Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Doughs and Bread Preparation
2.3. Microbial Quantification during the Process
2.4. pH Changes during the Process
2.5. Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry (SPME-GC-MS)
2.6. Statistical Analyses
3. Results
3.1. Microbial Quantification and pH Values during the Process
3.2. Analysis of the Volatilome
3.2.1. Quantification of VOCs before fermentation
3.2.2. Effect of Fermentation
3.2.3. Quantifications of the Main Fermentation Metabolites
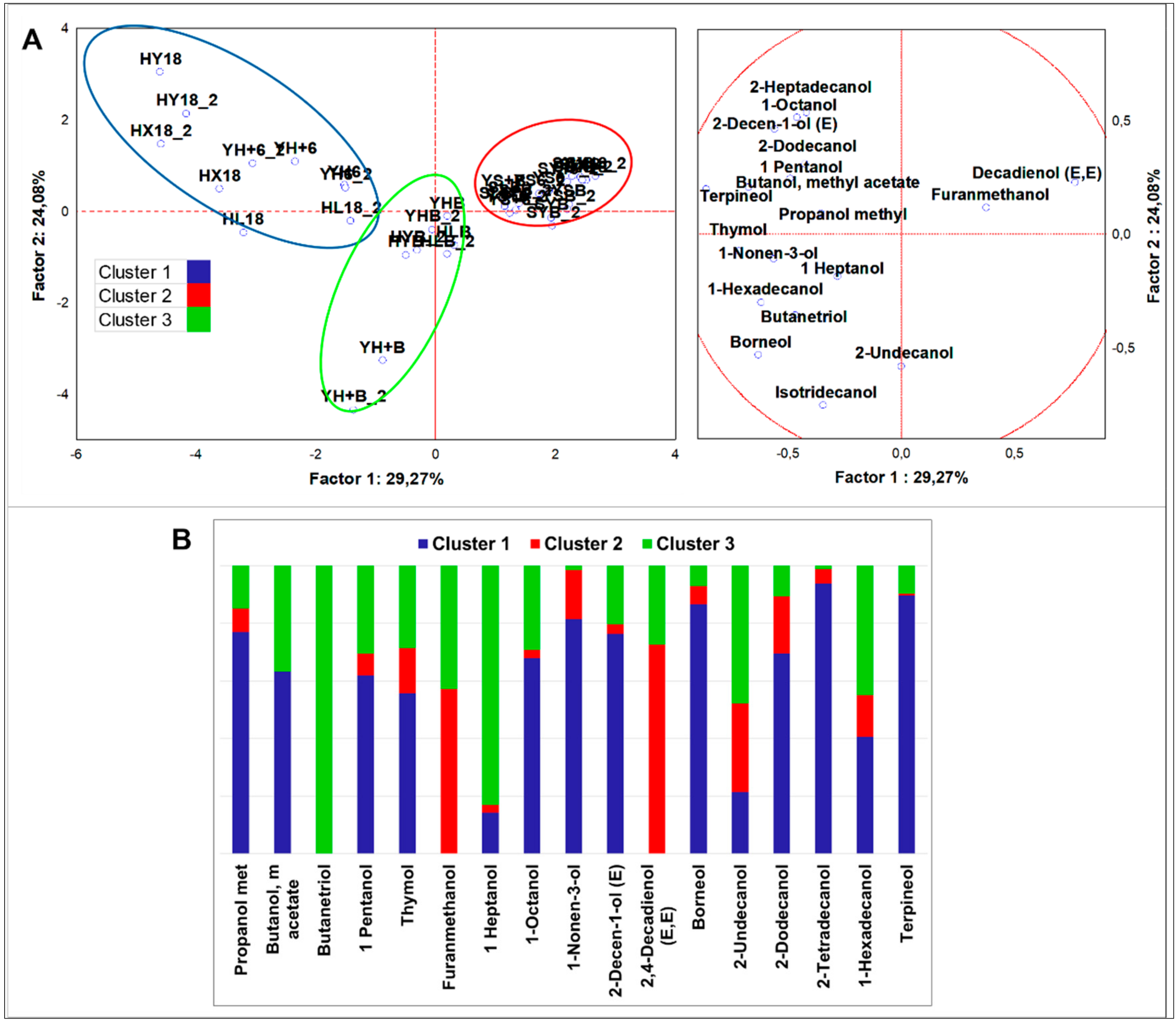
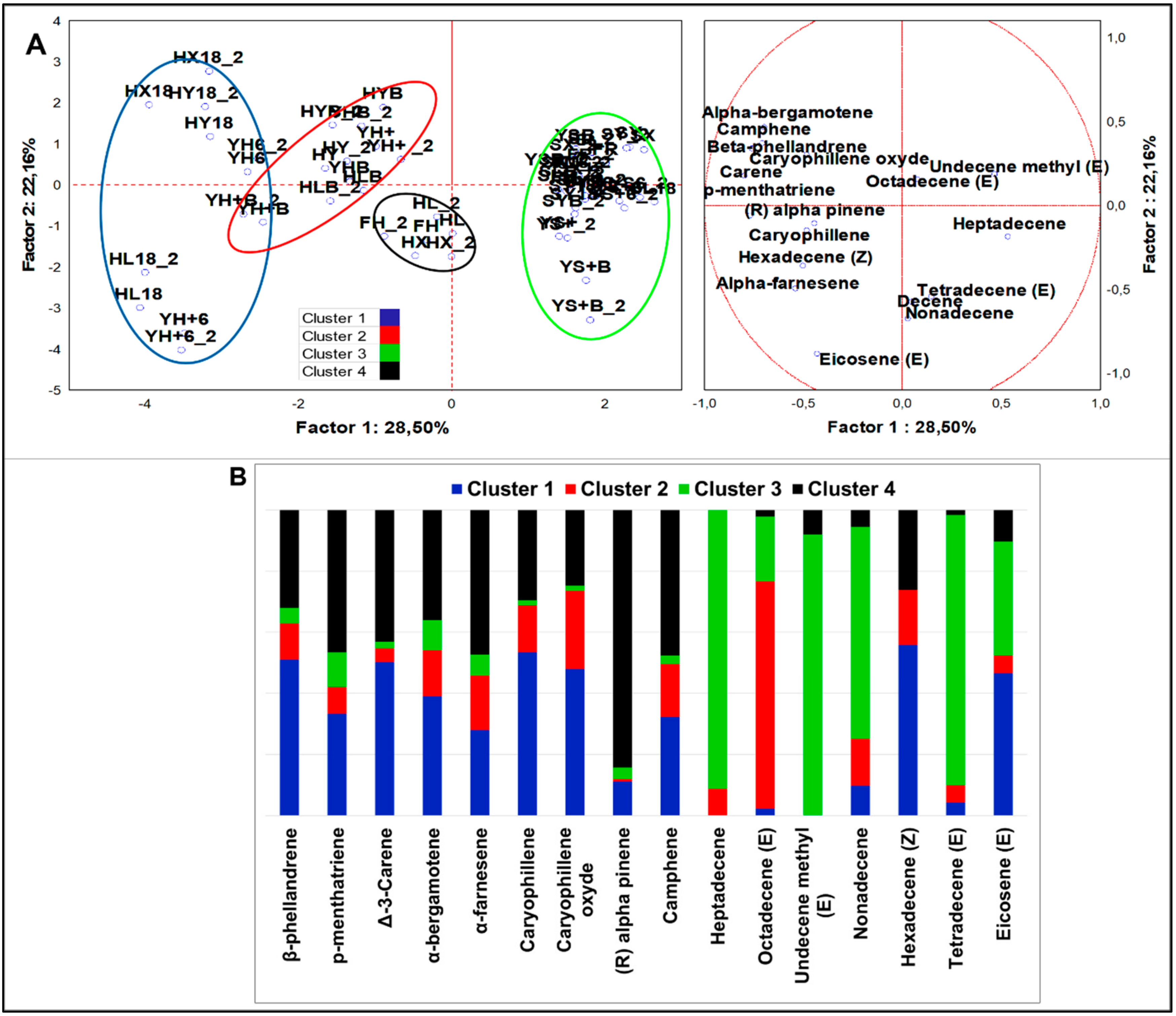
3.2.4. Multivariate Analysis of VOCs Organized by Different Chemical Classes
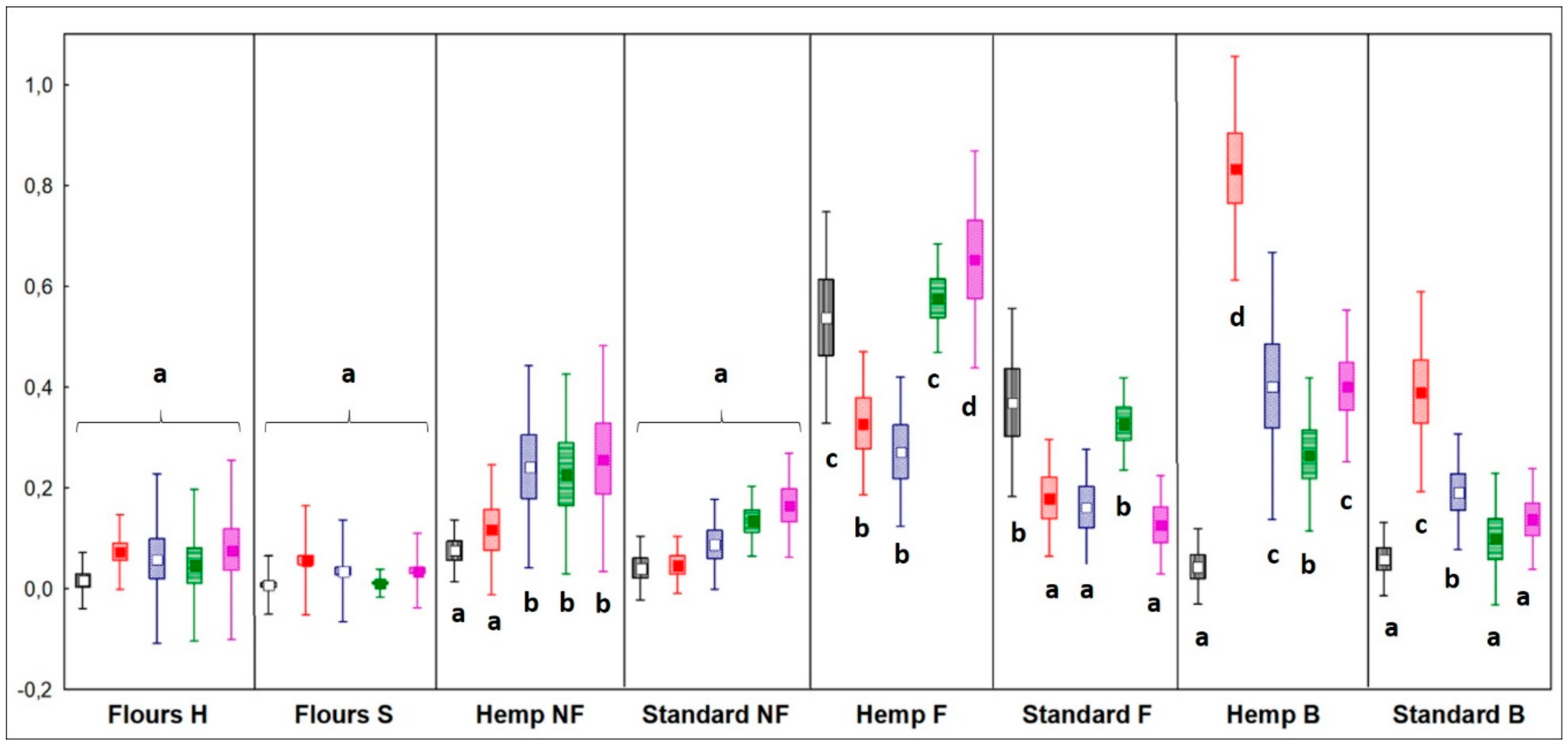
Alcohols
Aldehydes
Ketones
Alkenes
Organic Acids
4. Discussion
4.1. Microbial Growth and Major Fermentation Metabolites
4.2. Multivariate Analysis of VOCs Sorted by Chemical Class
4.2.1. Alcohols
4.2.2. Aldehydes
4.2.3. Alkenes
4.2.4. Ketones
4.2.5. Organic Acids
4.2.6. Overall Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allen, B.; Orfila, C. The Availability and Nutritional Adequacy of Gluten-Free Bread and Pasta. Nutrients 2018, 10, E1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.A.; EI Sohly, H.N.; EI Kashoury, E.A.; El Sohly, M.A. Fatty acids of Cannabis seeds. Phytochem. Anal. 1996, 7, 279–283. [Google Scholar] [CrossRef]
- El Sohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten-free bread. LWT-Food Sci. Technol 2017. [Google Scholar] [CrossRef]
- Gobbetti, M.; Di Cagno, R.; de Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Ipek Ayseli, Y. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci Technol. 2016, 48, 69–77. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Barbosa-Pereira, L.; Ferrocino, I.; Cocolin, L. Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits. Nutrients 2019, 11, 884. [Google Scholar] [CrossRef] [Green Version]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Taneyo-Saa, D.T.; Nissen, L.; Gianotti, A. Metabolomic approach to study the impact of flour type and fermentation process on volatile profile of bakery products. Food Res. Int. 2019, 119, 510–516. [Google Scholar]
- Kim, E.J.; Park, S.E.; Seo, S.H.; Kweon, O.C.; Son, H.S. A GC–MS based metabolic profiling of fermented tomato by lactic acid bacteria. Appl. Biol. Chem. 2019, 62, 2. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Farrés, A.; Díaz-Ruiz, G.; Sánchez, S.; Wacher, C.; Rodríguez-Sanoja, R. Omics in traditional vegetable fermented foods and beverages. Crit. Rev. Food Sci. Nutr. 2018, 60, 791–809. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Dei Più, L.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Taneyo-Saa, D.T.; Di Silvestro, R.; Nissen, L.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on bioactive fiber compounds in immature and ripe wheat flour bread. Food Sci. Technol. 2018, 89, 322–328. [Google Scholar]
- Castillo, M.; Martin-Orue, S.M.; Manzanilla, E.G.; Badiola, I.; Martin, M.; Gasa, M.J. Quantification of total bacteria, enterobacteria and LAB populations in pig digesta by real-time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef]
- Foschino, R.; Gallina, S.; Andrighetto, C.; Rossetti, L.; Galli, A. Comparison of cultural methods for the identification and molecular investigation of yeasts from sourdoughs for Italian sweet baked products. FEMS Yeast Res. 2004, 4, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Nissen, L.; Demircan, B.; Taneyo-Saa, D.L.; Gianotti, A. Shift of Aromatic Profile in Probiotic Hemp Drink Formulations: A Metabolomic Approach. Microorganisms 2019, 7, 509. [Google Scholar] [CrossRef] [Green Version]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic potential of hemp blended drinks fermented by probiotics. Food Research International. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef]
- Granato, D.; de Araujo Calado, M.V.; Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Vernia, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, L.; Gu, R.; Zhao, J.; Hou, X.; Zhu, H. Hydroxy-pentanones production by Bacillus sp. H15-1 and its complete genome sequence. J. Biotechnol. 2017, 259, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Petel, C.; Prost, C.; Onno, B. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In vitro antimicrobial activities and mechanism of 1-octen-3-ol against food-related bacteria and pathogenic fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Diniz-Silva, H.T.; Ramalho Brandão, L.; de Sousa Galvão, M.; Madruga, M.S.; Ferreira Maciela, J.; Leite de Souza, E.; Magnani, M. Survival of Lactobacillus acidophilus LA-5 and Escherichia coli O157:H7 in Minas Frescal cheese made with oregano and rosemary essential oils. Food Microbiol. 2020, 86, 103348. [Google Scholar] [CrossRef]
- Stan, M.S.; Voicu, S.N.; Caruntu, S.; Nica, I.C.; Olah, N.-K.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; et al. Antioxidant and Anti-Inflammatory Properties of a Thuja occidentalis Mother Tincture for the Treatment of Ulcerative Colitis. Antioxidants 2019, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Horvathova, E.; Kozics, K.; Srancikova, A.; Hunakova, L.; Galova, E.; Sevcovicova, A.; Slamenova, D. Borneol administration protects primary rat hepatocytes against exogenous oxidative DNA damage. Mutagenesis 2012, 27, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Cusimano, M.G.; Di Stefano, V.; La Giglia, M.; Di Marco Lo Presti, V.; Schillaci, D.; Pomilio, F.; Vitale, M. Control of Growth and Persistence of Listeria monocytogenes and β-Lactam-Resistant Escherichia coli by Thymol in Food Processing Settings. Molecules 2020, 25, 383. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef] [Green Version]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 25 February 2020).
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Yang, J.-J.; Song, X.-Z.; Wang, Y.-F.; Corlett, R.T.; Xu, Y.-K.; Hu, H.-B. Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules 2020, 25, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Habib, S.; Gupta, P.; Bhat, S.; Gupta, J. In silico, in-vitro and in vivo screening of biological activities of citral. Int. J. Vitam. Nutr. Res. 2020. [Google Scholar] [CrossRef]
- Garcia, M.V.; Copetti, M.V. Alternative methods for mould spoilage control in bread and bakery products. Int. Food Res. J. 2019, 26, 737–749. [Google Scholar]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Synergistic inhibition effect of citral and eugenol against Aspergillus niger and their application in bread preservation. Food Chem. 2020, 310, 25. [Google Scholar] [CrossRef]
- Martins, H.B.; Selis, N.D.; Souza, C.L.; Nascimento, F.S.; Pacheco de Carvalho, S.; D’Oliveira Gusmão, L.; dos Santos Nascimento, J.; Pereira Brito, K.; Itana de Souza, S.; Vasconcelos de Oliveira, M.; et al. Anti-Inflammatory Activity of the Essential Oil Citral in Experimental Infection with Staphylococcus aureus in a Model Air Pouch. Evid. Based Complementary Altern. Med. 2017. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.A.; Lima, B.S.; Trindade, G.G.G.; Souza, E.P.B.S.S.; Mota, D.S.A.; Heimfarth, L.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Sussuchi, E.M.; Sarmento, V.H.V.; et al. Anti-hyperalgesic and anti-inflammatory effects of citral with β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes in animal models. Life Sci. 2019, 229, 139–148. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical composition of commercial cold-pressed pomegranate (Punica granatum) seed oil from Turkey and Israel, and the use of bioactive compounds for samples’ origin preliminary discrimination. J. Food Compos. Anal. 2019, 75, 8–16. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus species (Ericaceae) as source of valuable bioactive products. Crit. Rev. Food Sci. Nutr. 2019, 59, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Badeka, A.V. Prickly pear seed oil by shelf-grown cactus fruits: Waste or Maste? Processes 2020, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Božik, M.; Cejnar, P.; Šašková, M.; Nový, P.; Maršík, P.; Klouček, P. Stress response of Escherichia coli to essential oil components—Insights on low-molecular-weight proteins from MALDI-TOF. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hermier, D.; Lan, A.; Tellier, F.; Blais, A.; Grauso Culetto, M.; Mathé, V.; Bellec, Y.; Gissot, L.; Schmidely, P.; Faure, J.D. Intestinal Availability and Metabolic Effects of Dietary Camelina Sphingolipids during the Metabolic Syndrome Onset in Mice. J. Agric. Food Chem. 2020, 68, 788–798. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.; Vieira, J.L.; Raposo, N.R.; Dutra, R.C. (-)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Aly, E.; Khajah, M.A.; Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2020, 25, 106. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.J.; Yang, J.Y.; Lee, M.H.; Kim, H.W.; Kwon, H.J.; Park, M.; Kim, S.-K.; Park, S.-Y.; Kim, S.-H.; Kim, J.-B. Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 1008. [Google Scholar] [CrossRef] [Green Version]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Geddo, F.; Scandiffio, R.; Antoniotti, S.; Cottone, E.; Querio, G.; Maffei, M.E.; Bovolin, P.; Gallo, M.P. PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients 2019, 11, 2788. [Google Scholar] [CrossRef] [Green Version]
- Kumawat, V.S.; Kaur, G. Insulinotropic and antidiabetic effects of β-caryophyllene with l-arginine in type 2 diabetic rats. J. Food Biochem. 2020, 2020, e13156. [Google Scholar] [CrossRef]
- Nissen, L.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Al-Taee, H.; Azimullah, S.; Nagoor Meeran, M.F.; Alaraj Almheiri, M.K.; Al Jasmi, R.A.; Tariq, S.; Khan, M.A.B.; Adeghate, E.; Ojha, S. Beta-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Sonnino, S.; Mauri, L. GM1 Ganglioside Is A Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, C.L.; Norris, G.H.; Vitols, A.; Garcia, C.; Seibel, S.; Anto, L.; Blesso, C.N. Dietary Egg Sphingomyelin Prevents Aortic Root Plaque Accumulation in Apolipoprotein-E Knockout Mice. Nutrients 2019, 11, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuser, F.; Zorn, H.; Berger, R.G. Generation of Odorous Acyloins by Yeast Pyruvate Decarboxylases and Their Occurrence in Sherry and Soy Sauce. J. Agric. Food Chem. 2000, 48, 6191–6195. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Schleining, G.; Krungleviciute, V.; Zadeike, D.; Zavistanaviciute, P.; Dimaite, I.; Kuzmaite, I.; Riskeviciene, V.; Joudeikine, G. Development and quality evaluation of lacto-fermented product based on hulled and not hulled hempseed (Cannabis sativa L.). Food Sci. Technol. 2016, 72, 544–551. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Martena, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef]
- Rego Costa, A.C.; Rosado, E.L.; Soares-Mota, M. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: A systematic review. Nutr. Hosp. 2012, 27, 103–108. [Google Scholar] [PubMed]
- Ji, H.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic antimicrobial activities of essential oil vapours against Penicillium corylophilum on a laboratory medium and beef jerky. Int. J. Food Microbiol. 2019, 291, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Císarová, M.; Hleb, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboňc, J.; Kováčikd, A.; Foltinová, D.; Božike, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapour phase against bread spoilage toxicogenic aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Matulyte, I.; Jekabsone, A.; Jankauskaite, L.; Zavistanaviciute, P.; Sakiene, V.; Bartkiene, E.; Ruzauskas, M.; Kopustinskiene, D.M.; Santini, A.; Bernatoniene, J. The Essential Oil and Hydrolats from Myristica fragrans Seeds with Magnesium Aluminometasilicate as Excipient: Antioxidant, Antibacterial, and Anti-inflammatory Activity. Foods 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Gao, J.; Wang, X.; Wang, W.; Dong, J.; Zhang, Y.; Wang, S. Formation and Alterations of the Potentially Harmful Maillard Reaction Products during the Production and Storage of Brown Fermented Milk. Molecules 2019, 24, 272. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, A.S.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Chemical Composition of Pyroligneous Acid Obtained from Eucalyptus GG100 Clone. Molecules 2018, 23, 426. [Google Scholar] [CrossRef] [Green Version]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, L.; Tabaszewska, M.; Wywrocka-Gurgu, A. Hemp flour as a valuable component for enriching physicochemical andantioxidant properties of wheat bread. LWT-Food Sci. Technol. 2019, 102, 164–172. [Google Scholar] [CrossRef]
- Srivastava, R.; Bousquières, J.; Cepeda-Vázquez, M.; Roux, S.; Bonazzi, C.; Rega, B. Kinetic study of furan and furfural generation during baking of cake models. Food Chem. 2018, 267, 329–336. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, K.; Wang, Y.-T.; Wu, J.-K.; Sui, Y.; Liang, X.-Z.; Yu, L.-Z.; Wu, X.-C.; Wang, P.-M.; Xu, J.-Z.; et al. Global analysis of furfural induced genomic instability using a yeast model. Appl. Environ. Microbiol. 2019, 85, e01237-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, M.; Garde, S.; Fernández-García, E.; Medina, M.; Nuñez, M. Effect of High-Pressure Treatment and a Bacteriocin-Producing Lactic Culture on the Odor and Aroma of Hispánico Cheese: Correlation of Volatile Compounds and Sensory Analysis. J. Agric. Food Chem. 2006, 54, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Härtl, K.; McGraphery, K.; Hoffmann, T.; Schwab, W. Attractive but toxic: Emerging roles of glycosidically bound volatiles and glycosyltransferases involved in their formation. Mol. Plant 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample | Description |
|---|---|
| FH | Hemp seed flour |
| FM | Maize flour |
| FR | Rice flour |
| HX | Hemp seed dough not inoculated (direct) |
| HL | Hemp seed dough LAB inoculated (direct) |
| HY | Hemp seed dough S. cerevisiae LBS inoculated (direct) |
| SX | Standard dough not inoculated (direct) |
| SL | Standard dough LAB inoculated (direct) |
| SY | Standard dough S. cerevisiae LBS inoculated (direct) |
| YH+ | Hemp seed dough added with sourdough |
| YS+ | Standard dough added with sourdough |
| HX18 | HX fermented 18 h |
| HL18 | HL fermented 18 h |
| HY18 | HY fermented 18 h |
| SX18 | SX fermented 18 h |
| SL18 | SL fermented 18 h |
| SY18 | SY fermented 18 h |
| YH+6 | YH+ fermented 6 h |
| YS+6 | YS+ fermented 6 h |
| YH6 | YH * fermented 6 h |
| YS6 | YS * fermented 6 h |
| YH+B | Bread from YH+6 |
| YS+B | Bread from YS+6 |
| YHB | Bread from YH6 |
| YSB | Bread from YS6 |
| HLB | Bread from HL18 |
| HYB | Bread from HY18 |
| SLB | Bread from SL18 |
| SYB | Bread from SY18 |
| Sample | LAB | S. cerevisiae LBS |
|---|---|---|
| SX | 2.60 ± 0.04 a | 3.90 ± 0.05 b |
| SL | 7.02 ± 0.26 c | 2.30 ± 0.09 a |
| SY | 5.30 ± 0.06 b | 6.60 ± 0.07 c |
| HX | 3.78 ± 0.08 b | 4.48 ± 0.04 b |
| HL | 7.15 ± 0.25 c | 3.48 ± 0.05 a |
| HY | 3.30 ± 0.08 a | 6.60 ± 0.09 c |
| SX18 | 6.32 ± 0.10 b | 5.90 ± 0.11 b |
| SL18 | 9.58 ± 0.34 d | 5.00 ± 0.11 b |
| SY18 | 6.45 ± 0.14 c | 7.84 ± 0.04 c |
| HX18 | 7.31 ± 0.29 c | 6.23 ± 0.04 c |
| HL18 | 9.74 ± 0.19 d | 6.90 ± 0.14 c |
| HY18 | 6.60 ± 0.15 c | 8.72 ± 0.16 d |
| YS+ | 9.86 ± 0.24 d | 6.92 ± 0.07 c |
| YH+ | 9.81 ± 0.21 d | 6.99 ± 0.13 c |
| YS | 5.71 ± 0.22 c | 6.33 ± 0.14 b |
| YH | 4.31 ± 0.15 b | 6.20 ± 0.07 b |
| YS+6 | 10.97 ± 0.31 d | 7.27 ± 0.11 c |
| YH+6 | 11.16 ± 0.23 d | 8.23 ± 0.19 c |
| YS6 | 6.53 ± 0.19 c | 7.14 ± 0.14 c |
| YH6 | 5.42 ± 0.09 b | 6.89 ± 0.14 c |
| Sample | Ethyl alcohol | Acetic acid | 2-butanone-3-hydroxy | 1,4-Butanediol |
|---|---|---|---|---|
| FR | tr. * | n.d. | n.d. | tr. |
| FM | n.d.† | n.d. | n.d. | n.d. |
| FH | n.d. | n.d. | n.d. | n.d. |
| HX | n.d. | n.d. | n.d. | n.d. |
| HL | tr. | 0.34 ± 0.03 a | n.d. | n.d. |
| HY | tr. | 0.13 ± 0.05 a | n.d. | n.d. |
| SX | tr. | n.d. | n.d. | n.d. |
| SL | tr. | n.d. | n.d. | tr. |
| SY | tr. | n.d. | n.d. | tr. |
| YH+ | 6.79 ± 1.06 b | 1.82 ± 0.82 a | 0.42 ± 0.17 a | 7.67 ± 1.02 c |
| YS+ | 4.03 ± 0.72 b | 0.33 ± 0.11 a | 0.37 ± 0.09 a | 6.46 ± 1.32 b |
| HX18 | 14.10 ± 0.79 c | 11.98 ± 0.68 c | 9.40 ± 0.69 c | 3.33 ± 0.56 b |
| HL18 | 25.97 ± 0.69 c | 25.18 ± 2.26 d | 12.44 ± 1.69 c | 8.85 ± 1.58 c |
| HY18 | 29.19 ± 3.00 c | 8.53 ± 1.83 c | 16.61 ± 1.99 c | 9.64 ± 1.23 c |
| SX18 | 16.17 ± 2.08 c | 2.94 ± 0.07 b | 7.11 ± 2.02 b | 2.02 ± 0.34 a |
| SL18 | 23.82 ± 1.54 c | 13.33 ± 1.57 c | 8.84 ± 0.99 c | 7.86 ± 0.49 c |
| SY18 | 21.30 ± 2.65 c | 6.31 ± 1.06 b | 9.08 ± 0.85 c | 9.90 ± 1.37 c |
| YH6 | 12.77 ± 1.90 c | 7.94 ± 0.42 c | 10.61 ± 1.44 c | 7.98 ± 1.28 c |
| YS6 | 13.74 ± 2.32 c | 6.44 ± 0.55 b | 6.12 ± 0.55 b | 8.40 ± 1.19 c |
| YH+6 | 15.03 ± 3.16 c | 18.60 ± 3.32 d | 10.14 ± 1.69 c | 23.75 ± 3.21 d |
| YS+6 | 16.19 ± 2.13 c | 13.26 ± 2.41 d | 11.84 ± 1.05 c | 18.93 ± 2.03 d |
| HLB | 0.11 ± 0.04 a | 8.04 ± 1.07 c | 6.34 ± 0.18 b | 3.13 ± 0.64 b |
| HYB | 0.17 ± 0.02 a | 4.10 ± 0.11 b | 10.29 ± 1.54 c | 3.56 ± 1.04 b |
| SLB | 0.45 ± 0.09 a | 2.73 ± 0.66 b | 4.45 ± 0.44 b | 2.22 ± 0.43 a |
| SYB | 0.39 ± 0.08 a | n.d. | 6.77 ± 0.99 b | 2.95 ± 0.78 b |
| YHB | 0.63 ± 0.12 a | 0.45 ± 0.28 a | 6.76 ± 1.12 b | 1.95 ± 0.32 a |
| YSB | 0.14 ± 0.09 a | tr. | 5.45 ± 1.30 b | 1.45 ± 0.78 a |
| YH+B | 3.63 ± 0.98 b | 7.99 ± 1.51 c | 8.88 ± 0.87 c | 6.99 ± 1.21c |
| YS+B | 2.87 ± 0.34 b | 5.33 ± 1.10 b | 6.87 ± 0.55 b | 4.33 ± 2.65 b |
| Compounds | Flavoring | Bioactivity | References |
|---|---|---|---|
| 1-heptanol | musty, pungent, leafy green | [18,23] | |
| 1-octen-3-ol | antimicrobial activity against spoilage and opportunistic microbes | [24] | |
| borneol | pine, wood, camphor | contrast spoilage microorganism bacterial foodborne and entero-pathogens; anti-inflammatory and antioxidant capacities for the treatment of ulcerative colitis; added to drinking water of rats for 7 days lowered the level of oxidative DNA lesions induced in their hepatocytes. | [23,25,26,27] |
| thymol | herbal, thyme, phenolic, medicinal, camphor | contrast spoilage microorganism bacterial foodborne and entero-pathogens; anti-inflammatory and antioxidant in human preadipocytes and in neuroprotection of rotenone-induced rat model of Parkinson’s disease. | [28,29,30,31] |
| terpineol | pine, terpene, lilac, citrus, woody, floral | contrast spoilage microorganism bacterial foodborne and entero-pathogens; anti-inflammatory and antioxidant in LPS-induced cell line. | [32,33,34] |
| octadienal dimethyl | nice aroma of lemon | counteract spoilage molds of breads; in vitro is reported to have antimicrobial potential to food borne and spoilage fungi; anti-inflammatory activity in experimental infection with pathogenic Staphylococcus aureus; anti-hyperalgesic effects in combination with β-cyclodextrins in animal models | [35,36,37,38,39,40] |
| 2-heptenal (Z) | pleasant almond flavor | associated to different plant-based products with anti-inflammatory and anti-oxidant activities. | [23,41,42,43] |
| ∆-3-carene | harsh, terpene-like, coniferous | active against spoilage microbes, food-borne pathogens, and pathogenic E. coli. | [31,44,45] |
| β-caryophillene oxide | dry, wood, cedarwood, carrot | anti-inflammatory and analgesic effects in different mouse models of inflammatory pain; antibacterial capacity versus Helicobacter pylori. | [31,46,47,48] |
| β-caryophillene | woody-spicy, dry and tenacious | known as “dietary cannabinoid”, it has been shown to be orally bioavailable; C. sativa essential oils bearing up to 13% of this compounds is effective against several opportunistic and spoilage microorganisms including Helicobacter pylori; prevents structural alteration of the myocardium; effective against LPS-induced oligodendrocyte toxicity; prevention of lipid accumulation and improvement of glucose uptake; insulinotropic and antidiabetic effects | [31,34,48,49,50,51,52,53] |
| Eicosene (E) | is a part constituting ceramide (Sphingosine); cardioprotective effects; on mouse model can be effective on treating metabolic disorder; in human plasma binds to high-density lipoprotein and exhibit anti-atherogenic properties | [45,54,55] | |
| 1-pentanone-3-hydroxy | caramel-sweet, buttery, and hay-like | is converted during glycosylation of toxic furanones | [56] |
| propanoic acid | typical sharp, acrid, vinegar, sour taste | Inhibition of ubiquitous bacilli, spoilage microbes and food-borne pathogens; prebiotics; fostering of the selective growth of probiotics in the gut; stimulation of epithelial immune function | [18,23,57,58] |
| lactic acid | sharp, acrid, vinegar, sour taste buttery nuance | inhibition of ubiquitous bacilli, spoilage microbes and food-borne pathogens | [18,23,57] |
| hexanoic acid | rancid-like | inhibition of molds in bread | [23,59] |
| heptanoic acid | rancid-like | [23] | |
| octanoic acid | rancid-like | binding to -OH of serine residues of ghrelin activate the hormone and regulate hunger; in combination to antioxidant compounds produces esters lipophenols that have stronger and more stable host antioxidant activity; | [23,59,60,61] |
| nonanoic acid | fatty, waxy, and cheesy with a mild sweet creamy background | effective on excessive calorie burning, inducing weight loss | [18,23,59,62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, L.; Bordoni, A.; Gianotti, A. Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach. Nutrients 2020, 12, 1050. https://doi.org/10.3390/nu12041050
Nissen L, Bordoni A, Gianotti A. Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach. Nutrients. 2020; 12(4):1050. https://doi.org/10.3390/nu12041050
Chicago/Turabian StyleNissen, Lorenzo, Alessandra Bordoni, and Andrea Gianotti. 2020. "Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach" Nutrients 12, no. 4: 1050. https://doi.org/10.3390/nu12041050
APA StyleNissen, L., Bordoni, A., & Gianotti, A. (2020). Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach. Nutrients, 12(4), 1050. https://doi.org/10.3390/nu12041050







