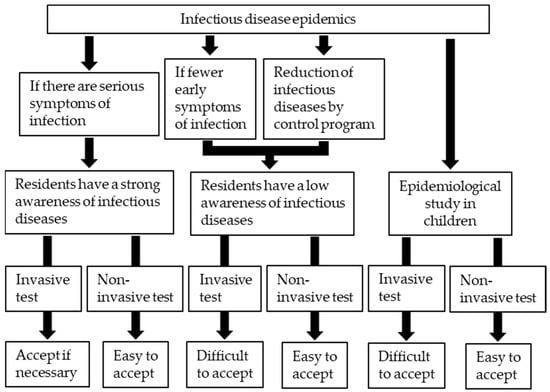
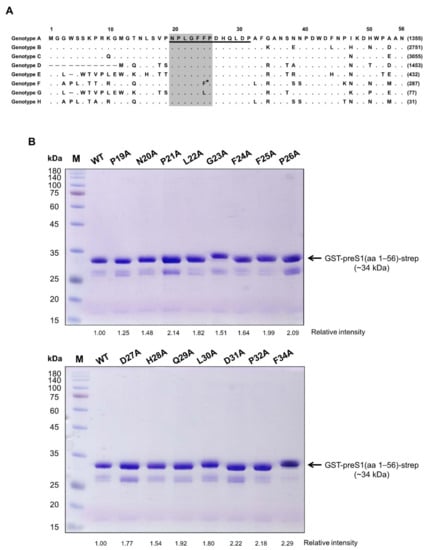
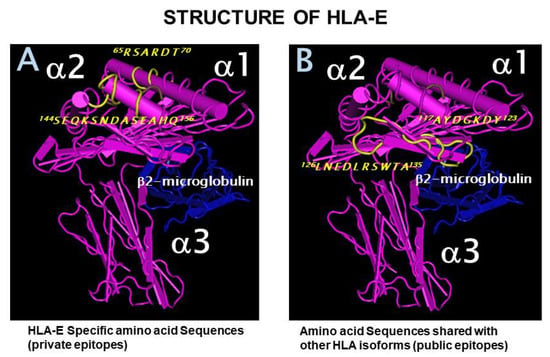
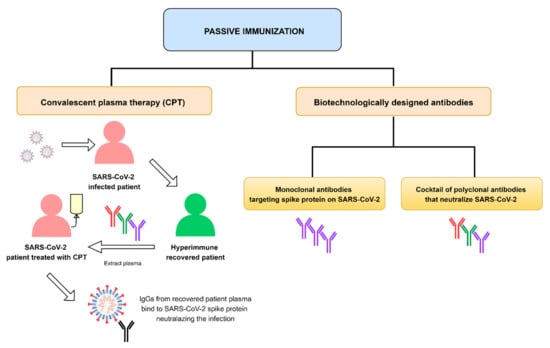
Research on Monoclonal Antibodies and Antibody Engineering
A topical collection in Vaccines (ISSN 2076-393X). This collection belongs to the section "Therapeutic Vaccines and Antibody Therapeutics".
Viewed by 78381Editor
Interests: antibody gene-based prophylaxis and therapy; physiology of antibody; DNA immunization; gene therapy; gene transfer; vaccine; adjuvant; influenza virus; immunology; virology
Topical Collection Information
Dear Colleagues,
Our Topic Collection, focuses on passive immune prophylaxis and therapy using monoclonal antibodies (mAbs) for a variety of diseases such as infection, cancer, and autoimmune disease. Passive immunization has a long history, dating back to the production of anti-tetanus and anti-diphtheria serum in the late 19th century by Kitasato and Behring. Inoculation with neutralizing antibodies is generally expected to induce a rapid and potent protective and therapeutic effect, independent of the individual’s immunocompetence. As you well know, current mAb-based products have attracted much attention for being one of the most effective molecular target drugs, and have also financially succeeded in developed markets. However, several obstacles hinder the widespread use of antibody drugs, such as a high production cost, the necessity of weekly or biweekly infusions due to short half-life (approximately 20 days), quality control, laborious development of the effective mAbs, shedding the target, antigenic variations, and unwanted side effects.
To address the above problems, and to share your interesting knowledge in the field of passive immunization, we are inviting you to submit an original research or review to this Topic Collection on one of the following topics:
(i) The methods of administration; e.g., antibody gene based-passive prophylaxis or therapy.
(ii) Antibody engineering; e.g., bispecific T-cell engager (BiTE), camelid variable domain of heavy chain only antibody (VHH), multivalent multidomain antibody (MDAb), defucosylated antibodies, and engineered Fc domain.
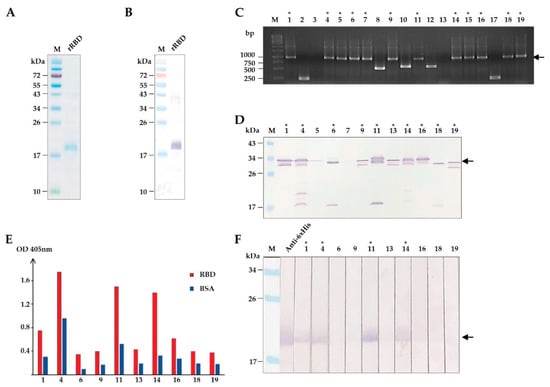
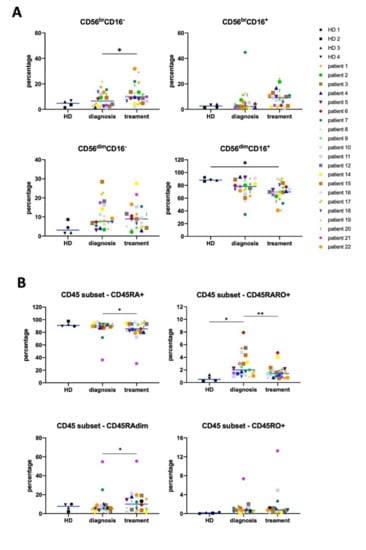
(iii) How to develop the effective mAbs; e.g., single-cell RNA sequencing (scRNA-seq), DNA immunization, adjuvant, double trans-chromosomic mouse, and ex vivo affinity maturation.
We also welcome to general remarks or details about your interesting mAbs on the following topic:
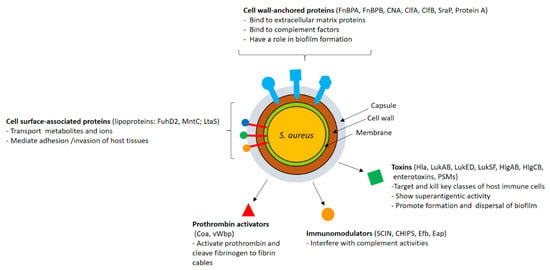
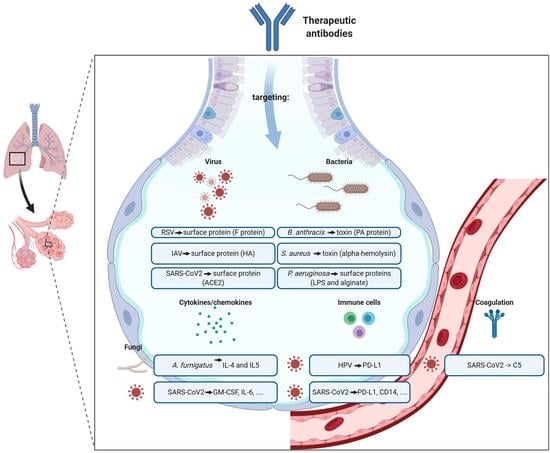
(iv) Prophylactic or therapeutic mAb against virus (e.g., influenza virus, SARS-CoV-2, HIV, dengue virus, and RS virus), bacteria (e.g., Clostridium difficile and Bacillus anthracis), parasite (e.g. malaria), fungus (e.g. Aspergillus), cancer, autoimmune disease, allergy, and obesity.
We are looking forward to your interesting manuscript.
Dr. Tatsuya Yamazaki
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Vaccines is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- passive immunization
- antibody gene-based passive prophylaxis or therapy
- antibody engineering
- monoclonal antibody
- infectious diseases
- cancer
- autoimmune diseases
- allergy
- obesity