Overcoming the Elusiveness of Neurosarcoidosis: Learning from Five Complex Cases
Abstract
1. Introduction
2. Design/Methods
3. Results
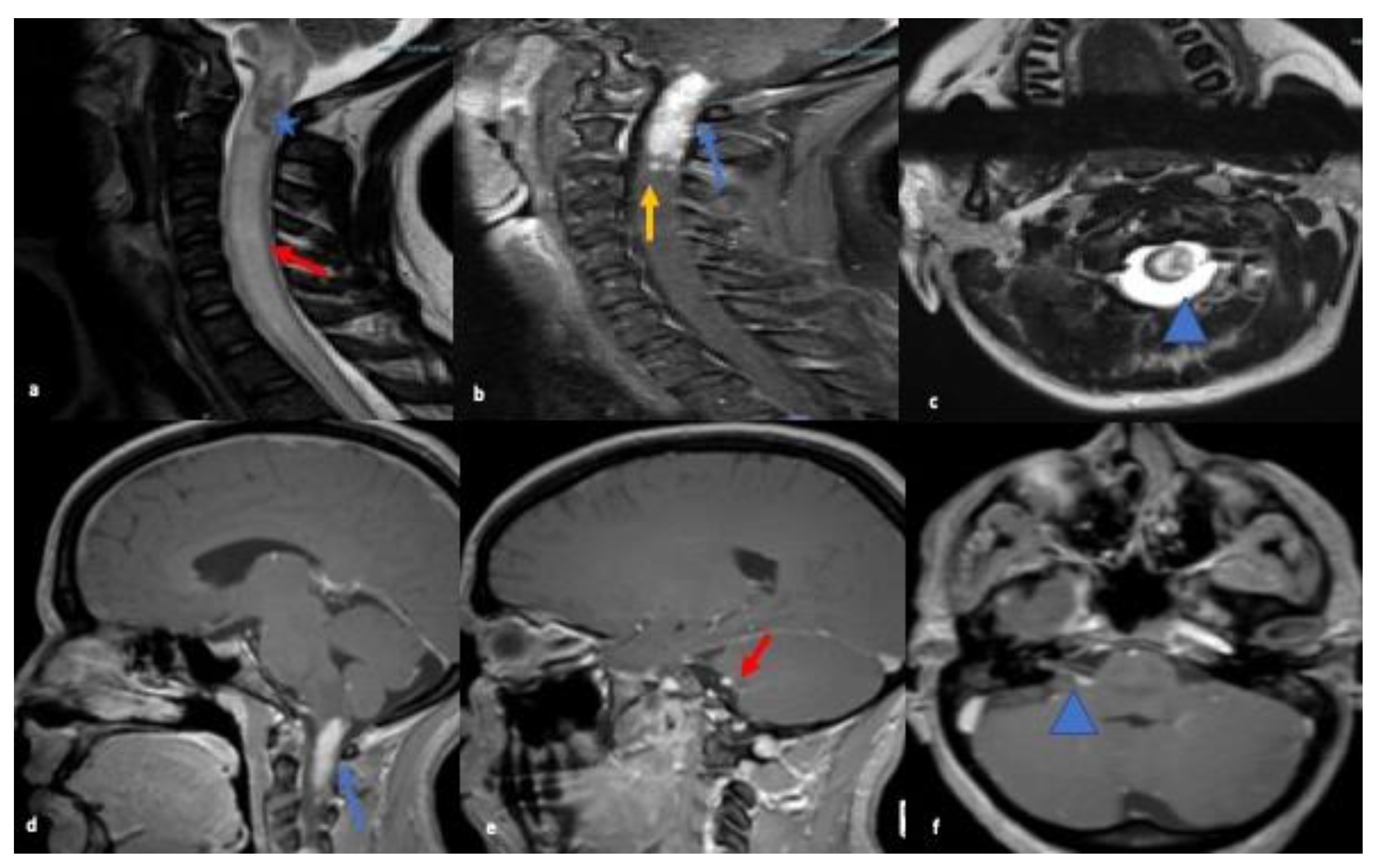
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
3.5. Case 5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| CSF | Cerebrospinal fluid |
| EVD | External Ventricular drain |
| ACE | Angiotensin converting enzymes |
| CINS | Clinically isolated primary Neurosarcoidosis |
| LP | Lumbar Puncture |
| TTE | Transthoracic Echocardiography |
| ICP | Intracranial Pressure; CNS, Central Nervous System |
| NMO | Neuromyelitis Optica |
| MS | Multiple Sclerosis |
| CN | Cranial Nerve |
References
- Christoforidis, G.A.; Spickler, E.M.; Recio, M.V.; Mehta, B.M. MR of CNS Sarcoidosis: Correlation of Imaging Features to Clinical Symptoms and Response to Treatment. Am. J. Neuroradiol. 1999, 20, 655–669. [Google Scholar]
- Pickuth, D.; Spielmann, R.P.; Heywang-Köbrunner, S.H. Heywang-Köbrunner, Role of radiology in the diagnosis of neurosarcoidosis. Eur. Radiol. 2000, 10, 941–944. [Google Scholar] [CrossRef]
- Nozaki, K.; Judson, M.A. Neurosarcoidosis: Clinical manifestations, diagnosis and treatment. Presse Méd. 2012, 41, e331–e348. [Google Scholar] [CrossRef]
- Tana, C.; Wegener, S.; Borys, E.; Pambuccian, S.; Tchernev, G.; Tana, M.; Giamberardino, M.A.; Silingardi, M. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann. Med. 2015, 47, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, R.T.; Wilkins, A.; Scolding, N.J. Neurosarcoidosis: A clinical approach to diagnosis and management. J. Neurol. 2017, 264, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Fritz, D.; Van De Beek, D.; Brouwer, M.C. Brouwer, Clinical features, treatment and outcome in neurosarcoidosis: Systematic review and meta-analysis. BMC Neurol. 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.; Salcman, M. Neurosarcoidosis: A review of the rarer manifestations. Surg. Neurol. 1981, 15, 204–211. [Google Scholar] [CrossRef]
- Hoitsma, E.; Faber, C.G.; Drent, M.; Sharma, O.P. Neurosarcoidosis: A clinical dilemma. Lancet Neurol. 2004, 3, 397–407. [Google Scholar] [CrossRef]
- Fritz, D.; Voortman, M.; van de Beek, D.; Drent, M.; Brouwer, M.C. Many faces of neurosarcoidosis: From chronic meningitis to myelopathy. Curr. Opin. Pulm. Med. 2017, 23, 439–446. [Google Scholar] [CrossRef]
- Wegener, S.; Linnebank, M.; Martin, R.; Valavanis, A.; Weller, M. Clinically isolated neurosarcoidosis: A recommended diagnostic path. Eur. Neurol. 2014, 73, 71–77. [Google Scholar] [CrossRef]
- Nowak, D.A.; Widenka, D.C. Neurosarcoidosis: A review of its intracranial manifestation. J. Neurol. 2001, 248, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, S.; Argentiero, V.; Tavolato, B. Neurosarcoidosis. J. Neurol. 2006, 253, 488–495. [Google Scholar] [CrossRef]
- Kidd, D.A.-O. Neurosarcoidosis: Clinical manifestations, investigation and treatment. Pract. Neurol. 2020, 20, 199–212. [Google Scholar] [CrossRef]
- Stern, B.J.; Royal, W.; Gelfand, J.M.; Clifford, D.B.; Tavee, J.; Pawate, S.; Berger, J.R.; Aksamit, A.J.; Krumholz, A.; Pardo, C.A.; et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018, 75, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Wellik, K.E.; Demaerschalk, B.M.; Wingerchuk, D.M. Cerebrospinal fluid angiotensin-converting enzyme for diagnosis of central nervous system sarcoidosis. Neurologist 2009, 15, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Matheus, M.G.; Castillo, M. Imaging manifestations of neurosarcoidosis. Am. J. Roentgenol. 2004, 182, 289–295. [Google Scholar] [CrossRef]
- Soni, N.; Bathla, G.; Maheshwarappa, R.P. Imaging findings in spinal sarcoidosis: A report of 18 cases and review of the current literature. Neuroradiol. J. 2018, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Durel, C.-A.; Marignier, R.; Maucort-Boulch, D.; Iwaz, J.; Berthoux, E.; Ruivard, M.; André, M.; Le Guenno, G.; Perard, L.; Dufour, J.-F.; et al. Clinical features and prognostic factors of spinal cord sarcoidosis: A multicenter observational study of 20 BIOPSY-PROVEN patients. J. Neurol. 2016, 263, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Vighetto, A.; Fischer, G.; Collet, P.; Bady, B.; Trillet, M. Intramedullary sarcoidosis of the cervical spinal cord. J. Neurol. Neurosurg. Psychiatry 1985, 48, 477–479. [Google Scholar] [CrossRef]
- Junger, S.S.; Stern, B.J.; Levine, S.R.; Sipos, E.; Marti-Masso, J.F. Intramedullary spinal sarcoidosis: Clinical and magnetic resonance imaging characteristics. Neurology 1993, 43, 333. [Google Scholar] [CrossRef] [PubMed]
- Kanoff, R.B.; Ruberg, R.L. Intramedullary sarcoidosis of the spinal cord: Report of a case. J. Am. Osteopat. Assoc. 1978, 77, 868–875. [Google Scholar]
- Flanagan, E.P.; Kaufmann, T.J.; Krecke, K.N.; Aksamit, A.J.; Pittock, S.J.; Keegan, B.M.; Giannini, C.; Weinshenker, B.G. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann. Neurol. 2016, 79, 437–447. [Google Scholar] [CrossRef]
- Jolliffe, E.A.; Keegan, B.M.; Flanagan, E.P. Trident sign trumps Aquaporin-4-IgG ELISA in diagnostic value in a case of longitudinally extensive transverse myelitis. Mult. Scler. Relat. Disord. 2018, 23, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.P.; Scolding, N.; Foster, O.; Rovaris, M.; Evanson, J.; Moseley, I.; Scadding, J.; Thompson, E.; Chamoun, V.; Miller, D.; et al. Central nervous system sarcoidosis—Diagnosis and management. QJM 1999, 92, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.Z.; Shaikh, F.; Siddiqui, A.A. Primary chiasmal sarcoid granuloma masquerading as glioma of the optic chiasm. J. Coll. Phys. Surg. Pak. 2010, 20, 695. [Google Scholar]
- Jennings, J.W.; Rojiani, A.M.; Brem, S.S.; Murtagh, F.R. Necrotizing neurosarcoidosis masquerading as a left optic nerve meningioma: Case report. Am. J. Neuroradiol. 2002, 23, 660–662. [Google Scholar]
- Kefella, H.; Luther, D.; Hainline, C. Ophthalmic and neuro-ophthalmic manifestations of sarcoidosis. Curr. Opin. Ophthalmol. 2017, 28, 587–594. [Google Scholar] [CrossRef]
- Acharya, N.R.; Browne, E.N.; Rao, N.; Mochizuki, M. Distinguishing Features of Ocular Sarcoidosis in an International Cohort of Uveitis Patients. Ophthalmology 2018, 125, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.J.; Quhill, F.; Pepper, I.M. The Evolution of an Optic Nerve Head Granuloma Due to Sarcoidosis. Neuro Ophthalmol. 2016, 40, 59–68. [Google Scholar] [CrossRef]
- Mafee, M.F.; Dorodi, S.; Pai, E. Sarcoidosis of the eye, orbit, and central nervous system: Role of MR imaging. Radiol. Clin. N. Am. 1999, 37, 73–87. [Google Scholar] [CrossRef]
- van Rooijen, J.M.; Mijnhout, G.S.; Aalders, T.T.; de Bondt, R.B. Hydrocephalus, a rare manifestation of sarcoidosis. Clin. Pract. 2011, 1, 136–138. [Google Scholar] [CrossRef]
- Brouwer, M.C.; De Gans, J.; Willemse, R.B.; Van De Beek, D. Sarcoidosis presenting with hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2009, 80, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Vandesteen, L.; Drier, A.; Galanaud, D.; Clarençon, F.; Leclercq, D.; Karachi, C.; Dormont, D. Imaging findings of intraventricular and ependymal lesions. J. Neuroradiol. 2013, 40, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Takai, K.; Ota, M.; Shimizu, T.; Komori, T.; Taniguchi, M. Isolated neurosarcoidosis in the medulla oblongata involving the fourth ventricle: A case report. Br. J. Neurosurg. 2012, 27, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Ginat, D.T.; Dhillon, G.; Almast, J. Magnetic resonance imaging of neurosarcoidosis. J. Clin. Imaging Sci. 2011, 1, 15. [Google Scholar] [PubMed]
- Smirniotopoulos, J.G.; Murphy, F.M.; Rushing, E.J.; Rees, J.H.; Schroeder, J.W. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007, 27, 525–551. [Google Scholar] [CrossRef]
- Raees, M.; Lilien, D.; Pedro, G.S.S. Unusual Appearance of Neurosarcoidosis on Magnetic Resonance and Positron Emission Tomographic Image. Chest 2003, 124, 250S. [Google Scholar] [CrossRef]
- Zalewski, N.L.; Krecke, K.N.; Weinshenker, B.G.; Aksamit, A.J.; Conway, B.L.; McKeon, A.; Flanagan, E.P. Central canal enhancement and the trident sign in spinal cord sarcoidosis. Neurology 2016, 87, 743–744. [Google Scholar] [CrossRef]
- Shah, R.; Roberson, G.H.; Curé, J.K. Correlation of MR imaging findings and clinical manifestations in neurosarcoidosis. Am. J. Neuroradiol. 2009, 30, 953–961. [Google Scholar] [CrossRef]
- Kumar, N.; Frohman, E.M. Spinal neurosarcoidosis mimicking an idiopathic inflammatory demyelinating syndrome. Arch. Neurol. 2004, 61, 586–589. [Google Scholar] [CrossRef]




| Case | Age/ Gender | Neurological Presentation | Serum Autoimmune and Infectious Panel | CSF Findings | MRI/CT Imaging Findings | Treatment and Outcome |
|---|---|---|---|---|---|---|
| 1 | 27 y/F | 1-week history of facial droop, numbness on right side of face and left sided weakness | Unremarkable for ANA, ANCA panel, ENA screen SS-A/Ro and SS-B/La antibodies, Anti-Smith antibodies, RNP antibodies, Anti-Scl-70 antibodies, Anti-double stranded DNA antibodies, Anti-chromatin antibodies, Anti-centromere antibodies, and antimitochondrial antibodies. VDRL, Human immunodeficiency virus antibody and lyme serum antibody were all negative. | CSF showed elevated WBC 10/mm3, predominant lymphocytes, protein 77 mg/dL, CSF glucose 69 mg/dL, serum glucose 157 mg/dL AQ-4 antibody was negative. Oligoclonal bands absent. CSF ACE not elevated values 0.6 U/L (ref range 0.0 to 2.5) | MRI brain and cervical spine abnormal enhancement identified within the right internal auditory canal with extension to the right cerebellopontine angle to the root of the VII, VIII cranial nerve complex with hyper-enhancing non-hemorrhagic intramedullary cervical spinal cord spanning the C1 and C2. Multiple separated subtle foci of enhancement at the C3 and within the lateral aspect of the medulla. | Findings from extensive radiological investigations, increased suspicion of sarcoidosis, for which confirmatory biopsy of intradural intramedullary lesions was done. Patient showed some improvement in muscle strength on day 4 of treatment with iv methylprednisolone 1 gm daily for 5 days. She was, then discharged to inpatient rehab with continuing oral steroids. |
| 2 | 51 y/F | 3 weeks of gradually progressive left eye swelling and pain with extra-ocular movements and diplopia with decreased visual acuity. | Unremarkable including ANA, ANCA, ENA screen SS-A/Ro and SS-B/La antibodies, Anti-Smith antibodies, RNP antibodies, Anti-Scl-70 antibodies, Anti-ds-DNA antibodies, Anti-chromatin antibodies, Anti-centromere antibodies, and antimitochondrial antibodies, VDRL, HIV antibody and lyme serum antibody were negative. | CSF study was not performed | MRI orbits showed soft tissue thickening and enhancement with enlarged recti muscles, lacrimal gland and enhancing nodule in the greater wing of left sphenoid concerning for osseous involvement | After subsidence of acute inflammation, the patients were subjected to surgical excision of the mass. Its biopsy confirmed the diagnosis of sarcoidosis which was treated with steroids and showed improvement. Prednisolone 10 mg daily, methotrexate 15 mg weekly maintenance dosage. |
| 3 | 23 y/M | 1-day history of worsening headache with decreased level of consciousness. Also, bowel and bladder incontinence | All infectious and autoimmune disease lab panels were negative as other cases. | CSF WBC 4/mm3, protein 67 mg/dL, CSF glucose 56 mg/dL, serum glucose 101 mg/dL AQP-4 antibody was negative. Oligoclonal bands absent. CSF ACE not elevated values 0.7 U/L (ref range 0.0 to 2.5) | CT brain showed enlarged lateral ventricles and hyper-density around the ventricles with effacement of sulci likely from raised intracranial pressure secondary to obstructive hydrocephalus. Also noted to have calcification in anteroinferior aspect of third ventricle and a nodular mass-like lesion in the foramen of Monro. | Patient underwent an External Ventricular drain (EVD) placement. He was started on prednisone post-biopsy confirmation during admission. On follow up evaluation at 2 months after discharge, patient’s symptoms had resolved and was recommended to continue prednisone 10 mg daily. |
| 4 | 47 y/F | 1-week history of fall at home Referral from another facility due to concerning lesions in the brain Weakness on the left upper and lower extremity | All infectious and autoimmune disease lab panels were negative as other cases. | CSF study was not performed | Multiple peripheral enhancing lesions involving the brainstem and supratentorial brain region. Some of the lesions demonstrated surrounding vasogenic edema. | During admission, biopsy confirmed the diagnosis of sarcoidosis which was well managed by steroids. Methylprednisolone 1 g daily for a total of 5 days. The patient reported an improvement in the left sided weakness on his follow-up visit. In addition to prednisone 60 mg daily |
| 5 | 50 y/M | 8-weeks history of headache behind the eyes with bilateral and progressively worsening vision and blurriness persisting despite refractory correction. | All infectious and autoimmune disease lab panels were negative as other cases. | CSF WBC 12/mm3 predominant lymphocytes, protein >400 mg/dL, CSF glucose 92 mg/dL, serum glucose 188 mg/dL AQP-4 antibody was negative. Oligoclonal bands absent. CSF ACE elevated values 3.7 U/L (ref range0.0 to 2.5) | MRI orbit images reveal enhancement of bilateral optic nerve sheaths. MRI Brain image reveals ill-defined patchy parenchymal hyperintensity in the left cerebellar hemisphere extending to the cerebellar peduncle. | The patient received left optic nerve sheath fenestration in effort to decrease the swelling in the optic nerve. Biopsy of the left optic nerve sheath confirm the diagnosis of sarcoidosis Patient was started on 1 dose of cyclophosphamide with Mesna and discharged with oral tapering prednisone starting at 60 mg dose and follow up in neurology and ophthalmology clinic. Following the treatment, the patient continued to have no light perception in left eye but the visual acuity improved on right eye to 20/40, and recommendation was to follow-up with the neurology outpatient clinic. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feizi, P.; Tandon, M.; Khan, E.; Subedi, R.; Prasad, A.; Chowdhary, A.; Sriwastava, S. Overcoming the Elusiveness of Neurosarcoidosis: Learning from Five Complex Cases. Neurol. Int. 2021, 13, 130-142. https://doi.org/10.3390/neurolint13020013
Feizi P, Tandon M, Khan E, Subedi R, Prasad A, Chowdhary A, Sriwastava S. Overcoming the Elusiveness of Neurosarcoidosis: Learning from Five Complex Cases. Neurology International. 2021; 13(2):130-142. https://doi.org/10.3390/neurolint13020013
Chicago/Turabian StyleFeizi, Parissa, Medha Tandon, Erum Khan, Roshan Subedi, Apoorv Prasad, Anisa Chowdhary, and Shitiz Sriwastava. 2021. "Overcoming the Elusiveness of Neurosarcoidosis: Learning from Five Complex Cases" Neurology International 13, no. 2: 130-142. https://doi.org/10.3390/neurolint13020013
APA StyleFeizi, P., Tandon, M., Khan, E., Subedi, R., Prasad, A., Chowdhary, A., & Sriwastava, S. (2021). Overcoming the Elusiveness of Neurosarcoidosis: Learning from Five Complex Cases. Neurology International, 13(2), 130-142. https://doi.org/10.3390/neurolint13020013






