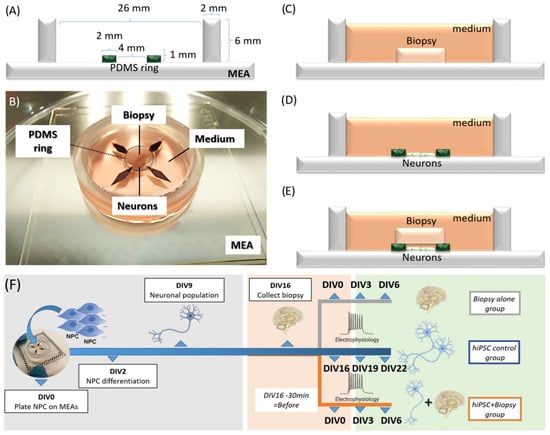
BioMEMS
A topical collection in Applied Sciences (ISSN 2076-3417). This collection belongs to the section "Applied Biosciences and Bioengineering".
Viewed by 6419Editor
Interests: medical devices; MEMS; microfluidics; insulin micropumps; implantable pumps; passive flow control valves; hydrocephalus shunts; microneedles; wearable bolus injectors
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
BioMEMS, in its wider acceptance, refers to any biomedical device that is partly or fully made using any microfabrication process and which therefore has at least one micrometric or submicrometric feature. Examples of bioMEMS devices include micropumps, lab-on-a-chip, organ-on-a-chip, DNA microarray, chemical sensor array, retina array, neuroMEMS, cell chips, etc.
The introduction of Clearblue, a paper-based pregnancy test, in the 1980s and, more importantly, the development of polydimethylsiloxane (PDMS) by George Whitesides’s group in the 1990s, which became the material of choice for rapid prototyping of microdevices for use with biological samples, gave a major boost to bioMEMS research. Today, the BioMEMS market continues to grow at an accelerated rate due to the increasing demand for homecare devices, in vitro diagnostics, and other wearable and implantable analytical systems.
In the biomedical field, miniaturized devices offer many advantages over conventional methods, including, among others, improved mass transfer and heat exchange, precise control of mixing, small sample size and reagent use, greater reliability and sensitivity, and lower manufacturing costs.
This Topical Collection covers research in diagnostic and therapeutic applications of bioMEMS and explores the design, characterization, modeling, and integration of microdevices with DNA, cells, and tissues.
Researchers are invited to submit original research papers, review articles, and short communications covering all aspects of bioMEMS, including but not limited to recent developments in the following areas:
- MEMS/NEMS for biomedical applications;
- Microfluidics and electrokinetics;
- Drug delivery devices;
- Wearable devices, implantable devices;
- Cell chips, cell-related studies;
- Organ-on-a-chip, Tissue microengineering;
- DNA amplification and detection;
- Diagnostics, physiological monitoring;
- Biosensors, bioelectronics;
- Wireless sensors, RF safety;
- Microactuators, micro-robots;
- Biocompatible materials, packaging.
We particularly encourage researchers to address and discuss the technical challenges associated with the development, manufacture, and testing of the different parts (actuators, sensors, materials, packaging, etc.) of bioMEMS devices.
Dr. Eric Chappel
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Applied Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- bioMEMS
- drug delivery
- diagnostics
- cells
- DNA
- organs and tissues