; Torres Filho, R.d.A.; Fontes, P.R.; Ramos, E.M.; Ramos, A.d.L.S. A Comparative Study on Meat Quality Characteristics of Murrah Buffalo and Nellore Cattle Commercialized in Southeastern Brazil. Ruminants 2023, 3, 172-181. https://doi.org/10.3390/ruminants3030016
Andrade BF, Rodrigues LM, De Paula LMAF, Torres Filho RdA, Fontes PR, Ramos EM, Ramos AdLS. A Comparative Study on Meat Quality Characteristics of Murrah Buffalo and Nellore Cattle Commercialized in Southeastern Brazil. Ruminants. 2023; 3(3):172-181. https://doi.org/10.3390/ruminants3030016
Chicago/Turabian StyleAndrade, Bruna Fernandes, Lorena Mendes Rodrigues, Luiza Maria Amaral Frossard De Paula, Robledo de Almeida Torres Filho, Paulo Rogério Fontes, Eduardo Mendes Ramos, and Alcinéia de Lemos Souza Ramos. 2023. "A Comparative Study on Meat Quality Characteristics of Murrah Buffalo and Nellore Cattle Commercialized in Southeastern Brazil" Ruminants 3, no. 3: 172-181. https://doi.org/10.3390/ruminants3030016





