Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina
Abstract
:1. Introduction
2. Clinical Significance of Neurodegeneration in the Retina
2.1. Inherited Retinal Dystrophies
2.2. Optic Neuropathies
3. The Use of Zebrafish for Retinal Neuroprotection and Regeneration Studies
3.1. Advantages of the Zebrafish Animal Model
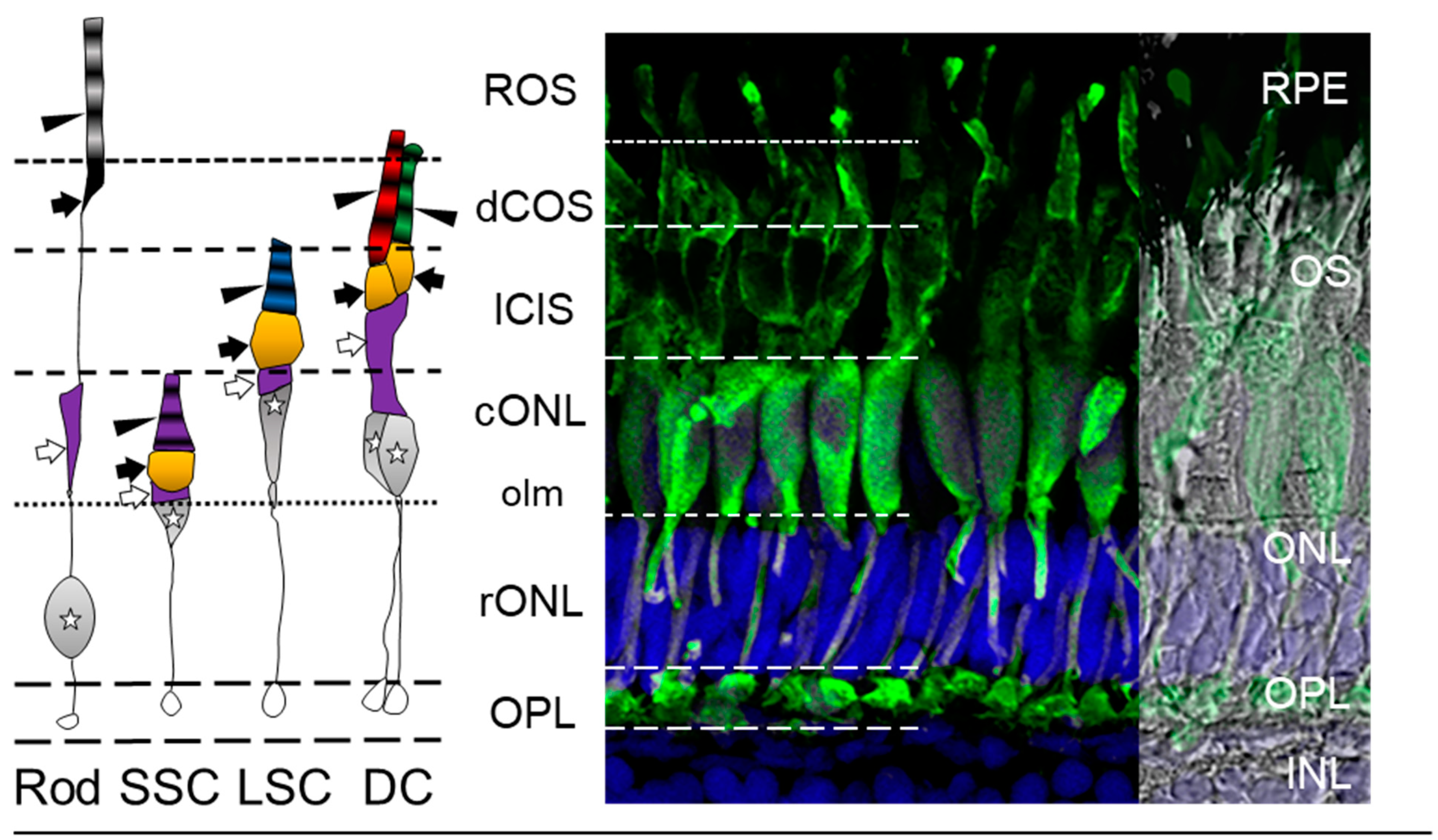
3.2. The Zebrafish Visual System
3.3. Zebrafish Visual Acuity
3.4. Müller Glia: Source of Regenerated Neurons in the Retina
4. Robust Endpoints for Retinal Neuroprotection Studies in Zebrafish
4.1. Behavioral Paradigms
4.1.1. Optokinetic/Optomotor Response (OKR/OMR)
4.1.2. Startle Response (SR)
4.1.3. Phototaxis or Phototactile Behavioral Response
4.2. Retinal Imaging
Optical Coherence Tomography
4.3. Functional Endpoints
4.3.1. Electroretinography
4.3.2. Ex Vivo Ca2+ Imaging of RGCs/Retinal Neurons
5. Neuroprotection and Regeneration in Zebrafish Retinal Injury Paradigms
5.1. Light-Induced Retinal Damage
| Retinal Injury Paradigm | Model | Age | Neuroprotective Agent or Mechanism | Reference |
|---|---|---|---|---|
| Light-Induced | ||||
| LIRD | Retinal degeneration | Larvae | EP300 (Histone acetyltransferase) | [164] |
| LIRD and ouabain | Retinal degeneration | Adult | SHH-N recombinant protein | [169] |
| Rose Bengal Light lesion | Retinal degeneration | Adult | Thiokynurenate (NMDA inhibitor) | [160] |
| Mechanical | ||||
| Optic nerve injury | RGC loss/injury | - | Neuroglobin | [195] |
| Chemical-Induced | ||||
| NMDA-induced neurodegeneration | Retinal degeneration | Adult | Resveratrol and MK-801 | [180] |
| Glaucoma | Adults | Resveratrol | [185] | |
| Acrylamide toxicity | Retinal Toxicity | Embryo | Carnosic acid | [176] |
| 6-OHDA | Night blindness | Larvae/adult | Stil-mediated Shh signaling | [179] |
| Oxidative Stress | ||||
| Hypoxia/reperfusion | Retinal degeneration | Embryo | HSF1 | [198] |
| Hypoxia | Hypoxia-driven retinal angiogenesis | Adult | Sunitinib and ZN323881 (anti-VEGF drugs) | [199] |
| Hydrogen peroxide | RGC degeneration | Larvae | Neurotrophins-magnetic nanoparticles | [200] |
| Paclobutrazol | Hypoxia | Embryo | Retinoic Acid | [186] |
| Age | Age-related oculopathy | Adult | Resveratrol | [201] |
| Diet-Induced | ||||
| MeHg-diet exposure | Retinal Toxicity | Embryo | Selenium | [95] |
| Genetically Targeted | ||||
| von Hippel-Lindau mutants | Vascular-driven retinopathies | Embryo | Sunitinib and 676475 | [202] |
| Gdf6 zebrafish mutants | Early onset retinal dystrophies | Embryo | Aminopropyl Carbazole, P7C3 | [203] |
| AMD, Tg(rho:hsa.HTRA1); RP, Tg(rho:hsa.RHO_Q344ter) | AMD and RP | Larvae | 6-boroV (HTRA1 inhibitor) | [204] |
| Tg line dyeucd6 | RP | Larvae | Tubastatin A (TST) | [205] |
| Retinal Injury Paradigm | Retinal Model | Age | Regenerative Target or Mechanism | Reference |
|---|---|---|---|---|
| Light-Induced | ||||
| LIRD | Retinal degeneration | Adult | Shh signaling | [169] |
| Several miRNAs | [168,206] | |||
| TGFβ signaling | [91] | |||
| β-catenin/Wnt signaling | [86] | |||
| Identified markers for stages of regeneration | [87] | |||
| Reported numerous gene expression profiles | [162] | |||
| Photoreceptor degeneration | Adult | Rho-associated coiled-coil kinase 2 (a and b) | [166] | |
| FGF signaling | [161] | |||
| Drgal1-L2 secretion | [159] | |||
| Reported numerous gene expression profiles | [158] | |||
| Adult/Larvae | Capn5 | [157] | ||
| Adult/Larvae/Embryo | Her4 expression | [174] | ||
| Laser Focal injury | Retinal injury | Adult | Microglia and Müller cell signaling | [153] |
| Mechanical, Light and Chemical retinal lesions | Retinal degeneration | Adult | Müller glia-derived progenitors | [193] |
| Retinal lesions and UV light damage | Retinal degeneration | Adult | Jak/Stat signaling and MG reprogramming | [175] |
| Mechanical | ||||
| Retinal stab injury | Retinal degeneration | Adult | Granuin 1 | [196] |
| Wnt signaling and GSK-3β inhibition | [194] | |||
| Retinal stab injury and optic nerve crush | Retinal degeneration | Adult | α1 Tubulin-expressing Muller glia | [92] |
| Rod photoreceptor ablation and retinal puncture | Age-related oculopathy | |||
| Optic nerve injury | Oxidative stress | Adult | Neuroglobin | [189] |
| RGC axon degeneration | Adult | Leukemia inhibitory factor | [192] | |
| RGC loss/injury | - | Neuroglobin | [195] | |
| Optic nerve crush | Optic nerve degeneration | Adult | Acute inflammatory response | [207] |
| Optic nerve injury | Adult | zRICH protein | [187] | |
| Larvae/Adult | Calretinin expression | [188] | ||
| Chemical-Induced | ||||
| Intravitreal injections of ouabain | Retinal degeneration | Adult | Microglia and the immune system | [182] |
| Purinergic signalling | [181] | |||
| Protemic profiles reported | [155] | |||
| Surviving Neurons | [208] | |||
| ADP | [177] | |||
| Light-damage and ouabain injections | Retinal degeneration | Adult | N-cadherin | [89] |
| Geneticcally Targeted | ||||
| Pde6cw59 mutants | Photoreceptor degeneration | Adult | Rip3 Kinase signalling | [209] |
| Embryo | Schisandrin B | [210] | ||
| Cell-specific ablation | Rod photoreceptor ablation and retinal puncture | Larvae/Adult | Microglial signaling | [211] |
| RPE ablation | Larvae/Adult | Wnt Signaling | [212] | |
| UV cone ablation | Larvae | H3 horizontal cells | [213] | |
| Neuronal Injury Paradigm | Model | Age | Reference |
|---|---|---|---|
| Light-Induced | |||
| LIRD | Photoreceptor degeneration | Adult | [165,170,172] |
| Focused Light lesion | Retinal degeneration and regeneration | Adult | [129,173] |
| Mechanical | |||
| Optic nerve crush | Optic nerve injury | Adults | [190] |
| Optic nerve remyelination | Adults | [191] | |
| Chemical | |||
| Ouabain | Inner retinal neuron regeneration | Adults | [88,143,214] |
| Acrylamide toxicity | Photoreceptor degeneration and regeneration | Adults | [178] |
| N-methyl-Nnitrosourea | Photoreceptor degeneration and regeneration | Adults | [103] |
| Cypermethrin | Retinal Toxicity | Adult | [183] |
| Diet-induced | |||
| SeMet-diet exposure | Retinal toxicity | Adult/Embryo | [184] |
| Glucose immersion | Diabetic Retinopathy | Adults | [144,145,215] |
| Gestational hyperglycemia | Embryo | [216] | |
| Genetically Targeted | |||
| ZFcerkl morpholino knockdown | Retinal dystrophies | Larvae/Adults | [217] |
| Tg lines Pde6cw59 and Xops:mCFPq13 | RP and cone-rod dystrophy | Embryos/Adults | [218] |
| pcare1rmc100/rmc100 | Diabetic Retinopathy | Adults | [219] |
| Tg bugeye mutants | Glaucoma | Larvae | [106,220,221] |
| NTR/MTZ Cell specific ablation | Rod photoreceptor ablation | - | [222] |
| Cone photoreceptor ablation | Larvae | [104,223] | |
| Bipolar Cell ablation | Larvae | [224,225] | |
5.2. Mechanical Retinal Damage
5.2.1. Retinal Stab Injury
5.2.2. Optic Nerve Crush/Injury
5.3. Chemical-Induced Retinal Damage
5.4. Oxidative Stress-Induced Retinal Injury
5.5. Diet-Induced Retinal Damage
5.6. Genetically Targeted Retinal Damage
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angueyra, J.M.; Kindt, K.S. Leveraging Zebrafish to Study Retinal Degenerations. Front. Cell Dev. Biol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Link, B.A.; Collery, R.F. Zebrafish Models of Retinal Disease. Annu Rev. Vis. Sci 2015, 1, 125–153. [Google Scholar] [CrossRef]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Maurer, C.M.; Huang, Y.Y.; Neuhauss, S.C. Application of zebrafish oculomotor behavior to model human disorders. Rev. Neurosci. 2011, 22, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef]
- Hood, D.C.; Ramachandran, R.; Holopigian, K.; Lazow, M.; Birch, D.G.; Greenstein, V.C. Method for deriving visual field boundaries from OCT scans of patients with retinitis pigmentosa. Biomed. Opt. Express 2011, 2, 1106–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolsley, C.J.; Silvestri, G.; O’Neill, J.; Saunders, K.J.; Anderson, R.S. The association between multifocal electroretinograms and OCT retinal thickness in retinitis pigmentosa patients with good visual acuity. Eye 2009, 23, 1524–1531. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.D.; Fleischhauer, J.C.; Gillies, M.C.; Sutter, F.K.; Helbig, H.; Barthelmes, D. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Krol, J.; Nelidova, D.; Daum, J.; Szikra, T.; Tsuda, B.; Juttner, J.; Farrow, K.; Scherf, B.G.; Alvarez, C.P.; et al. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 2014, 83, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Munch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Chizzolini, M.; Galan, A.; Milan, E.; Sebastiani, A.; Costagliola, C.; Parmeggiani, F. Good epidemiologic practice in retinitis pigmentosa: From phenotyping to biobanking. Curr. Genom. 2011, 12, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Na, K.H.; Kim, H.J.; Kim, K.H.; Han, S.; Kim, P.; Hann, H.J.; Ahn, H.S. Prevalence, Age at Diagnosis, Mortality, and Cause of Death in Retinitis Pigmentosa in Korea-A Nationwide Population-based Study. Am. J. Ophthalmol. 2017, 176, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha, A., Jr.; Woo, S.J.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Kapetanakis, V.V.; Chan, M.P.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2016, 100, 86–93. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Burr, J.; Ramsay, C.; Cooper, D.; Foster, P.J.; Friedman, D.S.; Scotland, G.; Javanbakht, M.; Cochrane, C.; Norrie, J.; et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. Lancet 2016, 388, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004, 82, 887–888. [Google Scholar]
- Quigley, H.A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Whitmore, A.V.; Libby, R.T.; John, S.W. Glaucoma: Thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog. Retin. Eye Res. 2005, 24, 639–662. [Google Scholar] [CrossRef]
- Gupta, N.; Yucel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef]
- Doozandeh, A.; Yazdani, S. Neuroprotection in Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindler, K.S.; Ventura, E.; Dutt, M.; Rostami, A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp. Eye Res. 2008, 87, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Balcer, L.J. Clinical practice. Optic neuritis. N. Engl. J. Med. 2006, 354, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Kale, N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain 2016, 8, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaphiades, M.S.; Kline, L.B. Optic neuritis. Compr. Ophthalmol. Update 2007, 8, 67–75; discussion 77–78. [Google Scholar] [PubMed]
- Nikoskelainen, E. Later course and prognosis of optic neuritis. Acta Ophthalmol. 1975, 53, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Cleary, P.A.; Anderson, M.M., Jr.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R.; et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N. Engl. J. Med. 1992, 326, 581–588. [Google Scholar] [CrossRef]
- Steel, D.H.; Waldock, A. Measurement of the retinal nerve fibre layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 505–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, V.; Manni, G.; Spadaro, M.; Colacino, G.; Restuccia, R.; Marchi, S.; Bucci, M.G.; Pierelli, F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2520–2527. [Google Scholar]
- Costello, F.; Coupland, S.; Hodge, W.; Lorello, G.R.; Koroluk, J.; Pan, Y.I.; Freedman, M.S.; Zackon, D.H.; Kardon, R.H. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 2006, 59, 963–969. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984, 91, 1464–1474. [Google Scholar] [CrossRef]
- Kempen, J.H.; O’Colmain, B.J.; Leske, M.C.; Haffner, S.M.; Klein, R.; Moss, S.E.; Taylor, H.R.; Hamman, R.F.; Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch. Ophthalmol. 2004, 122, 552–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Saaddine, J.B.; Chou, C.F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.; Klein, R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010, 304, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. European Consortium for the Early Treatment of Diabetic, R. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the pathogenesis of diabetic retinopathy: Molecular mechanisms and therapeutic implications. Curr. Med. Chem. 2013, 20, 3241–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, A.J. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundstrom, J.M.; Hernandez, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simo-Servat, O.; Garcia-Ramirez, M.; Barber, A.J.; et al. Proteomic Analysis of Early Diabetic Retinopathy Reveals Mediators of Neurodegenerative Brain Diseases. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trauma. In The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 5th ed.; Ehlers, J.P.; Shah, C.P. (Eds.) Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 12. [Google Scholar]
- Pirouzmand, F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J. Craniofac. Surg. 2012, 23, 516–520. [Google Scholar] [CrossRef]
- Berkelaar, M.; Clarke, D.B.; Wang, Y.C.; Bray, G.M.; Aguayo, A.J. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 1994, 14, 4368–4374. [Google Scholar] [CrossRef] [Green Version]
- Kermer, P.; Klocker, N.; Labes, M.; Thomsen, S.; Srinivasan, A.; Bahr, M. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. Febs Lett. 1999, 453, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic Retinal Ganglion Cell Death After Optic Nerve Transection or Crush in Mice: Delayed RGC Loss With BDNF or a Caspase 3 Inhibitor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Nicolas, F.M.; Sobrado-Calvo, P.; Jimenez-Lopez, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6095–6112. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Romero, C.; Aviles-Trigueros, M.; Jimenez-Lopez, M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolas, F.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-induced retinal ganglion cell death in adult mice: Quantitative and topographic time course analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef]
- Villegas-Perez, M.P.; Vidal-Sanz, M.; Rasminsky, M.; Bray, G.M.; Aguayo, A.J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J. Neurobiol. 1993, 24, 23–36. [Google Scholar] [CrossRef]
- Levin, L.A.; Beck, R.W.; Joseph, M.P.; Seiff, S.; Kraker, R. The treatment of traumatic optic neuropathy: The International Optic Nerve Trauma Study. Ophthalmology 1999, 106, 1268–1277. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 2014, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Elsaeidi, F.; Bemben, M.A.; Zhao, X.F.; Goldman, D. Jak/Stat signaling stimulates zebrafish optic nerve regeneration and overcomes the inhibitory actions of Socs3 and Sfpq. J. Neurosci. 2014, 34, 2632–2644. [Google Scholar] [CrossRef]
- Fleisch, V.C.; Fraser, B.; Allison, W.T. Investigating regeneration and functional integration of CNS neurons: Lessons from zebrafish genetics and other fish species. Biochim. Biophys. Acta 2011, 1812, 364–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jin, Y. Genetic dissection of axon regeneration. Curr. Opin. Neurobiol. 2011, 21, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef]
- Fu, Y.; Yau, K.W. Phototransduction in mouse rods and cones. Pflug. Arch. 2007, 454, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Andermann, M.L.; Ritt, J.; Neimark, M.A.; Moore, C.I. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron 2004, 42, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Neimark, M.A.; Andermann, M.L.; Hopfield, J.J.; Moore, C.I. Vibrissa resonance as a transduction mechanism for tactile encoding. J. Neurosci. 2003, 23, 6499–6509. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Yoshida, M. Auditory cued spatial learning in mice. Physiol. Behav. 2007, 92, 906–910. [Google Scholar] [CrossRef]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef] [Green Version]
- Lagman, D.; Callado-Perez, A.; Franzen, I.E.; Larhammar, D.; Abalo, X.M. Transducin duplicates in the zebrafish retina and pineal complex: Differential specialisation after the teleost tetraploidisation. PLoS ONE 2015, 10, e0121330. [Google Scholar] [CrossRef] [Green Version]
- Bilotta, J.; Saszik, S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 2001, 19, 621–629. [Google Scholar] [CrossRef]
- Chinen, A.; Hamaoka, T.; Yamada, Y.; Kawamura, S. Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics 2003, 163, 663–675. [Google Scholar] [PubMed]
- Mochizuki, A. Pattern formation of the cone mosaic in the zebrafish retina: A cell rearrangement model. J. Biol. 2002, 215, 345–361. [Google Scholar] [CrossRef]
- Applebury, M.L.; Antoch, M.P.; Baxter, L.C.; Chun, L.L.; Falk, J.D.; Farhangfar, F.; Kage, K.; Krzystolik, M.G.; Lyass, L.A.; Robbins, J.T. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 2000, 27, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.; Hurley, J.B.; Dierks, B.; Srinivas, M.; Salto, C.; Vennstrom, B.; Reh, T.A.; Forrest, D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 2001, 27, 94–98. [Google Scholar] [CrossRef]
- Wang, Y.V.; Weick, M.; Demb, J.B. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J. Neurosci. 2011, 31, 7670–7681. [Google Scholar] [CrossRef]
- Haverkamp, S.; Wassle, H.; Duebel, J.; Kuner, T.; Augustine, G.J.; Feng, G.; Euler, T. The primordial, blue-cone color system of the mouse retina. J. Neurosci. 2005, 25, 5438–5445. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Kunze, V.P.; Ball, J.M.; Peng, B.T.; Krishnan, A.; Zhou, G.; Dong, L.; Li, W. True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field. Elife 2020, 9. [Google Scholar] [CrossRef]
- Denman, D.J.; Luviano, J.A.; Ollerenshaw, D.R.; Cross, S.; Williams, D.; Buice, M.A.; Olsen, S.R.; Reid, R.C. Mouse color and wavelength-specific luminance contrast sensitivity are non-uniform across visual space. Elife 2018, 7. [Google Scholar] [CrossRef]
- Zimmermann, M.J.Y.; Nevala, N.E.; Yoshimatsu, T.; Osorio, D.; Nilsson, D.E.; Berens, P.; Baden, T. Zebrafish Differentially Process Color across Visual Space to Match Natural Scenes. Curr. Biol. 2018, 28, 2018–2032.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caves, E.M.; Brandley, N.C.; Johnsen, S. Visual Acuity and the Evolution of Signals. Trends Ecol. Evol. 2018, 33, 358–372. [Google Scholar] [CrossRef]
- Collin, S.P.; Pettigrew, J.D. Quantitative comparison of the limits on visual spatial resolution set by the ganglion cell layer in twelve species of reef teleosts. Brain Behav. Evol. 1989, 34, 184–192. [Google Scholar] [CrossRef]
- Pettigrew, J.D.; Dreher, B.; Hopkins, C.S.; McCall, M.J.; Brown, M. Peak density and distribution of ganglion cells in the retinae of microchiropteran bats: Implications for visual acuity. Brain Behav. Evol. 1988, 32, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Popovic, Z.; Sjostrand, J. The relation between resolution measurements and numbers of retinal ganglion cells in the same human subjects. Vis. Res. 2005, 45, 2331–2338. [Google Scholar] [CrossRef] [Green Version]
- Sjostrand, J.; Olsson, V.; Popovic, Z.; Conradi, N. Quantitative estimations of foveal and extra-foveal retinal circuitry in humans. Vis. Res. 1999, 39, 2987–2998. [Google Scholar] [CrossRef] [Green Version]
- Mangrum, W.I.; Dowling, J.E.; Cohen, E.D. A morphological classification of ganglion cells in the zebrafish retina. Vis. Neurosci. 2002, 19, 767–779. [Google Scholar] [CrossRef]
- Danias, J.; Shen, F.; Goldblum, D.; Chen, B.; Ramos-Esteban, J.; Podos, S.M.; Mittag, T. Cytoarchitecture of the retinal ganglion cells in the rat. Investig. Ophthalmol. Vis. Sci. 2002, 43, 587–594. [Google Scholar]
- Salinas-Navarro, M.; Mayor-Torroglosa, S.; Jimenez-Lopez, M.; Aviles-Trigueros, M.; Holmes, T.M.; Lund, R.D.; Villegas-Perez, M.P.; Vidal-Sanz, M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vis. Res. 2009, 49, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Displaced retinal ganglion cells in albino and pigmented rats. Front. Neuroanat 2014, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Navarro, M.; Jimenez-Lopez, M.; Valiente-Soriano, F.J.; Alarcon-Martinez, L.; Aviles-Trigueros, M.; Mayor, S.; Holmes, T.; Lund, R.D.; Villegas-Perez, M.P.; Vidal-Sanz, M. Retinal ganglion cell population in adult albino and pigmented mice: A computerized analysis of the entire population and its spatial distribution. Vis. Res. 2009, 49, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, M.F.; Biehlmaier, O.; Mueller, K.P.; Neuhauss, S.C. Visual acuity in larval zebrafish: Behavior and histology. Front. Zool 2010, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Meyers, J.R.; Hu, L.; Moses, A.; Kaboli, K.; Papandrea, A.; Raymond, P.A. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008, 87, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Fimbel, S.M.; Montgomery, J.E.; Burket, C.T.; Hyde, D.R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 2007, 27, 1712–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, T.J.; Fossum, S.L.; Fimbel, S.M.; Montgomery, J.E.; Hyde, D.R. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp. Eye Res. 2010, 91, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenkowski, J.R.; Qin, Z.; Sifuentes, C.J.; Thummel, R.; Soto, C.M.; Moens, C.B.; Raymond, P.A. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia 2013, 61, 1687–1697. [Google Scholar] [CrossRef] [Green Version]
- Fausett, B.V.; Goldman, D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef] [Green Version]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [Green Version]
- Portugues, R.; Engert, F. The neural basis of visual behaviors in the larval zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.N.; Connaughton, V.P.; Dellinger, J.A.; Klemer, D.; Udvadia, A.; Carvan, M.J., 3rd. Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol. Behav. 2008, 93, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Neuhauss, S.C. The optokinetic response in zebrafish and its applications. Front. Biosci. 2008, 13, 1899–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximov, V.; Maximova, E.; Damjanovic, I.; Maximov, P. Detection and resolution of drifting gratings by motion detectors in the fish retina. J. Integr. Neurosci. 2013, 12, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Komori, A.; Sagara, H.; Suzuki, E.; Manabe, T.; Hosoya, T.; Nojima, Y.; Wada, H.; Tanaka, H.; Okamoto, H.; et al. Mutation of cGMP phosphodiesterase 6alpha′-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech. Dev. 2008, 125, 932–946. [Google Scholar] [CrossRef]
- Rainy, N.; Etzion, T.; Alon, S.; Pomeranz, A.; Nisgav, Y.; Livnat, T.; Bach, M.; Gerstner, C.D.; Baehr, W.; Gothilf, Y.; et al. Knockdown of unc119c results in visual impairment and early-onset retinal dystrophy in zebrafish. Biochem. Biophys. Res. Commun. 2016, 473, 1211–1217. [Google Scholar] [CrossRef]
- Lobo, G.P.; Pauer, G.; Lipschutz, J.H.; Hagstrom, S.A. The Retinol-Binding Protein Receptor 2 (Rbpr2) Is Required for Photoreceptor Survival and Visual Function in the Zebrafish. Adv. Exp. Med. Biol. 2018, 1074, 569–576. [Google Scholar]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.L.; O’Connor, J.J.; Dillon, E.T.; Duffy, D.J.; Kolch, W.; Cagney, G.; et al. A Brain-Derived Neurotrophic Factor Mimetic Is Sufficient to Restore Cone Photoreceptor Visual Function in an Inherited Blindness Model. Sci. Rep. 2017, 7, 11320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, E.; Tschopp, M.; Tappeiner, C.; Sallin, P.; Jazwinska, A.; Enzmann, V. Methylnitrosourea (MNU)-induced retinal degeneration and regeneration in the zebrafish: Histological and functional characteristics. J. Vis. Exp. 2014, e51909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagerman, G.F.; Noel, N.C.; Cao, S.Y.; DuVal, M.G.; Oel, A.P.; Allison, W.T. Rapid Recovery of Visual Function Associated with Blue Cone Ablation in Zebrafish. PLoS ONE 2016, 11, e0166932. [Google Scholar] [CrossRef]
- Lewis, A.; Wilson, N.; Stearns, G.; Johnson, N.; Nelson, R.; Brockerhoff, S.E. Celsr3 is required for normal development of GABA circuits in the inner retina. Plos Genet. 2011, 7, e1002239. [Google Scholar] [CrossRef] [Green Version]
- Stujenske, J.M.; Dowling, J.E.; Emran, F. The bugeye mutant zebrafish exhibits visual deficits that arise with the onset of an enlarged eye phenotype. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4200–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portugues, R.; Feierstein, C.E.; Engert, F.; Orger, M.B. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron 2014, 81, 1328–1343. [Google Scholar] [CrossRef] [Green Version]
- Feierstein, C.E.; Portugues, R.; Orger, M.B. Seeing the whole picture: A comprehensive imaging approach to functional mapping of circuits in behaving zebrafish. Neuroscience 2015, 296, 26–38. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Easter, S.S., Jr.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [Green Version]
- Canfield, J.G. Functional evidence for visuospatial coding in the Mauthner neuron. Brain Behav. Evol. 2006; 67, 188–202. [Google Scholar]
- Del Bene, F.; Wyart, C.; Robles, E.; Tran, A.; Looger, L.; Scott, E.K.; Isacoff, E.Y.; Baier, H. Filtering of visual information in the tectum by an identified neural circuit. Science 2010, 330, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; Del Bene, F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Heap, L.A.L.; Vanwalleghem, G.; Thompson, A.W.; Favre-Bulle, I.A.; Scott, E.K. Luminance Changes Drive Directional Startle through a Thalamic Pathway. Neuron 2018, 99, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, S.S.; An, S.; Hamil, T. Visual detection of UV cues by adult zebrafish (Danio rerio). J. Vis. 2011, 11, 2. [Google Scholar] [CrossRef]
- Emran, F.; Rihel, J.; Dowling, J.E. A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J. Vis. Exp. 2008. [Google Scholar] [CrossRef]
- Allwardt, B.A.; Lall, A.B.; Brockerhoff, S.E.; Dowling, J.E. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J. Neurosci. 2001, 21, 2330–2342. [Google Scholar] [CrossRef] [Green Version]
- Emran, F.; Rihel, J.; Adolph, A.R.; Wong, K.Y.; Kraves, S.; Dowling, J.E. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 19126–19131. [Google Scholar] [CrossRef] [Green Version]
- Burgess, H.A.; Johnson, S.L.; Granato, M. Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors in robo3 mutant zebrafish. Genes Brain Behav. 2009, 8, 500–511. [Google Scholar] [CrossRef]
- Wolman, M.A.; Jain, R.A.; Liss, L.; Granato, M. Chemical modulation of memory formation in larval zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 15468–15473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsden, K.C.; Granato, M. In Vivo Ca(2+) Imaging Reveals that Decreased Dendritic Excitability Drives Startle Habituation. Cell Rep. 2015, 13, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banote, R.K.; Edling, M.; Eliassen, F.; Kettunen, P.; Zetterberg, H.; Abramsson, A. beta-Amyloid precursor protein-b is essential for Mauthner cell development in the zebrafish in a Notch-dependent manner. Dev. Biol. 2016, 413, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Shen, H.F.; Shen, Y.Q.; Schachner, M. The Adhesion Molecule-Characteristic HNK-1 Carbohydrate Contributes to Functional Recovery After Spinal Cord Injury in Adult Zebrafish. Mol. Neurobiol. 2017, 54, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Dubreuil, A.M.; Bertoni, T.; Bohm, U.L.; Bormuth, V.; Candelier, R.; Karpenko, S.; Hildebrand, D.G.C.; Bianco, I.H.; Monasson, R.; et al. Sensorimotor computation underlying phototaxis in zebrafish. Nat. Commun. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Huckenpahler, A.L.; Wilk, M.A.; Cooper, R.F.; Moehring, F.; Link, B.A.; Carroll, J.; Collery, R.F. Imaging the adult zebrafish cone mosaic using optical coherence tomography. Vis. Neurosci. 2016, 33, E011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, B.A.; Yuan, A.; Dicicco, R.M.; Fogerty, J.; Lessieur, E.M.; Perkins, B.D. The adult zebrafish retina: In vivo optical sectioning with Confocal Scanning Laser Ophthalmoscopy and Spectral-Domain Optical Coherence Tomography. Exp. Eye Res. 2016, 153, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Huckenpahler, A.; Wilk, M.; Link, B.; Carroll, J.; Collery, R. Repeatability and Reproducibility of In Vivo Cone Density Measurements in the Adult Zebrafish Retina. Adv. Exp. Med. Biol. 2018, 1074, 151–156. [Google Scholar]
- Toms, M.; Tracey-White, D.; Muhundhakumar, D.; Sprogyte, L.; Dubis, A.M.; Moosajee, M. Spectral Domain Optical Coherence Tomography: An In Vivo Imaging Protocol for Assessing Retinal Morphology in Adult Zebrafish. Zebrafish 2017, 14, 118–125. [Google Scholar] [CrossRef]
- Conedera, F.M.; Arendt, P.; Trepp, C.; Tschopp, M.; Enzmann, V. Muller Glia Cell Activation in a Laser-induced Retinal Degeneration and Regeneration Model in Zebrafish. J. Vis. Exp. 2017, 128, 27. [Google Scholar]
- Lin, Y.; Xiang, X.; Chen, T.; Mao, G.; Deng, L.; Zeng, L.; Zhang, J. In vivo monitoring the dynamic process of acute retinal hemorrhage and repair in zebrafish with spectral-domain optical coherence tomography. J. Biophotonics 2019, 12, e201900235. [Google Scholar] [CrossRef]
- Makhankov, Y.V.; Rinner, O.; Neuhauss, S.C. An inexpensive device for non-invasive electroretinography in small aquatic vertebrates. J. Neurosci. Methods 2004, 135, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Chrispell, J.D.; Rebrik, T.I.; Weiss, E.R. Electroretinogram analysis of the visual response in zebrafish larvae. J. Vis. Exp. 2015, 97, 16. [Google Scholar] [CrossRef] [Green Version]
- Saszik, S.; Bilotta, J.; Givin, C.M. ERG assessment of zebrafish retinal development. Vis. Neurosci 1999, 16, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Le, H.G.; Dowling, J.E.; Cameron, D.J. Early retinoic acid deprivation in developing zebrafish results in microphthalmia. Vis. Neurosci. 2012, 29, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Moyano, M.; Porteros, A.; Dowling, J.E. The effects of nicotine on cone and rod b-wave responses in larval zebrafish. Vis. Neurosci. 2013, 30, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, H.; Matsui, Y.; Miyata, R.; Nishimura, Y.; Yamamoto, T.; Tanaka, T.; Kondo, M. New photic stimulating system with white light-emitting diodes to elicit electroretinograms from zebrafish larvae. Doc. Ophthalmol. 2017, 135, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jusuf, P.R.; Goodbourn, P.T.; Bui, B.V. Electroretinogram Recording in Larval Zebrafish using A Novel Cone-Shaped Sponge-tip Electrode. J. Vis. Exp. 2019, 145, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maaswinkel, H.; Riesbeck, L.E.; Riley, M.E.; Carr, A.L.; Mullin, J.P.; Nakamoto, A.T.; Li, L. Behavioral screening for nightblindness mutants in zebrafish reveals three new loci that cause dominant photoreceptor cell degeneration. Mech. Ageing Dev. 2005, 126, 1079–1089. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Matsui, J.I.; Miller, J.; Dowling, J.E.; Perkins, B.D. myosin 7aa(-/-) mutant zebrafish show mild photoreceptor degeneration and reduced electroretinographic responses. Exp. Eye Res. 2014, 122, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, M.; Slijkerman, R.; Lerner, K.; Broekman, S.; Wegner, J.; Howat, T.; Peters, T.; Hetterschijt, L.; Boon, N.; de Vrieze, E.; et al. Usherin defects lead to early-onset retinal dysfunction in zebrafish. Exp. Eye Res. 2018, 173, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiao, X.; D’Atri, I.; Ono, F.; Nelson, R.; Chan, C.C.; Nakaya, N.; Ma, Z.; Ma, Y.; Cai, X.; et al. Mutation in the intracellular chloride channel CLCC1 associated with autosomal recessive retinitis pigmentosa. PLoS Genet. 2018, 14, e1007504. [Google Scholar] [CrossRef] [Green Version]
- Messchaert, M.; Dona, M.; Broekman, S.; Peters, T.A.; Corral-Serrano, J.C.; Slijkerman, R.W.N.; van Wijk, E.; Collin, R.W.J. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS ONE 2018, 13, e0200789. [Google Scholar] [CrossRef] [Green Version]
- McGinn, T.E.; Mitchell, D.M.; Meighan, P.C.; Partington, N.; Leoni, D.C.; Jenkins, C.E.; Varnum, M.D.; Stenkamp, D.L. Restoration of Dendritic Complexity, Functional Connectivity, and Diversity of Regenerated Retinal Bipolar Neurons in Adult Zebrafish. J. Neurosci. 2018, 38, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Chen, K.; Reynolds, A.L.; Waghorne, N.; O’Connor, J.J.; Kennedy, B.N. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanvir, Z.; Nelson, R.F.; DeCicco-Skinner, K.; Connaughton, V.P. One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, R.A.; Dobler, S.; Schmitner, N.; Walsen, T.; Freudenblum, J.; Meyer, D. Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment. Sci. Rep. 2015, 5, 14241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Z.; Zang, J.; Lagali, N.; Schmitner, N.; Salvenmoser, W.; Mukwaya, A.; Neuhauss, S.C.F.; Jensen, L.D.; Kimmel, R.A. Photoreceptor Degeneration Accompanies Vascular Changes in a Zebrafish Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 43. [Google Scholar] [CrossRef]
- Wei, H.P.; Yao, Y.Y.; Zhang, R.W.; Zhao, X.F.; Du, J.L. Activity-induced long-term potentiation of excitatory synapses in developing zebrafish retina in vivo. Neuron 2012, 75, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P.R.; Lowe, A.S.; Thompson, I.D.; Meyer, M.P. Emergent properties of the optic tectum revealed by population analysis of direction and orientation selectivity. J. Neurosci. 2013, 33, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Semmelhack, J.L.; Donovan, J.C.; Thiele, T.R.; Kuehn, E.; Laurell, E.; Baier, H. A dedicated visual pathway for prey detection in larval zebrafish. eLife 2014, 3, 09. [Google Scholar] [CrossRef]
- Barker, A.J.; Baier, H. Sensorimotor decision making in the zebrafish tectum. Curr. Biol. 2015, 25, 2804–2814. [Google Scholar] [CrossRef] [Green Version]
- Antinucci, P.; Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 2016, 6, 29490. [Google Scholar] [CrossRef] [Green Version]
- Conedera, F.M.; Pousa, A.M.Q.; Mercader, N.; Tschopp, M.; Enzmann, V. Retinal microglia signaling affects Muller cell behavior in the zebrafish following laser injury induction. Glia 2019, 67, 1150–1166. [Google Scholar] [CrossRef] [PubMed]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nat. Commun 2018, 9, 4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastlake, K.; Heywood, W.E.; Tracey-White, D.; Aquino, E.; Bliss, E.; Vasta, G.R.; Mills, K.; Khaw, P.T.; Moosajee, M.; Limb, G.A. Comparison of proteomic profiles in the zebrafish retina during experimental degeneration and regeneration. Sci. Rep. 2017, 7, 44601. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.L.; Ranski, A.H.; Morgan, G.W.; Thummel, R. Reactive gliosis in the adult zebrafish retina. Exp. Eye Res. 2016, 143, 98–109. [Google Scholar] [CrossRef]
- Coomer, C.E.; Morris, A.C. Capn5 Expression in the Healthy and Regenerating Zebrafish Retina. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.E.; Calinescu, A.A.; Hitchcock, P.F. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J. Ocul. Biol. Dis. Infor. 2008, 1, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, S.E.; Thummel, R.; Ahmed, H.; Vasta, G.R.; Hyde, D.R.; Hitchcock, P.F. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3244–3252. [Google Scholar] [CrossRef]
- Eichenbaum, J.W.; Cinaroglu, A.; Eichenbaum, K.D.; Sadler, K.C. A zebrafish retinal graded photochemical stress model. J. Pharm. Toxicol. Methods 2009, 59, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Hochmann, S.; Kaslin, J.; Hans, S.; Weber, A.; Machate, A.; Geffarth, M.; Funk, R.H.; Brand, M. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS ONE 2012, 7, e30365. [Google Scholar] [CrossRef] [Green Version]
- Kassen, S.C.; Ramanan, V.; Montgomery, J.E.; Burket, T.C.; Liu, C.G.; Vihtelic, T.S.; Hyde, D.R. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 2007, 67, 1009–1031. [Google Scholar] [CrossRef]
- Kassen, S.C.; Thummel, R.; Campochiaro, L.A.; Harding, M.J.; Bennett, N.A.; Hyde, D.R. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp. Eye Res. 2009, 88, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Kawase, R.; Nishimura, Y.; Ashikawa, Y.; Sasagawa, S.; Murakami, S.; Yuge, M.; Okabe, S.; Kawaguchi, K.; Yamamoto, H.; Moriyuki, K.; et al. EP300 Protects from Light-Induced Retinopathy in Zebrafish. Front. Pharm. 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.S.; Friemel, T.D.; Grillo, S.L.; Stella, S.L., Jr. A short period of dark-adaptation is sufficient to generate light-induced photoreceptor degeneration in pigmented zebrafish. Neuroreport 2020, 31, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Li, J.; Marton, R.M.; Hyde, D.R. Actin-Cytoskeleton- and Rock-Mediated INM Are Required for Photoreceptor Regeneration in the Adult Zebrafish Retina. J. Neurosci. 2015, 35, 15612–15634. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Kidd, A.R., 3rd; Thomas, J.L.; Poss, K.D.; Hyde, D.R.; Raymond, P.A.; Thummel, R. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp. Eye Res. 2011, 93, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, K.; Harding, R.L.; Bailey, T.; Patton, J.G.; Hyde, D.R. Dynamic miRNA expression patterns during retinal regeneration in zebrafish: Reduced dicer or miRNA expression suppresses proliferation of Muller glia-derived neuronal progenitor cells. Dev. Dyn. 2014, 243, 1591–1605. [Google Scholar] [CrossRef]
- Thomas, J.L.; Morgan, G.W.; Dolinski, K.M.; Thummel, R. Characterization of the pleiotropic roles of Sonic Hedgehog during retinal regeneration in adult zebrafish. Exp. Eye Res. 2018, 166, 106–115. [Google Scholar] [CrossRef]
- Thomas, J.L.; Nelson, C.M.; Luo, X.; Hyde, D.R.; Thummel, R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp. Eye Res. 2012, 97, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.L.; Thummel, R. A novel light damage paradigm for use in retinal regeneration studies in adult zebrafish. J. Vis. Exp. 2013, e51017. [Google Scholar] [CrossRef] [Green Version]
- Vihtelic, T.S.; Hyde, D.R. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000, 44, 289–307. [Google Scholar] [CrossRef]
- Weber, A.; Hochmann, S.; Cimalla, P.; Gartner, M.; Kuscha, V.; Hans, S.; Geffarth, M.; Kaslin, J.; Koch, E.; Brand, M. Characterization of light lesion paradigms and optical coherence tomography as tools to study adult retina regeneration in zebrafish. PLoS ONE 2013, 8, e80483. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.G.; Wen, W.; Pillai-Kastoori, L.; Morris, A.C. Tracking the fate of her4 expressing cells in the regenerating retina using her4:Kaede zebrafish. Exp. Eye Res. 2016, 145, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.F.; Wan, J.; Powell, C.; Ramachandran, R.; Myers, M.G., Jr.; Goldman, D. Leptin and IL-6 family cytokines synergize to stimulate Muller glia reprogramming and retina regeneration. Cell Rep. 2014, 9, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albalawi, A.; Alhasani, R.H.A.; Biswas, L.; Reilly, J.; Akhtar, S.; Shu, X. Carnosic acid attenuates acrylamide-induced retinal toxicity in zebrafish embryos. Exp. Eye Res. 2018, 175, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battista, A.G.; Ricatti, M.J.; Pafundo, D.E.; Gautier, M.A.; Faillace, M.P. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J. Neurochem. 2009, 111, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Raghupathy, R.K.; Albalawi, A.; Zhao, Z.; Reilly, J.; Xiao, Q.; Shu, X. A colour preference technique to evaluate acrylamide-induced toxicity in zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharm. 2017, 199, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, P.; Carr, A.; Wang, X.; DeLaPaz, A.; Sun, L.; Lee, E.; Tomei, E.; Li, L. Functional expression of SCL/TAL1 interrupting locus (Stil) protects retinal dopaminergic cells from neurotoxin-induced degeneration. J. Biol. Chem. 2013, 288, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.W.; Wang, H.T.; Wang, N.; Sheng, W.W.; Jin, M.; Lu, Y.; Bai, Y.J.; Zou, S.Q.; Pang, Y.L.; Xu, H.; et al. Establishment of an adult zebrafish model of retinal neurodegeneration induced by NMDA. Int. J. Ophthalmol. 2019, 12, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.P.; Bejarano, C.A.; Battista, A.G.; Venera, G.D.; Bernabeu, R.O.; Faillace, M.P. Injury-induced purinergic signalling molecules upregulate pluripotency gene expression and mitotic activity of progenitor cells in the zebrafish retina. Purinergic. Signal. 2017, 13, 443–465. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.M.; Lovel, A.G.; Stenkamp, D.L. Dynamic changes in microglial and macrophage characteristics during degeneration and regeneration of the zebrafish retina. J. Neuroinflamm. 2018, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Zolessi, F.R.; Casco, V.H. Cypermethrin: Oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat Res. Genet. Toxicol. Env. Mutagen. 2018, 826, 25–32. [Google Scholar] [CrossRef]
- Raine, J.C.; Lallemand, L.; Pettem, C.M.; Janz, D.M. Effects of Chronic Dietary Selenomethionine Exposure on the Visual System of Adult and F1 Generation Zebrafish (Danio rerio). Bull. Env. Contam. Toxicol. 2016, 97, 331–336. [Google Scholar] [CrossRef]
- Sheng, W.; Lu, Y.; Mei, F.; Wang, N.; Liu, Z.Z.; Han, Y.Y.; Wang, H.T.; Zou, S.; Xu, H.; Zhang, X. Effect of Resveratrol on Sirtuins, OPA1, and Fis1 Expression in Adult Zebrafish Retina. Investig. Ophthalmol.Vis. Sci. 2018, 59, 4542–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.D.; Hsu, H.J.; Li, Y.F.; Wu, C.Y. Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol. Int. J. Mol. Sci. 2017, 18, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballestero, R.P.; Dybowski, J.A.; Levy, G.; Agranoff, B.W.; Uhler, M.D. Cloning and characterization of zRICH, a 2′,3′-cyclic-nucleotide 3′-phosphodiesterase induced during zebrafish optic nerve regeneration. J. Neurochem. 1999, 72, 1362–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Crespo, D.; Vecino, E. Differential expression of calretinin in the developing and regenerating zebrafish visual system. Histol. Histopathol. 2004, 19, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, Y.; Fujikawa, C.; Ogai, K.; Sugitani, K.; Watanabe, S.; Kato, S.; Wakasugi, K. Functional characterization of fish neuroglobin: Zebrafish neuroglobin is highly expressed in amacrine cells after optic nerve injury and can translocate into ZF4 cells. Biochim. Biophys. Acta 2013, 1834, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Koke, J.R.; Mosier, A.L.; Garcia, D.M. Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Muller glia: Differential distribution of cytokeratin and GFAP. BMC Res. Notes 2010, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Munzel, E.J.; Becker, C.G.; Becker, T.; Williams, A. Zebrafish regenerate full thickness optic nerve myelin after demyelination, but this fails with increasing age. Acta Neuropathol. Commun. 2014, 2, 77. [Google Scholar] [CrossRef]
- Ogai, K.; Kuwana, A.; Hisano, S.; Nagashima, M.; Koriyama, Y.; Sugitani, K.; Mawatari, K.; Nakashima, H.; Kato, S. Upregulation of leukemia inhibitory factor (LIF) during the early stage of optic nerve regeneration in zebrafish. PLoS ONE 2014, 9, e106010. [Google Scholar] [CrossRef]
- Powell, C.; Cornblath, E.; Elsaeidi, F.; Wan, J.; Goldman, D. Zebrafish Muller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Sci Rep. 2016, 6, 24851. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Zhao, X.F.; Goldman, D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 15858–15863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugitani, K.; Koriyama, Y.; Ogai, K.; Wakasugi, K.; Kato, S. A Possible Role of Neuroglobin in the Retina After Optic Nerve Injury: A Comparative Study of Zebrafish and Mouse Retina. Adv. Exp. Med. Biol. 2016, 854, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruma, K.; Saito, Y.; Okuyoshi, H.; Yamaguchi, A.; Shimazawa, M.; Goldman, D.; Hara, H. Granulin 1 Promotes Retinal Regeneration in Zebrafish. Investig. Ophthalmol Vis. Sci. 2018, 59, 6057–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Kuse, Y.; Inoue, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Transient acceleration of autophagic degradation by pharmacological Nrf2 activation is important for retinal pigment epithelium cell survival. Redox Biol. 2018, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; Middleton, R.C.; Le, Q.P.; Shelden, E.A. HSF1 is essential for the resistance of zebrafish eye and brain tissues to hypoxia/reperfusion injury. PLoS ONE 2011, 6, e22268. [Google Scholar] [CrossRef]
- Cao, R.; Jensen, L.D.; Soll, I.; Hauptmann, G.; Cao, Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE 2008, 3, e2748. [Google Scholar] [CrossRef] [Green Version]
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell. Mol. Life Sci. 2018, 75, 1255–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging (Albany Ny) 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- van Rooijen, E.; Voest, E.E.; Logister, I.; Bussmann, J.; Korving, J.; van Eeden, F.J.; Giles, R.H.; Schulte-Merker, S. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis. Model. Mech. 2010, 3, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Asai-Coakwell, M.; March, L.; Dai, X.H.; Duval, M.; Lopez, I.; French, C.R.; Famulski, J.; De Baere, E.; Francis, P.J.; Sundaresan, P.; et al. Contribution of growth differentiation factor 6-dependent cell survival to early-onset retinal dystrophies. Hum. Mol. Genet. 2013, 22, 1432–1442. [Google Scholar] [CrossRef] [Green Version]
- Oura, Y.; Nakamura, M.; Takigawa, T.; Fukushima, Y.; Wakabayashi, T.; Tsujikawa, M.; Nishida, K. High-Temperature Requirement A 1 Causes Photoreceptor Cell Death in Zebrafish Disease Models. Am. J. Pathol. 2018, 188, 2729–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyk, J.; Daly, C.; Janssen-Bienhold, U.; Kennedy, B.N.; Richter-Landsberg, C. HDAC6 inhibition by tubastatin A is protective against oxidative stress in a photoreceptor cell line and restores visual function in a zebrafish model of inherited blindness. Cell Death Dis. 2017, 8, e3028. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, K.; Harding, R.L.; Hyde, D.R.; Patton, J.G. miR-203 regulates progenitor cell proliferation during adult zebrafish retina regeneration. Dev. Biol. 2014, 392, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Bollaerts, I.; Van Houcke, J.; Beckers, A.; Lemmens, K.; Vanhunsel, S.; De Groef, L.; Moons, L. Prior Exposure to Immunosuppressors Sensitizes Retinal Microglia and Accelerates Optic Nerve Regeneration in Zebrafish. Mediat. Inflamm. 2019, 2019, 6135795. [Google Scholar] [CrossRef]
- Sherpa, T.; Lankford, T.; McGinn, T.E.; Hunter, S.S.; Frey, R.A.; Sun, C.; Ryan, M.; Robison, B.D.; Stenkamp, D.L. Retinal regeneration is facilitated by the presence of surviving neurons. Dev. Neurobiol. 2014, 74, 851–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viringipurampeer, I.A.; Shan, X.; Gregory-Evans, K.; Zhang, J.P.; Mohammadi, Z.; Gregory-Evans, C.Y. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Differ. 2014, 21, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.B.; Pang, C.P.; Ko, K.M.; et al. A Naturally-Derived Compound Schisandrin B Enhanced Light Sensation in the pde6c Zebrafish Model of Retinal Degeneration. PLoS ONE 2016, 11, e0149663. [Google Scholar] [CrossRef] [Green Version]
- White, D.T.; Sengupta, S.; Saxena, M.T.; Xu, Q.; Hanes, J.; Ding, D.; Ji, H.; Mumm, J.S. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proc. Natl. Acad. Sci. USA 2017, 114, E3719–E3728. [Google Scholar] [CrossRef] [Green Version]
- Hanovice, N.J.; Leach, L.L.; Slater, K.; Gabriel, A.E.; Romanovicz, D.; Shao, E.; Collery, R.; Burton, E.A.; Lathrop, K.L.; Link, B.A.; et al. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLoS Genet. 2019, 15, e1007939. [Google Scholar] [CrossRef] [Green Version]
- Yoshimatsu, T.; D’Orazi, F.D.; Gamlin, C.R.; Suzuki, S.C.; Suli, A.; Kimelman, D.; Raible, D.W.; Wong, R.O. Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nat. Commun. 2016, 7, 10590. [Google Scholar] [CrossRef] [Green Version]
- Sherpa, T.; Fimbel, S.M.; Mallory, D.E.; Maaswinkel, H.; Spritzer, S.D.; Sand, J.A.; Li, L.; Hyde, D.R.; Stenkamp, D.L. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 2008, 68, 166–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Singh, A.; Castillo, H.A.; Brown, J.; Kaslin, J.; Dwyer, K.M.; Gibert, Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci. Rep. 2019, 9, 4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera, M.; Burguera, D.; Garcia-Fernandez, J.; Gonzalez-Duarte, R. CERKL knockdown causes retinal degeneration in zebrafish. PLoS ONE 2013, 8, e64048. [Google Scholar] [CrossRef] [Green Version]
- Saade, C.J.; Alvarez-Delfin, K.; Fadool, J.M. Rod photoreceptors protect from cone degeneration-induced retinal remodeling and restore visual responses in zebrafish. J. Neurosci. 2013, 33, 1804–1814. [Google Scholar] [CrossRef]
- Corral-Serrano, J.C.; Messchaert, M.; Dona, M.; Peters, T.A.; Kamminga, L.M.; van Wijk, E.; Collin, R.W.J. C2orf71a/pcare1 is important for photoreceptor outer segment morphogenesis and visual function in zebrafish. Sci. Rep. 2018, 8, 9675. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, T.; Hunter, S.S.; Frey, R.A.; Robison, B.D.; Stenkamp, D.L. Retinal proliferation response in the buphthalmic zebrafish, bugeye. Exp. Eye Res. 2011, 93, 424–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veth, K.N.; Willer, J.R.; Collery, R.F.; Gray, M.P.; Willer, G.B.; Wagner, D.S.; Mullins, M.C.; Udvadia, A.J.; Smith, R.S.; John, S.W.; et al. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011, 7, e1001310. [Google Scholar] [CrossRef] [Green Version]
- White, D.T.; Mumm, J.S. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods 2013, 62, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Fraser, B.; DuVal, M.G.; Wang, H.; Allison, W.T. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS ONE 2013, 8, e55410. [Google Scholar] [CrossRef] [Green Version]
- D’Orazi, F.D.; Zhao, X.F.; Wong, R.O.; Yoshimatsu, T. Mismatch of Synaptic Patterns between Neurons Produced in Regeneration and during Development of the Vertebrate Retina. Curr. Biol. 2016, 26, 2268–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.F.; Ellingsen, S.; Fjose, A. Labelling and targeted ablation of specific bipolar cell types in the zebrafish retina. BMC Neurosci. 2009, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCurley, A.T.; Callard, G.V. Time Course Analysis of Gene Expression Patterns in Zebrafish Eye During Optic Nerve Regeneration. J. Exp. Neurosci 2010, 2010, 17–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.X.; Wang, Z.R. [Changes in number and distribution of retinal ganglion cells after optic nerve crush in zebrafish]. Shi Yan Sheng Wu Xue Bao 2002, 35, 159–162. [Google Scholar] [PubMed]
- Zou, S.; Tian, C.; Ge, S.; Hu, B. Neurogenesis of retinal ganglion cells is not essential to visual functional recovery after optic nerve injury in adult zebrafish. PLoS ONE 2013, 8, e57280. [Google Scholar] [CrossRef]
- Lemmens, K.; Bollaerts, I.; Bhumika, S.; de Groef, L.; Van Houcke, J.; Darras, V.M.; Van Hove, I.; Moons, L. Matrix metalloproteinases as promising regulators of axonal regrowth in the injured adult zebrafish retinotectal system. J. Comp. Neurol. 2016, 524, 1472–1493. [Google Scholar] [CrossRef]
- Bhumika, S.; Lemmens, K.; Vancamp, P.; Moons, L.; Darras, V.M. Decreased thyroid hormone signaling accelerates the reinnervation of the optic tectum following optic nerve crush in adult zebrafish. Mol. Cell. Neurosci. 2015, 68, 92–102. [Google Scholar] [CrossRef]
- Kaneda, M.; Nagashima, M.; Nunome, T.; Muramatsu, T.; Yamada, Y.; Kubo, M.; Muramoto, K.; Matsukawa, T.; Koriyama, Y.; Sugitani, K.; et al. Changes of phospho-growth-associated protein 43 (phospho-GAP43) in the zebrafish retina after optic nerve injury: A long-term observation. Neurosci. Res. 2008, 61, 281–288. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Growth and pathfinding of regenerating axons in the optic projection of adult fish. J. Neurosci. Res. 2007, 85, 2793–2799. [Google Scholar] [CrossRef]
- McGinn, T.E.; Galicia, C.A.; Leoni, D.C.; Partington, N.; Mitchell, D.M.; Stenkamp, D.L. Rewiring the Regenerated Zebrafish Retina: Reemergence of Bipolar Neurons and Cone-Bipolar Circuitry Following an Inner Retinal Lesion. Front. Cell Dev. Biol. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Mela, M.; Cambier, S.; Mesmer-Dudons, N.; Legeay, A.; Grotzner, S.R.; de Oliveira Ribeiro, C.A.; Ventura, D.F.; Massabuau, J.C. Methylmercury localization in Danio rerio retina after trophic and subchronic exposure: A basis for neurotoxicology. Neurotoxicology 2010, 31, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Didiano, D.; Abner, J.J.; Hinger, S.A.; Flickinger, Z.; Kent, M.; Clement, M.A.; Balaiya, S.; Liu, Q.; Dai, X.; Levine, E.M.; et al. Induction of a proliferative response in the zebrafish retina by injection of extracellular vesicles. Exp. Eye Res. 2020, 200, 108254. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, S.L., Jr.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633. https://doi.org/10.3390/cells10030633
Stella SL Jr., Geathers JS, Weber SR, Grillo MA, Barber AJ, Sundstrom JM, Grillo SL. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells. 2021; 10(3):633. https://doi.org/10.3390/cells10030633
Chicago/Turabian StyleStella, Salvatore L., Jr., Jasmine S. Geathers, Sarah R. Weber, Michael A. Grillo, Alistair J. Barber, Jeffrey M. Sundstrom, and Stephanie L. Grillo. 2021. "Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina" Cells 10, no. 3: 633. https://doi.org/10.3390/cells10030633
APA StyleStella, S. L., Jr., Geathers, J. S., Weber, S. R., Grillo, M. A., Barber, A. J., Sundstrom, J. M., & Grillo, S. L. (2021). Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells, 10(3), 633. https://doi.org/10.3390/cells10030633







