Abstract
Keratinocyte carcinoma (KC) is a form of skin cancer that develops in keratinocytes, which are the predominant cells present in the epidermis layer of the skin. Keratinocyte carcinoma comprises two sub-types, namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). This review provides a holistic literature assessment of the origin, diagnosis methods, contributing factors, and current topical treatments of KC. Additionally, it explores the increase in KC cases that occurred globally over the past ten years. One of the principal concepts highlighted in this article is the adverse effects linked to conventional treatment methods of KC and how novel treatment strategies that combine phytochemistry and transdermal drug delivery systems offer an alternative approach for treatment. However, more in vitro and in vivo studies are required to fully assess the efficacy, mechanism of action, and safety profile of these phytochemical based transdermal chemotherapeutics.
1. Introduction
Cutaneous carcinoma, or skin cancer, remains one of the highest occurring cancer types, with the number of incidences increasing globally. Although the number of cases differs significantly depending on the geographical region, it remains a major health care problem across the world, as it affects both men and women of every ethnicity/race [1].
There are two criteria that classify skin cancer; the cell from which they originate and their clinical behavior. Skin cancer is categorized into two major groups: non-melanoma skin cancer (NMSC) and malignant melanoma. The cases of non-melanoma skin cancer are generally substantially higher than melanoma cases and are often much less complex to treat compared to melanoma, as it has a lower metastatic potential [2]. Non-melanoma skin cancer, consisting of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is often referred to as keratinocyte carcinoma, as these two subtypes are derived from the epidermal keratinocytes [3].
2. Methodology
A scientific literature search was performed during 2018–2020, using several databases (Science Direct, Google Scholar, Scopus, and Pubmed). The literature search focused on the following topics; KC, BCC, and SCC. Review articles linked to these topics contributed extensively to the structure of the current review. To understand the severity of KC and the increase in cases and mortality, various research papers, and cancer registries were analyzed. Previous research papers that reported on in vitro and in vivo studies regarding the use of medicinal plants, phytochemicals, and transdermal drug carriers provided insights on novel treatments for KC. Literature that was reviewed ranged from the year 1987–2020, which included research articles, patents, book chapters, and review articles.
3. The Origin and History of Keratinocyte Carcinoma
Basal cell carcinoma and SCC originate within the epidermis layer of the skin [4]. The epidermis layer undergoes a process known as homeostasis, where new cells replace old or damaged cells. Homeostasis and the regeneration of skin cells are maintained by stem cells located within the epithelial tissue, which have the ability to differentiate into different cell lineages and are able to self-renew. The epidermis layer is comprised of three main compartments: the interfollicular epidermis, the hair follicle, and the sebaceous and sweat glands [5]. There have been numerous conflicting results on the epidermal lineage of BCC and SCC cells, which are discussed below.
3.1. Basal Cell Carcinoma
The origin of BCC has been debated since the early 1900s. Krompecher defined the origin of BCC as a tumor that arose from the basal cell layer of the interfollicular epidermis [6]. Several researchers over the years have endorsed Krompechers’ definition. However, Lever [7] had a contrary view, which explained that BCC is of follicular origin, derived from an epithelial hair germ. Immunohistochemical studies have been conducted that substantiated Levers’ view [8]. A study conducted by Van Scott and Reinertson in the year 1961, discovered that the growth of tumorous epithelial cells is dependent on their stroma. A later study validated the findings by Van Scott and Reinerston and further reported on the stromal properties, which are fundamental requirements for tumor growth, which include platelet-derived growth factor (PDGF) A and B, and their corresponding receptors α and β. Another study that focused on gene expression profiling of BCC stromal tissue demonstrated that PDGF receptor-like protein displayed an upregulation in BCC stroma. Extensive studies are necessary to accurately assess the effect of the stroma in relation to BCC tumor growth [9,10].
In the year 1824, Aurther Jacob, a member of the royal college of surgeons in Ireland, first described what we now term “basal cell carcinoma”. He conducted immunohistochemical studies that led him to define BCC as a mass of cancerous cells, which were germinative keratinocytes that emerged from hair follicles [11]. Further research conducted by a German pathologist, Krompecher Odon, in 1900 validated the discovery formed by Jacob. In the year 1903, Krompecher published a book called Der Basalzellenkrebs (The Basal Cell Cancer), which explained that these specific tumors arise from the lowest layer of cells present in the skin [6]. A recent study performed by Tan et al. confirmed that BCC originates within the basal layer of the epidermis and arises from the interfollicular epidermis [12].
3.2. Squamous Cell Carcinoma
A review by Kipling et al. provided a fundamental timeline that established the discovery of SCC. Heinrich Bass (Bassius) first described scrotum carcinoma in 1731, which was published in an article entitled ‘Scrotum sphacelo consumptum et fenatum’. In the year 1740, Treyling also confirmed the description of scrotum carcinoma in an article titled ‘Scrotum immaniter auctum scirrhoso scrophulosm’ [13]. However, Percivall Pott, an English surgeon and scientist, was the first to assign occupational cause to the disease. In the year 1775, Pott established that high incidence rates of scrotal cancer were related to chimney soot exposure. Subsequent studies termed this cancer chimney sweeps carcinoma, which can be described as an extremely rare sub-division of SCC. Potts’ discovery was the inception of a plethora of research articles focused on SCC [14,15]. Squamous cell carcinoma arises from the squamous cell layer, which is located in the epidermal layer of the skin [16]. Therefore, SCC can be described as an epithelial malignancy that occurs in organ covered squamous epithelium [17].
4. Incidence and Demographics of Keratinocyte Carcinoma
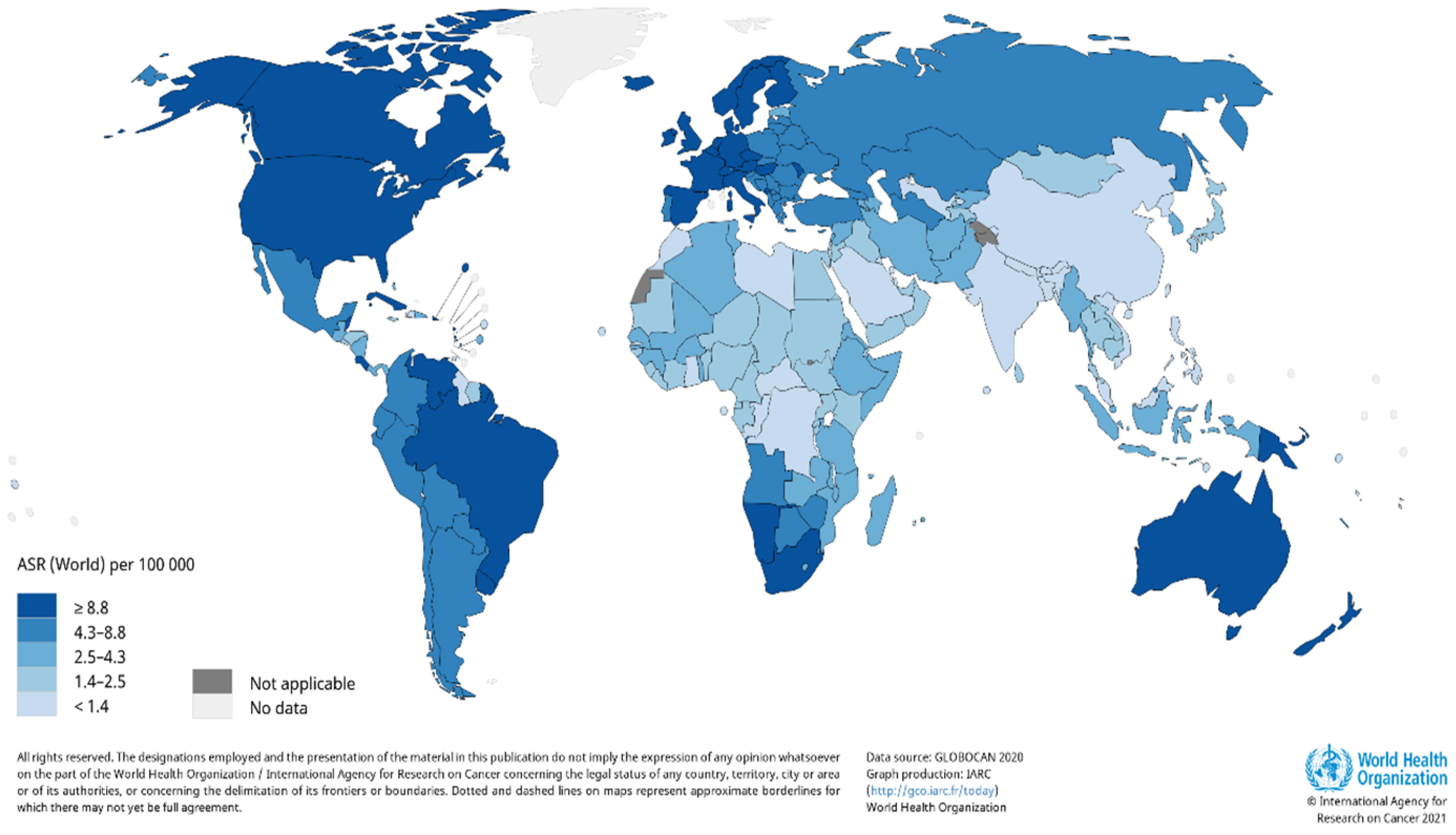
A report by the World Health Organization (WHO) reveals there is are estimated 2–3 million cases of KC cancer that occur globally each year [18]. Due to the incidence of skin cancer not always being reported, the estimated number of cases that occur globally each year are severely underestimated. It is also evident that there is a large variation in the number of reported cases that occur; therefore, the worldwide burden of KC remains unclear. The occurrence rate of KC in the United States (US) documented by the American Cancer Society revealed that an estimated 3.3 million people were diagnosed with KC; however, the number of cases is severely underestimated, as KC cases are not required to be reported to cancer registries [19]. A report by Rogers et al. substantiated the increase in the number of cases, where 5,434,193 million KC cases were diagnosed, and 3,315,554 individuals were treated in the US in 2015 [20]. According to GLOBOCAN, the estimated number of new KC cases in 2018, excluding basal cell carcinoma cases, was 1,042,056 with 65,155 deaths [21]. Estimates for the number of non-melanoma skin cancer incidence worldwide for 2020 were updated by Globocan (Figure 1) [22].
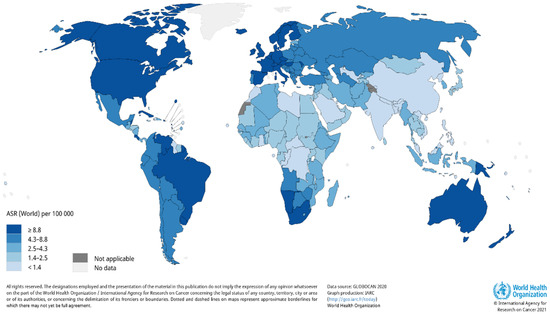
Figure 1.
Estimated age-standardized world-wide incidence rates of non-melanoma skin cancer in 2020. Reproduced from [22]. Copyright 2021, International Agency for Research on Cancer 2021, World Health Organization.
Basal cell carcinoma is the world’s most prevalent human cancer and is accountable for approximately 70–80% of all skin cancers [23,24]. A report by Dessinioti et al. revealed that Australia had the highest incidence of BCC (~1–2%) annually, with approximately 2448 of a 100,000 population diagnosed in 2011, followed by the US (450 per 100,000 in 2010) and Europe (220.1 per 100,000 yearly average) [25,26]. The South African National Cancer Registry reported approximately 14,414 BCC and 6950 SCC cases in 2016 [27]. Another study found that the lowest incidence rates of BCC were in Finland, in comparison to other European countries [28]. This trend is followed closely by Italy, with 88 out of 100,000 people diagnosed with BCC [29]. Squamous cell carcinoma has been reported to contribute approximately 20% of skin cancer cases [30]. Numerous reports indicate a significant incline in SCC incidence rates over the past three decades, with an approximately yearly increase of 3–10% [26]. Currently, SCC incidence rate is estimated to be between 15–35/100,000 people per year and is set to increase between 2–4% annually, due to chronic ultraviolet B (UVB) exposure and an aging population [31].
5. Diagnosis of Keratinocyte Carcinoma
The early diagnosis of KC, such as SCC, is crucial in controlling the risk of cancer becoming invasive and assists in minimizing the complexity of treatment plans. Approximately 70% of all BCCs are found on the head and neck, which can have detrimental effects such as facial deformities and the collapse of certain vital structures [32].
Dermatoscopy, an in vivo imaging diagnostic technique, is used to examine pigmented skin lesions under high magnification in order to distinguish between pigmented BCC and melanoma. Computer tomography or magnetic resonance imaging are used to determine the extent of abnormal cell tissue growth in cartilage, bone, large nerve, eyeball, or parotid gland, whereas skin biopsy aids in identifying KC subtypes and subsequent treatment plans [33]. Lesions that appear clinically abnormal can be tested via biopsy or excision. Abnormal lesions can be examined using incisional biopsy (incision, punch, or shave biopsy), where a section of tissue is removed, or excisional biopsy, where the entire lesion or tissue is removed. Appropriate treatment and prognosis is therefore based on tumor differentiation grade, histologic subtype, degree of dermal invasion, tumor depth, presence or absence of perineural, lymphatic, or vascular invasion, and extension of tumor cells to margins [34,35]. A tumor staging system, such as the Cancer Staging Manual, published by the American Joint Committee on Cancer (AJCC), is used to determine the prognosis and treatment plant based on the location and size of the tumor, whether it has spread to the lymph nodes, and the extent to which it has spread to other parts of the body [34]. There are two types of staging systems, the number staging system (Table 1) or the TNM (tumor, node, and metastasis) staging system. A full description of the TNM staging system has been described by Fahradyan et al. [35].

Table 1.
The number staging system of keratinocyte carcinoma [34].
6. Clinical Variants of BCC
Basal cell carcinoma accounts for approximately 70% of KC cases and most commonly develops on sun-exposed skin, predominantly on the head, but can also develop on the neck, trunk, and lower extremities. It is defined as a slow growing tumor, which rarely metastasizes but can cause facial deformities if left untreated. A characteristic feature of BCC is the formation of island or nests of basaloid cells found in the epidermis, which can invade the dermis depending on the variant of BCC [36,37]. There are numerous subtypes of BCC; however, several main variants are summarized in Table 2.

Table 2.
Clinical variants of basal cell carcinoma.
7. Actinic Keratosis as Precursor Lesions of SCC
Actinic keratosis (AK) is a principal precursor lesion for the formation of SCC. It is often found on parts of the body that are exposed to solar UV radiation such as the forearms, back, scalp, upper chest, face, neck, and back of the hands. Due to the correlation between the development of AK and exposure to UV radiation, it is often found in middle-aged people and the elderly. It can be clinically identified by poorly formed borders, flaky erythroderma, and uneven papules or patches. It is also found on surface areas of the body that exhibit various pre-existing impairments such as uneven pigmentation, telangiectasias, and atrophy. The formation of AK can lead to the development of invasive SCC; however, it can also spontaneously regress or remain a benign AK lesion. Although most cases of SCC have been linked to AK, it has been reported that only 5–10% of AK lesions develop into invasive SCC. Histologically, actinic keratosis is the exponential growth of abnormal keratinocyte cells, which predominately occurs in the lower layers of the epidermis [38]. Additionally, these atypical keratinocytes display an assembly of distinct characteristics such as pleomorphic and hyperchromatic nuclei, polarity deficiency, cell size enlargement, and mitosis enhancement. Several subtypes of AK have been identified that exhibit a broad range of histologic patterns (Table 3) [38,39].
Reports indicate that the rate of progression from AK to SCC ranges from approximately 12% to 20% [40]. A study by Sahin et al. assessed the degree of AK advancement and its link to SCC formation. In this study, evaluations were performed on 115 lesions from 82 patients diagnosed with AK, over a period of eight years, in which the percentage of male patients was 51% and female patients was 48%. This study revealed that the highest percentage of AKs were located on the nose (30.4%), followed by the face (23.5%), lips (8.7%), and ears (7.8%) [41]. Furthermore, when comparing AK localization in males and females, it was observed that AKs frequently occur on the nose of males, whereas in females, they occur on the facial skin. When comparing the subtypes of AK, research indicates that proliferative AK has the highest rate of occurrence at 29.6%. Additionally, acantholytic AKs display a 4% progression rate to SCC [41].

Table 3.
Various subtypes of actinic keratosis.
Table 3.
Various subtypes of actinic keratosis.
| Actinic Keratosis Subtype | Characteristics | Occurrence Percentage (%) | References |
|---|---|---|---|
| Pigmented actinic keratosis |
| 1.7 | [39,41,42] |
| Lichenoid actinic keratosis |
| - | [39,43] |
| Bowenoid actinic keratosis |
| 9.6 | [39,44] |
| Proliferative actinic keratosis |
| 29.6 | [39,44] |
| Hypertrophic actinic keratosis |
| 27 | [39,45] |
| Atrophic actinic keratosis |
| 8.7 | [39,46] |
| Acantholytic actinic keratosis |
| 18.3 | [39,47] |
| Actinic cheilitis/cheilosis (rare variant) |
| 3.5 | [48] |
| Cutaneous horn (uncommon variant) |
| 1.7 | [49] |
8. Squamous Cell Carcinoma
8.1. Squamous Cell Carcinoma In Situ
Squamous cell carcinoma in situ (SCCIS) can be viewed as a fundamental transitional phase from AK to invasive SCC. Currently, the predominant trend in oncology research is the synonymity that exists between Bowen’s disease and SCCIS, exclusively for lesions occurring on non-genital areas [38,39].
Squamous cell carcinoma in situ, which is also known as Bowen’s disease, can be histologically identified by the following characteristics: atypia, which extends through the complete thickness of the epidermis, excluding the adnexal structures, and hyperparakeratosis, which can either be nominal or extremely abundant, which gives rise to a cutaneous horn. Atypical keratinocytes exhibit apoptosis, hyperchromasia, nuclear pleomorphism, as well as a “windblown” appearance when polarity is lost. It is generally defined as a skin disease that does not possess the ability to invade the dermis layer of the skin. Squamous cell carcinoma in situ can develop on any epidermal body site; however, studies indicate that approximately 72% of SCCIS cases occur on sun-exposed skin surfaces, namely the hands, neck, and head. It is therefore often diagnosed in elderly people aged over 60 years old and is rarely diagnosed in individuals under the age of 30. Other areas in which SCCIS can be found include the nail bed, soles of the feet, and palms of the hand. Squamous cell carcinoma in situ can be clinically distinguished by the following features; presence of a distinct plaque or scaly patch, which is benign and displays erythema. Lesions may develop unfavorable properties which include crustation, fissures, hyperkeratosis, and ulcerations. Reports indicate that the risk of SCCIS progressing to invasive SCC is approximately 3–5%. Statistics further revealed that 20% of tumors that evolve to invasive SCC will ultimately metastasize [38,39].
8.2. Invasive Squamous Cell Carcinoma (SCCI)
Invasive squamous cell carcinoma is often referred to as standard SCC. Histologically, it is defined as the vertical invasion of abnormal cells, which originates at the basement membrane and advances into the dermis. It has been reported that approximately 97% of SCCI cases are linked to the malignant progression of AK [50]. There are several variants of SCC (Table 4).

Table 4.
Clinical variants of squamous cell carcinoma.
9. Recurrent and Metastatic KC
Tumor recurrence and incomplete excision of the primary tumor are major risk factors that can increase the possibility of developing metastatic SCC. Studies have revealed that aggressive tumors are susceptible to recurrence and are accountable for approximately 25–30% of SCC metastasis [60]. In a study by Clayman et al., 130 patients, which exhibited advanced or aggressive SCC tumors, displayed a recurrence of 27.5% [61]. Leading characteristics of recurrent tumors include large tumor size, invasion of lympho-vascular or perineural system, and tumor infiltration into subcutaneous tissue [62]. A study that comprised of 603 patients with cutaneous SCC revealed that 89% of patients died from distant metastasis, which is consistent with current SCC metastatic reports [63]. Key features that are linked to metastasis include cancerous cells that disseminate via the lymphatic, which are responsible for 80% of metastases [64], and the fact that the vast majority of metastatic SCC cases occurred on the head and neck of patients [60]. A report by Lazarus et al. presented a study in which 6900 patients had SCC; however, only 142 patients exhibited metastatic lesions. An evaluation of these lesions revealed that lymph nodes are the primary site for metastases occurrence (Table 5) [65]. On the contrary, metastatic BCC cases are rare, with a percentage rate of approximately 0.0028–0.55% [66]. Metastatic BCC follows the same trend as SCC, where 85% of cases originate from tumors that are located in the head and neck region of the patient. Moreover, statistics reveal that a minimum of two-thirds of metastasis cases arise from tumors that are exclusively situated on the face. Tumors that exhibit a specific set of characteristics can be categorized as having a high metastatic potential, such as tumors located in the mid-face or ear, a tumor that has been present for a long period of time, tumor diameter size (>2 cm), and previous radiation treatment [67]. A study by Freitas et al. evaluated 25 BCC cases between the time period of January 2012 and March 2017 and concluded that metastases occur most frequently in lymph nodes (Table 5).

Table 5.
Anatomical sites of metastases occurrence in squamous cell carcinoma and basal cell carcinoma.
10. Risk Factors Associated with the Development of KC
There are various environmental and biological factors that contribute to the development of KC, which are divided into external and internal factors. Identification of high-risk patients allows for early diagnosis and an efficient treatment regime to be established.
10.1. External Risk Factors
10.1.1. Solar UV Radiation
The main external risk factor for developing KC is exposure to solar UV radiation [68]. However, specific patterns of UV radiation exposure result in development of various types of KC. The development of SCC can be linked to long-term sun exposure, whereas BCC formation is associated with excessive sun exposure that transpired in early life as well as intermittent exposure [69]. Furthermore, Kim et al. highlighted that 90% of KC cases are linked to high levels of UV radiation exposure [70]. Solar UV radiation can be divided into three categories, according to a difference in wavelength, namely UVA (320–400 nm), UVB (280–320 nm), and UVC (200–280 nm) [71]. A study conducted by Grossman et al. showed that solar UV radiation induces KC development through DNA damage and immunosuppression [72]. Previously, UVA was reported to primarily be responsible for skin aging; however, recently, UVA has been coupled with UVB, where both are implicated in the development of cutaneous skin cancer. There are different mechanisms by which UVA and UVB cause DNA damage. UVB is known to play a greater role in KC development, due to wavelength penetration depth. UVB radiation is absorbed by cellular components that are present in the epidermis, whereas UVA radiation infiltrates into the basal layer of the epidermis and dermal fibroblasts [73,74]. A study conducted by Boukamp [75], confirmed the findings by Grossman et al. [72] with regards to UVB being a major contributing factor for KC development [75]. This study revealed that the formation of photoproducts, which damage the DNA present in keratinocytes, occurs due to long-term UVB exposure [75]. One of the major photoproducts that form is cyclobutene pyrimidine dimer (CPD). CPDs form thymine dimers (T/T) and pyrimidine-pyrimidone lesions (6-4PP), which, if unrepaired, are mutagenic. This specific type of DNA damage can be repaired by the nucleotide excision repair mechanism (NER); however, malfunction of this mechanism can result in multifocal skin cancer [76]. Additionally, UV radiation has been reported to cause mutations in the suppressor gene existing in the p53 protein. The central function of the p53 tumor suppressor gene is to encode for a protein, which regulates the cell cycle and induces apoptosis [76]. Previous studies have indicated that mutations present in the p53 gene are associated with numerous human cancers and are most prevalent in KCs [77]. According to Kooy et al., UV radiation is able to induce immunosuppression by depleting epidermal dendritic Langerhans cells (CD1a+). As a result, T helper type-1 converts to T helper type-2 response, which inhibits the ability of cell-containing-antigens to induce antitumor immunity [78].
10.1.2. Indoor Tanning
Artificial sources of UV radiation have been categorized as cancer causing agents by the International Agency for Research on Cancer (IARC) in the year 2012 [79]. Indoor tanning generates similar adverse effects in human skin as exposure to solar UVB radiation; however, reports indicate that indoor tanning is 10–15 times stronger than exposure to solar UVA radiation [80]. A review conducted by Wehner et al. investigated approximately 9300 cases of keratinocyte carcinoma and was able to correlate the development of KC to indoor tanning. This report revealed a 67% higher risk of developing SCC and a 29% higher risk for BCC development when exposed to indoor tanning. Studies indicate that exposure to indoor tanning between 16–25 years leads to a greater risk of developing BCC [81].
10.1.3. Ionizing Radiation
The association between exposure to ionizing radiation and skin cancer formation was discovered by radiologists that were working with X-ray analysis. Furthermore, SCC development was observed in skin locations that exhibited dermatitis and ulceration, which was caused due to high-level ionizing radiation exposure, whereas formation of BCC was seen in low to moderate exposure to ionizing radiation [82]. Ionizing radiation is used as a form of treatment for various types of cancers; however, this is often associated with the development of radiation dermatitis, which occurs in approximately 95% of patients receiving radiation therapy. This is due to damage caused to the basal keratinocytes and hair follicle stem cells, which is followed by double-stranded DNA breaks and inflammation caused by the production of reactive oxygen species [83].
10.1.4. Arsenic Exposure
Studies indicate that the two leading characteristics that arsenic exhibits are its ability to act as a toxin and as a carcinogen. The mechanism of action is to target and negatively alter the cellular processes within various organ systems based on a dose and time-dependent manner. The toxic effects of arsenic are first displayed in the skin, which can include the development of BCC and SCC. Development of SCC from arsenic exposure is said to be more aggressive in nature when compared to chronic UV-induced SCC. Statistics reveal that 33% of untreated arsenic-induced SCC demonstrates metastatic behavior [84].
10.2. Internal Factors
10.2.1. Age
Studies have shown that the development of KC is more prevalent in elderly patients with a median age diagnosis of 70 years and older [85]. There are several factors that potentially contribute to the frequent occurrence of KC in the elderly, namely prolonged exposure to solar UV radiation and repair mechanisms of the cell that are not functioning at optimum levels [86]. Transformations that occur in the immune system of elderly patients result in immune suppression, which can lead to opportunistic disease development, of which malignancies are frequently observed [87].
10.2.2. Skin Type
Skin type and pigmentation is a crucial factor regarding the development of KC. Skin type 1 is represented by people who have fair skin, light blue, and grey eyes, with light red and blonde hair, and are more susceptible to KC formation. The number of KC cases occur more frequently in fair-skinned individuals as compared to dark-skinned individuals. This is due to low levels of melanin present in the skin. Melanin, which is responsible for skin pigmentation, has photoprotective properties. It protects the skin by acting as a physical barrier to UV radiation and is furthermore able to absorb UV radiation, thereby limiting the amount of UV that penetrates the skin. Melanin is produced in the melanosomes, which is translocated to adjacent keratinocytes via dendrites [88,89].
10.2.3. Immunosuppression
Immunosuppression is a significant contributing factor for the development of KC, more specifically SCC. The development of KC occurs more frequently in patients that exhibit immunodeficiency, which includes patients that use immunosuppressive drug therapy in cases such as solid-organ transplantation, auto-immune inflammatory diseases, and human immunodeficiency virus (HIV) infection. Patients who have received organ transplants have an increased risk (20–200-fold) of developing SCC. A study revealed the incidence ratio of SCC caused by solid-organ transplantation to be 1355/100,000, whereas the incidence ratio of SCC in the general population was 38/100,000 [90].
11. Topical Pharmacotherapies Currently Used for the Treatment of KC
Topical chemotherapy is a form of non-surgical therapy used to remove or eliminate localized skin cancer cells. There are several topical treatments available (Table 6), which can induce cell death through the direct damage to DNA/RNA, such as 5-fluorouracil, or act as immune-modulators, such as imiquimod, which stimulates the production of various cytokines, thereby inducing antitumor activity. Topical treatments are often a consideration when patients are elderly or unhealthy; therefore, surgery may not be an option, or for individuals who have tumors/lesions on areas that cosmetically sensitive, and therefore surgery may result in a disfiguring scar [91].

Table 6.
Existing topical pharmacotherapies for the treatment of KC.
12. Transdermal Delivery of Drugs
A fundamental approach used to enhance bioavailability of pharmaceutical drugs is developing novel drug delivery systems. Although oral delivery systems are preferred for pharmaceutical drug administration, these systems are linked to several disadvantages such as insufficient drug stability within the gastrointestinal tract, reduced drug concentration upon reaching its site of action due to metabolism, and decreased drug solubility in intestinal fluid resulting in poor permeability through the intestinal membrane [117]. Due to the large surface area and accessibility of the skin, extensive investigations of drug delivery via the skin have been conducted. Administration of drugs through the skin can result in the drug being confined within the skin (topical application) or the drug can penetrate through the skin, allowing it to reach the blood circulatory system (transdermal delivery). There are numerous advantages associated with transdermal drug delivery when compared to conventional drug administration routes, these include; non-invasiveness, increased patient compliance, enhanced drug bioavailability, more cost effective and decreased drug plasma fluctuations [118]. However, it is imperative to consider physiological and physiochemical factors, as these factors influence a drug’s movement through skin. A drug’s absorption rate is primarily controlled by skin age, moisture contents, and anatomical location. Aged skin has a decreased moisture contents, resulting in reduced and slower absorption when compared to younger skin. An increase in temperature leads to an enhanced absorption rate, whereas a reduction in blood flow results in a drug’s influx being negatively impacted [119]. An ideal transdermal drug should possess characteristics such as a short half-life, low molecular weight (less than 1000 Da), bio-compatible, low melting point, and an affinity for both hydrophilic and lipophilic phases [120].
12.1. Targeted Delivery Through the Skin
The main objective of drugs that exert their pharmaceutical effect topically is to target a multitude of sites that exist in different skin layers, skin appendages, and underlying tissue. Studies indicate that the systemic circulatory system is predominately targeted by transdermal drug compounds; moreover, the anatomical structures of interest are hair follicles, nerves, Langerhans cells, keratinocytes, and melanocytes within the epidermis. It is imperative that drug candidates for transdermal delivery include the following characteristics: limited sites for hydrogen bonding, have a low molecular weight, average lipophilicity, and a low melting point [121]. The permeation of a drug occurs through the stratum corneum and can be calculated by Fick’s second law:
where J represents the transport flux, Dm is the diffusion coefficient of the drug present in the membrane, Cv represents the drug concentration that exists in the vehicle, P is the drug partition coefficient, and L represents the thickness of the stratum corneum [122].
12.2. Transdermal Drug Permeation Routes
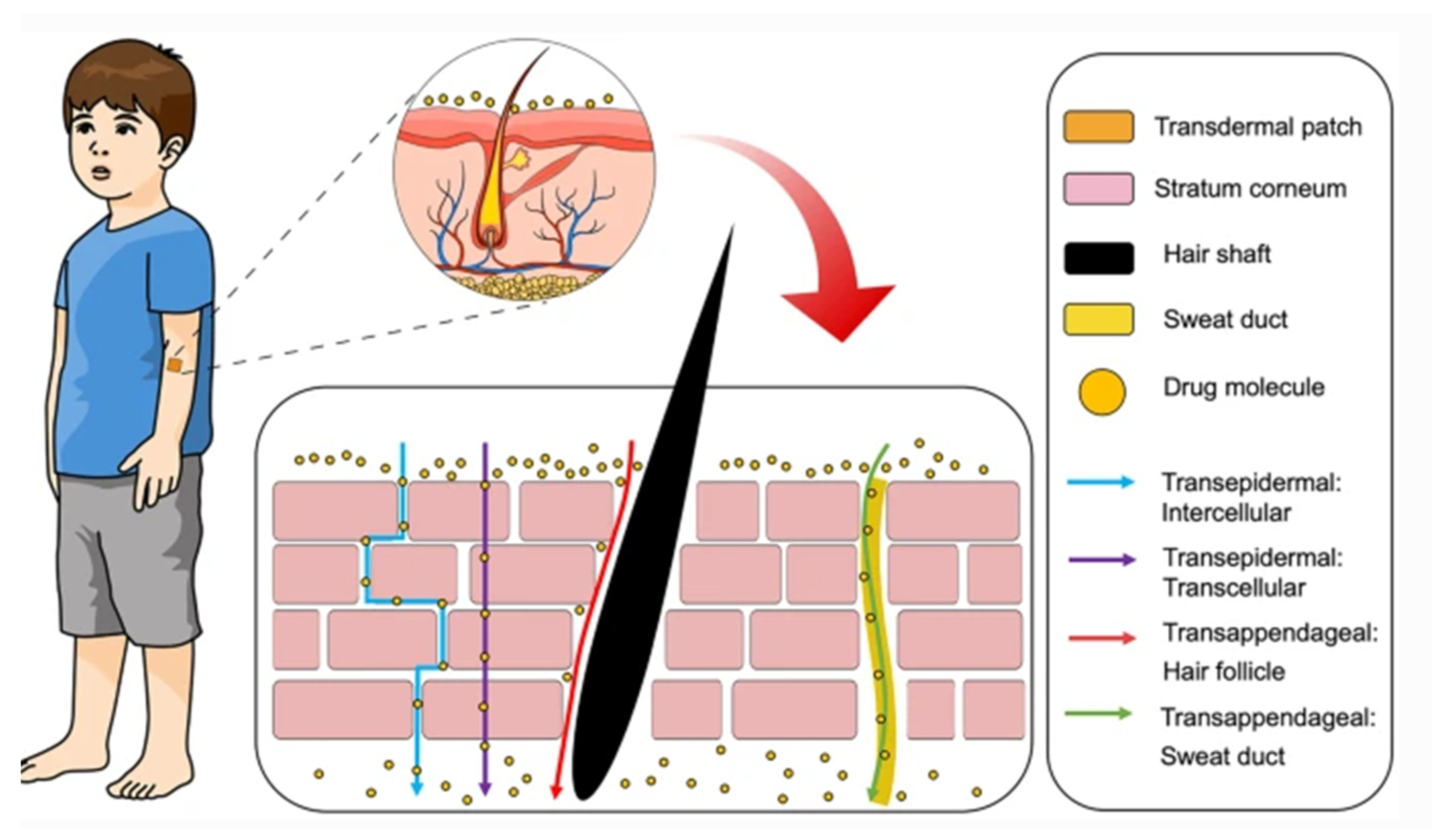
Administration of drugs through the skin can follow two potential routes, trans-epidermal and trans-appendegeal (Figure 2) [117]. The trans-epidermal pathway allows for molecules to pass through the stratum corneum and can be further sub-divided into intracellular and intercellular pathways [123]. The intracellular route depicts a pathway that permits the transport of hydrophilic or polar solutes through differentiated keratinocytes known as corneocytes, whereas the intercellular route allows the transport of lipophilic or non-polar solutes via intercellular spaces in the lipid matrix. The second transdermal drug route (trans-appendageal route) represents a pathway that allows the permeation of molecules via hair follicles that are associated with sebaceous glands as well as sweat glands [123]. This route is suitable for ions and large polar molecules, which experience difficulty in stratum corneum permeation [124].
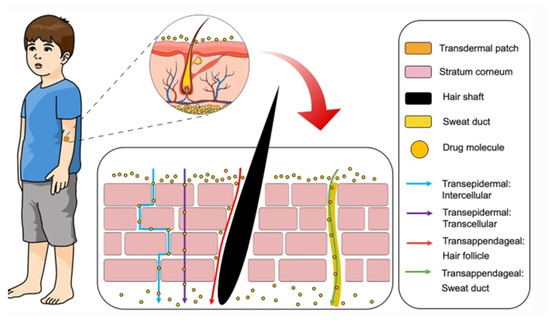
Figure 2.
Transdermal delivery pathways in the skin. Reproduced with permission from [117]. This is an open access article distributed under the terms of the Creative Commons CC BY license.
12.3. Transdermal Delivery of Skin Cancer Drugs
The majority of chemotherapeutic drugs are distributed using the systemic circulatory system and are capable of generating cytotoxic effects on healthy cells. Therefore, transdermal drug delivery of anticancer compounds can be viewed as an attractive alternative, due to the two essential advantages of transdermal drug delivery systems, namely increased therapeutic benefit and enhanced drug targeting. However, there are several challenges that have been linked to this treatment method, including increased concentration, lack of bioavailability, and penetration of drug compounds possessing antineoplastic properties [125,126]. Due to the advancements in technology, anticancer macromolecules, inclusive of proteins and nucleic acids, that experience difficulty penetrating the stratum corneum can be delivered with the assistance of penetration enhancers, physical enhancement devices, and micro-carriers [127].
Studies have reported on two types of penetration enhancers, namely chemical and biological. Chemical penetration enhancers can be described as compounds that facilitate increased drug penetration through the skin, such as alcohol, terpenes, esters, fatty acids, polyols, and surfactants. Reports on various mechanisms of action for this type of enhancer include facilitating disturbances within the stratum corneum, intercellular protein interaction, and enhanced drug segmentation within the stratum corneum [128]. Biological enhancers are peptide-based and are capable of transporting an array of compounds, such as nucleic acids, proteins, polymers, and nanoparticles, throughout the skin with minimal toxicity. Physical enhancement devices can be characterized by the implementation of electric fields such as electroporation, iontophoresis, and sonophoresis [127]. Further literature explained that the fundamental purpose of these techniques is based on the ability to exert a momentary and reversible disintegration of the stratum corneum, which results in enhanced permeation of an antineoplastic drug at a tumor site [129].
In recent times, micro-nanocarrier systems that include inorganic nanoparticles, nano-emulsions, dendritic nanocarriers, and liposomes have attracted increased attention in the field of transdermal drug delivery, as they offer various advantages, such as increased skin penetration, enhanced solubility, and implementation of controlled release [127]. Within the micro-nanocarrier system, there exist several elements that greatly contribute to skin permeability, such as charge, shape, and size of nanomaterials [130].
This review explored several transdermal drug delivery and micro-nanocarrier systems for their application in skin disorders and skin cancer (Table 7).

Table 7.
Transdermal drug delivery and micro-nanocarrier systems in skin disorders and skin cancer.
13. Potential Therapeutic Effects of Phytochemical/Medicinal Plants against KC
Due to the considerable incline of KC cases worldwide, researchers have a vested interest in developing novel treatment options [139]. The fundamental purpose of an anticancer therapeutic strategy is the ability to selectivity target malignant tumor cells, that either inhibits their growth or induces cell death [140]. However, the vast majority of KC treatments that are currently available, also have a detrimental effect on non-tumors cells. In an effort to create cutting-edge, more effective and non-toxic anticancer treatments, researchers have explored the possibility of isolating compounds from natural sources, more specifically phytochemicals [141].
Studies have reported that phytochemicals and medicinal plants exhibit potential anticancer properties. From the year 1940 to 2014, approximately 50% of approved anticancer therapeutic agents were either obtained or derived from natural sources [142]. Several medicinal plants, and their bioactive compounds, have been investigated for potential anticancer activity. These have been tested against skin tumors, in both in vitro and in vivo studies, and have exhibited noteworthy activity by arresting the development and proliferation of skin tumor cells [143]. Phytochemicals have the potential to alter various molecular processes associated with the development of skin cancer and subsequently inhibit tumor proliferation [144]. Some of the reported studies on medicinal plants and phytochemicals have been summarized in Table 8. Additionally, emerging KC treatments that are currently in the clinical trial phase have been summarized in Table 9.

Table 8.
The therapeutic effect of medicinal plants and phytochemicals against keratinocyte carcinoma.

Table 9.
Plant derived bioactives and their biological effect on keratinocyte carcinoma.
Often, public perception is that herbal or medicinal plant treatments are deemed safe and effective, as is the case with the use of black salve. Black salve has not undergone any clinical trials in order to evaluate its safety or efficacy against skin cancer and is available to purchase over-the-counter or online. A review by Lim summarizes numerous case reports on the use of black salve for skin neoplasms; however, almost each of the cases reported side effects that included severe tissue necrosis and damage as well as scarring ulceration [176]. A major alkaloid compound present within bloodroot is sanguinarine, which has been linked to the potential carcinogenic effect of bloodroot; however, due to contradictory in vitro and in vivo results, the carcinogenic classification of sanguinarine has not yet been established [177].
Furthermore, there is a lack of quality control and quantification or standardization of the active ingredients within these “home” remedies. Black salve has been largely linked to the occurrence of facial deformities, which needs to be corrected through cosmetic reconstruction, which emphasizes the need for the correct safety and efficacy trials to be conducted before these types of remedies are made available on the market for use.
Similarly, in a pilot trial, betulin oleogel-S10 was initially shown to be effective in treating AK, with a clearance rate of 64%; however, this was a small trial and did not include the use of a placebo control [178]. Consequently, a larger study on the use of betulin oleogel-S10 was conducted by Pflugfelder et al. [175], including a placebo control group, which concluded that the topical use of betulin-oleaogel-S10 did not significantly clear AK when compared to the placebo group.
Camptothecin, an alkaloid isolated from Camptotheca acuminata Decne. is a cytotoxic compound that inhibits DNA topoisomerase I [179]; however, due to its insolubility in biocompatible solvents, it remains a challenge to effectively administer it intravenously. However, in a study by Lin et al. [132], α-MSH liposomes that encapsulate camptothecin showed an enhanced antiproliferative activity against melanoma cells, when compared to camptothecin alone, due to the targeted delivery and controlled release of camptothecin, which emphasizes the importance of using appropriate drug delivery systems. Similarly, a study by Kessels et al. [171] showed that a 10% sinecatechin ointment, which contained EGCG, was not effective in treating superficial BCC; however, it was concluded that this may be due to a lack of EGCG uptake by the cancerous cells and that using liposomes as a drug delivery mechanism may enhance the uptake and effectiveness of EGCG.
14. Conclusions
The number of KC cases is increasing at an alarming rate globally. Moreover, statistics revealed that South Africa is one of the leading countries in the world, with the highest rates of skin cancer cases. Although the current topical treatments available for KC have shown promising results, they have also been associated with numerous adverse effects. Therefore, the discovery, development, and treatment of KC using plants and their natural products are becoming increasingly sort after. Natural plant compounds, such as polyphenolics and alkaloids, have in numerous studies demonstrated increased antiproliferative and anticancer activity. Studies have shown that these natural compounds are capable of functioning both independently and interdependently. Various reports further indicated the emergence of nanocarrier and transdermal delivery systems and the numerous advantages they offer, specifically to overcome current challenges faced in cancer treatment, such as bioavailability, targeted delivery, and systemic toxicity. However, it is important to note that case studies alone are not considered conclusive to determine or evaluate the safety and efficacy of a test substance such as a plant extract or natural product. Large clinical trials are required that include a placebo control group and different skin types in order to determine the effectiveness of a test substance.
Although there have been numerous reports on the antiproliferative activity of plant extracts and their natural products on non-melanoma skin cancer cell lines, there have been relatively few studies that have evaluated the potential of these plants/natural products in clinical trials. Additionally, although some plant extracts or natural products may not have shown significant in vitro activity or anticancer activity in animal models when used alone, a suitable drug delivery system may provide for enhanced delivery and efficacy and therefore should further be explored.
The topical application of EGCG and caffeine, using different transdermal delivery systems, should be considered for further evaluation, as this may result in increased uptake of phytochemicals by the cancerous cells. In addition, the use of capsaicin against skin cancer should be further assessed, as it showed promising results in two patients but has not been assessed in larger clinical trials. The potential of synthesizing capsaicin derivatives may provide a source of novel compounds with decreased irritant effects and increased efficacy. Ursolic acid, which has extensively been studied for its activity against melanoma, has not been well documented for its activity against keratinocyte carcinomas and therefore should be considered for further assessment.
It is, however, important to note that upon identification of a plant extract or compound showing significant activity, the mechanism of action, as well as the efficacy and toxicity potential of the identified extract or compounds requires further evaluation using clinical trials, which includes the pharmacodynamics and pharmacokinetic properties of the plant extract/compound.
Author Contributions
Conceptulisation and preparation of original draft (A.J.J., D.T.). Provided expert opinion and critical review (D.T., S.K.P., N.L. and S.S.R.). All authors have read and agreed to the published version of the manuscript.
Funding
A.J.J., S.K.P. and S.S.R. thank the Department of Science and Innovation and the Council for Scientific and Industrial Research for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Nehal, K.S.; Bichakjian, C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef] [PubMed]
- ‘Skin Cancer Types|the Woodruff Institute’. Available online: https://www.thewoodruffinstitute.com/skin-cancer-types/ (accessed on 10 March 2021).
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [PubMed]
- Crouch, H.E. History of basal cell carcinoma and its treatment. J. R. Soc. Med. 1983, 76, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Lever, W.F. Pathogenesis of benign tumors of cutaneous appendages and of basal cell epithelioma: I. benign tumors of the cutaneous appendages. Arch. Derm. Syphilol. 1948, 57, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K. Basal cell carcinoma: Cell of origin, cancer stem cell hypothesis and stem cell markers. Br. J. Dermatol. 2011, 164, 696–711. [Google Scholar] [CrossRef]
- Ponten, F.; Ren, Z.; Nister, M.; Westermark, B.; Ponten, J. Epithelial-Stromal Interactions in Basal Cell Cancer: The PDGF System. J. Investig. Dermatol. 1994, 102. [Google Scholar] [CrossRef]
- Micke, P.; Kappert, K.; Ohshima, M.; Sundquist, C.; Scheidl, S.; Lindahl, P.; Heldin, C.H.; Botling, J.; Ponten, F.; Östman, A. In situ identification of genes regulated specifically in fibroblasts of human basal cell carcinoma. J. Investig. Dermatol. 2007, 127, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Jaks, V.; Hohl, D.; Toftgård, R. Basal cell carcinoma—Molecular biology and potential new therapies. J. Clin. Investig. 2012, 122, 455–463. [Google Scholar] [CrossRef]
- Tan, S.T.; Ghaznawie, M.; Heenan, P.J.; Dosan, R. Basal cell carcinoma arises from interfollicular layer of epidermis. J. Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Kipling, M.D.; Usherwood, R.; Varley, R. A monstrous growth: An historical note on carcinoma of the scrotum. Br. J. Ind. Med. 1970, 27, 382–384. [Google Scholar] [CrossRef][Green Version]
- Azike, J.E. A Review of the History, Epidemiology and Treatment of Squamous Cell Carcinoma of the Scrotum. Rare Tumors 2009, 1, 47–49. [Google Scholar] [CrossRef]
- Johnson, T.M.; Rowe, D.E.; Nelson, B.R.; Swanson, N.A. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J. Am. Acad. Dermatol. 1992, 26, 467–484. [Google Scholar] [CrossRef]
- Centres for Disease Control and Prevention, ‘What Is Skin Cancer?|CDC’. Available online: https://www.cdc.gov/cancer/skin/basic_info/what-is-skin-cancer.htm (accessed on 11 November 2020).
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar] [PubMed]
- World Health Organization, ‘Radiation: Ultraviolet (UV) Radiation and Skin Cancer. 2017. Available online: https://www.who.int/news-room/q-a-detail/ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 11 November 2020).
- Choquet, H.; Ashrafzadeh, S.; Kim, Y.; Asgari, M.M.; Jorgenson, E. Genetic and environmental factors underlying keratinocyte carcinoma risk. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- World Health Organization—International Agency for Research on Cancer, ‘Cancer Today’. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=17&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D (accessed on 10 March 2021).
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146, 1–6. [Google Scholar] [CrossRef]
- Ciaźyńska, M.; Narbutt, J.; Woźniacka, A.; Lesiak, A. Trends in basal cell carcinoma incidence rates: A 16-yearretrospective study of a population in central Poland. Postep. Dermatol. Alergol. 2018, 35, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Antoniou, C.; Katsambas, A.; Stratigos, A.J. Basal cell carcinoma: What’s new under the sun. Photochem. Photobiol. 2010, 86, 481–491. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef]
- South African National Health Laboratory Service, ‘National Cancer Registry’. 2016. Available online: https://cansa.org.za/files/2020/10/NCR_2016_Report_updated_14April2020.pdf (accessed on 11 November 2020).
- Hannuksela-Svahn, A.; Pukkala, E.; Karvonen, J. Basal cell skin carcinoma and other nonmelanoma skin cancers in Finland from 1956 through 1995. Arch. Dermatol. 1999, 135, 781–786. [Google Scholar] [CrossRef]
- Boi, S.; Cristofolini, M.; Micciolo, R.; Polla, E.; Palma, P.D. Epidemiology of Skin Tumors: Data from the Cutaneous Cancer Registry in Trentino, Italy. J. Cutan. Med. Surg. Inc. Med. Surg. Dermatol. 2020, 7, 300–305. Available online: https://www.academia.edu/5028523/Epidemiology_of_Skin_Tumors_Data_from_the_Cutaneous_Cancer_Registry_in_Trentino_Italy (accessed on 11 November 2020). [CrossRef]
- Smoller, B.R. Lever’s Histopathology of the Skin, 10th edition. J. Cutan. Pathol. 2009, 36, 605. [Google Scholar]
- Motaparthi, K.; Kapil, J.P.; Velazquez, E.F. Cutaneous Squamous Cell Carcinoma: Review of the Eighth Edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv. Anat. Pathol. 2017, 24, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Tirumani, S.H.; Giardino, A.; Rosenthal, M.H.; Tirumani, H.; Krajewski, K.; Ramaiya, N.H. Basal Cell Carcinoma: A Comprehensive Review for the Radiologist. Am. J. Roentgenol. 2015, 204, W132–W140. [Google Scholar] [CrossRef]
- Dębski, T.; Lembas, L.; Jethon, J. Basal Cell Carcinoma, Current Concepts in Plastic Surgery; Agullo, F., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0398-1. [Google Scholar]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. AJCC Cancer Staging Handbook—From the AJCC Cancer Staging Manual|Stephen Edge|Springer. 2010. Available online: https://www.springer.com/la/book/9780387884424 (accessed on 27 March 2019).
- Fahradyan, A.; Howell, A.; Wolfswinkel, E.; Tsuha, M.; Sheth, P.; Wong, A. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, L.; Rusinova, D.; Botev, I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol. Online J. 2013, 4, 12. [Google Scholar] [CrossRef]
- McDaniel, B.; Bermudez, R. Epitheliomas, Basal Cell. StatPearls 2018, 2, 161–168. [Google Scholar]
- Yanofsky, V.R.; Mercer, S.E.; Phelps, R.G. Histopathological Variants of Cutaneous Squamous Cell Carcinoma: A Review. J. Skin Cancer 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Parekh, V.; Seykora, J.T. Cutaneous Squamous Cell Carcinoma. Clin. Lab. Med. 2017, 37, 503–525. [Google Scholar] [CrossRef]
- McKee, P.H.; Calonje, E.; Granter, S.R. Pathology of the Skin with Clinical Correlations, 3rd ed.; Mosby Ltd.: St. Louis, MO, USA, 2005. [Google Scholar]
- Şahin, N.; Bozdaǧ, Z.; Erkiliç, S.; Aydin, N.E.; Şener, S. Histopathological subtyping of actinic keratosis and it’s coexistence with nonmelanotic skin cancers in Gaziantep and Malatya regions. Turkderm Turk. Arch. Dematol. Venereol. 2016, 50, 103–108. [Google Scholar] [CrossRef]
- Abudu, B.; Calame, A.; Cohen, P.R. Pigmented Actinic Keratosis: Case Report and Review of an Uncommon Actinic Keratosis Variant that can Mimic Melanoma. Cureus 2019, 11. [Google Scholar] [CrossRef]
- Maor, D.; Ondhia, C.; Yu, L.L.; Chan, J.J. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin. Exp. Dermatol. 2017, 42, 663–666. [Google Scholar] [CrossRef]
- Goldberg, L.H.; Joseph, A.K.; Tschen, J.A. Proliferative actinic keratosis. Int. J. Dermatol. 1994, 33, 341–345. [Google Scholar] [CrossRef]
- Billano, R.A.; Little, W.P. Hypertrophic actinic keratosis. J. Am. Acad. Dermatol. 1982, 7, 484–489. [Google Scholar] [CrossRef]
- Person, J.R. An actinic keratosis is neither malignant nor premalignant: It is an initiated tumor. J. Am. Acad. Dermatol. 2003, 48, 637–638. [Google Scholar] [CrossRef]
- Carapeto, F.J.; García-Pérez, A. Acantholytic Keratosis. Dermatology 1974, 148, 233–239. [Google Scholar] [CrossRef]
- Kim, S.M.; Myoung, H.; Eo, M.Y.; Cho, Y.J.; Lee, S.K. Proper management of suspicious actinic cheilitis. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 15. [Google Scholar] [CrossRef]
- Copcu, E.; Sivrioglu, N.; Culhaci, N. Cutaneous horns: Are these lesions as innocent as they seem to be? World J. Surg. Oncol. 2004, 2, 18. [Google Scholar] [CrossRef]
- Ulrich, M.; Stockfleth, E.; Roewert-Huber, J.; Astner, S. Noninvasive diagnostic tools for nonmelanoma skin cancer. Br. J. Dermatol. 2007, 157, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Hawrot, A.; Alam, M.; Ratner, D. Squamous cell carcinoma. Curr. Probl. Dermatol. 2003, 15, 91–133. [Google Scholar] [CrossRef]
- Ogawa, T.; Kiuru, M.; Konia, T.H.; Fung, M.A. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not “high risk”: Diagnosis, management, and clinical outcomes in a series of 115 cases. J. Am. Acad. Dermatol. 2017, 76, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Nappi, O.; Pettinato, G.; Wick, M.R. Adenoid (acantholytic) squamous cell carcinoma of the skin. J. Cutan. Pathol. 1989, 16, 114–121. [Google Scholar] [CrossRef]
- Silvis, N.G.; Swanson, P.E.; Manivel, J.C.; Kaye, V.N.; Wick, M.R. Spindle-cell and pleomorphic neoplasms of the skin. A clinicopathologic and immunohistochemical study of 30 cases, with emphasis on “atypical fibroxanthomas”. Am. J. Dermatopathol. 1988, 10, 9–19. [Google Scholar] [CrossRef]
- Schwartz, R.A. Verrucous carcinoma of the skin and mucosa. J. Am. Acad. Dermatol. 1995, 32, 1–21. [Google Scholar] [CrossRef]
- Kuo, T. Clear cell carcinoma of the skin. A variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am. J. Surg. Pathol. 1980, 4, 573–583. [Google Scholar] [CrossRef]
- Ko, C.J.; McNiff, J.M.; Glusac, E.J. Squamous cell carcinomas with single cell infiltration: A potential diagnostic pitfall and the utility of MNF116 and p63. J. Cutan. Pathol. 2008, 35, 353–357. [Google Scholar] [CrossRef]
- Weber, F.; Bauer, J.W.; Sepp, N.; Högler, W.; Salmhofer, W.; Hintner, H.; Fritsch, P. Squamous cell carcinoma in junctional and dystrophic epidermolysis bullosa. Acta Derm. Venereol. 2001, 81, 189–192. [Google Scholar] [CrossRef]
- Sabin, S.R.; Goldstein, G.; Rosenthal, H.G.; Haynes, K.K. Aggressive Squamous Cell Carcinoma Originating as a Marjolin’s Ulcer. Dermatol. Surg. 2004, 30, 229–230. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S.; et al. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Brunner, M.; Veness, M.J.; Ch’Ng, S.; Elliott, M.; Clark, J.R. Distant metastases from cutaneous squamous cell carcinoma-analysis of AJCC stage IV. Head Neck 2013, 35, 72–75. [Google Scholar] [CrossRef]
- Bonerandi, J.J.; Beauvillain, C.; Caquant, L.; Chassagne, J.F.; Chaussade, V.; Clavère, P.; Desouches, C.; Garnier, F.; Grolleau, J.L.; Grossin, M.; et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Herzig, R.H.; Bornstein, R.; Laipply, T.C. Metastatic squamous cell carcinoma of the skin. J. Natl. Med. Assoc. 1980, 72, 1196–1199. [Google Scholar] [PubMed]
- Piva De Freitas, P.; Senna, C.G.; Tabai, M.; Chone, C.T.; Altemani, A. Metastatic Basal Cell Carcinoma: A Rare Manifestation of a Common Disease. Case Rep. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Vu, A.; Laub, D., Jr. Metastatic Basal Cell Carcinoma. In Basal Cell Carcinoma; InTech: London, UK, 2012. [Google Scholar]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012 empirical relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef]
- Kim, I.Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar]
- Tran, T.N.T.; Schulman, J.; Fisher, D.E. UV and pigmentation: Molecular mechanisms and social controversies. Pigment. Cell Melanoma Res. 2008, 21, 509–516. [Google Scholar]
- Grossman, D. The Molecular Basis of Nonmelanoma Skin Cancer. Arch. Dermatol. 1997, 133, 1263. [Google Scholar]
- Ibrahim, S.F.; Brown, M.D. Tanning and Cutaneous Malignancy. Dermatol. Surg. 2008, 34, 460–474. [Google Scholar]
- Latonen, L.; Laiho, M. Cellular UV damage responses—Functions of tumor suppressor p53. Biochim. Biophys. Acta Rev. Cancer 2005, 1755, 71–89. [Google Scholar]
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar]
- Marks, R. The epidemiology of non-melanoma skin cancer: Who, why and what can we do about it. J. Dermatol. 1995, 22, 853–857. [Google Scholar] [CrossRef]
- Soehnge, H.; Ouhtit, A.; Ananthaswamy, O.N. Mechanisms of induction of skin cancer by UV radiation. Front. Biosci. 1997, 2. [Google Scholar] [CrossRef]
- Kooy, A.J.W.; Prens, E.P.; Van Heuklelum, A.; Vuzevski, V.D.; Van Joost, T.; Tank, B. Interferon-γ-induced ICAM-1 and CD40 expression, complete lack of HLA- DR and CD80 (B7.1), and inconsistent HLA-ABC expression in basal cell carcinoma: A possible role for interleukin-10? J. Pathol. 1999, 187, 351–357. [Google Scholar]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. Special Report: Policy A Review of Human Carcinogens-Part. D: Radiation. Lancet 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Gerber, B.; Mathys, P.; Moser, M.; Bressoud, D.; Braun-Fahrländer, C. Ultraviolet Emission Spectra of Sunbeds. Photochem. Photobiol. 2002, 76, 664. [Google Scholar] [CrossRef]
- Wehner, M.R.; Shive, M.L.; Chren, M.M.; Han, J.; Qureshi, A.A.; Linos, E. Indoor tanning and non-melanoma skin cancer: Systematic review and meta-analysis. BMJ 2012, 345, e5909. [Google Scholar] [CrossRef]
- Karagas, M.R.; Nelson, H.H.; Zens, M.S.; Linet, M.; Stukel, T.A.; Spencer, S.; Applebaum, K.M.; Mott, L.; Mabuchi, K. Squamous cell and basal cell carcinoma of the skin in relation to radiation therapy and potential modification of risk by sun exposure. Epidemiology 2007, 18, 776–784. [Google Scholar] [CrossRef]
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef]
- Hunt, K.M.; Srivastava, R.K.; Athar, M. Cutaneous Toxicology of Arsenic. In Handbook of Arsenic Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 301–314. ISBN 9780124199552. [Google Scholar]
- Leus, A.J.G.; Frie, M.; Haisma, M.S.; Terra, J.B.; Plaat, B.E.C.; Steenbakkers, R.J.H.M.; Halmos, G.B.; Rácz, E. Treatment of keratinocyte carcinoma in elderly patients—a review of the current literature. J. Eur. Acad. Dermatol. Venereol. 2020, jdv.16268. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Giordano, M.; Cappellani, A.; Berretta, M.; Malaguarnera, M.; Perrotta, R. Skin Cancers in Elderly Patients. Anticancer Agents Med. Chem. 2013, 13, 1406–1411. [Google Scholar] [CrossRef]
- Perrotta, R.E.; Giordano, M.; Malaguarnera, M. Non-melanoma skin cancers in elderly patients. Crit. Rev. Oncol. Hematol. 2011, 80, 474–480. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte carcinomas: Current concepts and future research priorities. Clin. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Ferraro, S.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part II. Clinical applications. J. Am. Acad. Dermatol. 2014, 70, e1–e979. [Google Scholar] [CrossRef]
- Lanoue, J.; Goldenberg, G. Basal cell carcinoma: A comprehensive review of existing and emerging nonsurgical therapies. J. Clin. Aesthet. Dermatol. 2016, 9, 26–36. [Google Scholar] [PubMed]
- Eaglstein, W.H.; Weinstein, G.D.; Frost, P. Fluorouracil: Mechanism of Action in Human Skin and Actinic Keratoses: I. Effect on DNA Synthesis in Vivo. Arch. Dermatol. 1970, 101, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- How to Use Fluorouracil and Imiquimod for Non-Melanoma Skin Cancer in a General Practice Setting. 2017. Available online: https://bpac.org.nz/2017/skin-cancer.aspx (accessed on 28 July 2018).
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef]
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557. [Google Scholar] [CrossRef]
- Chosidow, O.; Dummer, R. Imiquimod: Mode of action and therapeutic potential. Acta Derm. Venereol. Suppl. (Stockh.) 2003, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [CrossRef]
- Doan, H.Q.; Gulati, N.; Levis, W.R. Ingenol mebutate: Potential for further development of cancer immunotherapy. J. Drugs Dermatol. 2012, 11, 1156–1157. [Google Scholar]
- Lebwohl, M.; Sohn, A. Ingenol mebutate (ingenol 3-angelate, PEP005): Focus on its uses in the treatment of nonmelanoma skin cancer. Expert Rev. Dermatol. 2012, 7, 121–128. [Google Scholar] [CrossRef]
- Cozzi, S.J.; Le, T.T.; Ogbourne, S.M.; James, C.; Suhrbier, A. Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch. Dermatol. Res. 2013, 305, 79–83. [Google Scholar] [CrossRef]
- Moreno Romero, J.A.; Campoy, A.; Perez, N.; Garcia, F.; Grimalt, R. Rapidly-growing squamous cell carcinoma shortly after treatment with ingenol mebutate for actinic keratoses: Report of two cases. Br. J. Dermatol. 2015, 173, 1514–1517. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Vrouenraets, M.B.; Visser, G.W.M.; Snow, G.B.; Van Dongen, G.A.M.S. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar]
- Cohen, D.K.; Lee, P.K. Photodynamic therapy for non-melanoma skin cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef]
- Matei, C.; Tampa, M.; Poteca, T.; Panea-Paunica, G.; Georgescu, S.R.; Ion, R.M.; Popescu, S.M.; Giurcaneanu, C. Photodynamic therapy in the treatment of basal cell carcinoma. J. Med. Life 2013, 6, 50–54. [Google Scholar]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef]
- Christensen, E.; Mørk, C.; Skogvoll, E. High and sustained efficacy after two sessions of topical 5-aminolaevulinic acid photodynamic therapy for basal cell carcinoma: A prospective, clinical and histological 10-year follow-up study. Br. J. Dermatol. 2012, 166. [Google Scholar] [CrossRef]
- Ahmadi, S.; McCarron, P.A.; Donnelly, R.F.; Woolfson, A.D.; McKenna, K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: A pilot study. Exp. Dermatol. 2004, 13. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Mosterd, K.; Arits, A.H.M.M.; Roozeboom, M.H.; Sommer, A.; Essers, B.A.B.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Steijlen, P.M.; Nelemans, P.J.; et al. Five-Year Results of a Randomized Controlled Trial Comparing Effectiveness of Photodynamic Therapy, Topical Imiquimod, and Topical 5-Fluorouracil in Patients with Superficial Basal Cell Carcinoma. J. Investig. Dermatol. 2018, 138, 527–533. [Google Scholar] [CrossRef]
- Tschen, E.H.; Wong, D.S.; Pariser, D.M.; Dunlap, F.E.; Houlihan, A.; Ferdon, M.B. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: Phase IV multicentre clinical trial with 12-month follow up. Br. J. Dermatol. 2006, 155. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Chimenti, S. Preliminary Observations on the Use of Topical Tazarotene to Treat Basal-Cell Carcinoma. N. Engl. J. Med. 1999, 341, 1767–1768. [Google Scholar] [CrossRef]
- Bardazzi, F.; Bianchi, F.; Parente, G.; Guareschi, E.; Landi, C. A pilot study on the use of topical tazarotene to treat squamous cell carcinoma in situ. J. Am. Acad. Dermatol. 2005, 52, 1102–1104. [Google Scholar] [CrossRef]
- Bianchi, L.; Orlandi, A.; Campione, E.; Angeloni, C.; Costanzo, A.; Spagnoli, L.G.; Chimenti, S. Topical treatment of basal cell carcinoma with tazarotene: A clinicopathological study on a large series of cases. Br. J. Dermatol. 2004, 151, 148–156. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.S.; Lee, G.Y.; Kim, J.K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef]
- Yavuz, H.; Çetin, K.; Akgönüllü, S.; Battal, D.; Denizli, A. Therapeutic protein and drug imprinted nanostructures as controlled delivery tools. In Design and Development of New Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–473. [Google Scholar]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal Drug Delivery System: A Review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Delouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, G.; Chen, G.; Zheng, Y.; He, B.; Gu, Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020, 13, 1810–1824. [Google Scholar] [CrossRef]
- Roy, N.; Agrawal, M.; Chaudhary, S.; Tirkey, V.; Dhwaj, A.; Mishra, N. Review article on permeation enhancers: A major breakthrough in drug delivery technology. Int. J. Pharm. Sci. Res. 2017, 8, 1001. [Google Scholar]
- Fleury, S.; Vianna Lopez, R.F. Topical Administration of Anticancer Drugs for Skin Cancer Treatment. In Skin Cancers—Risk Factors, Prevention and Therapy; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Moses, M.A.; Brem, H.; Langer, R. Advancing the field of drug delivery: Taking aim at cancer. Cancer Cell 2003, 4, 337–341. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Ramzan, M.; Ahsan, M.N.; Ur Rehman, Z.; Ahmad, F.J. Elastic liposome-based gel for topical delivery of 5-fluorouracil: In vitro and in vivo investigation. Drug Deliv. 2016, 23, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Al-Suwayeh, S.A.; Hung, C.F.; Chen, C.C.; Fang, J.Y. Camptothecin-loaded liposomes with α-melanocyte-stimulating hormone enhance cytotoxicity toward and cellular uptake by melanomas: An application of nanomedicine on natural product. J. Tradit. Complement. Med. 2013, 3, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Ogilvie, J.; McKenna, J.; Segal, J.; Scurr, D.; Marlow, M. Intradermal Delivery of an Immunomodulator for Basal Cell Carcinoma; Expanding the Mechanistic Insight into Solid Microneedle-Enhanced Delivery of Hydrophobic Molecules. Mol. Pharm. 2020, 17, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Jin, L.M.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. Injectable intratumoral hydrogel as 5-fluorouracil drug depot. Biomaterials 2013, 34. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carriers for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 2395–2408. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Usoltseva (Menshova), R.V.; Silchenko, A.S.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural features and anticancer activity in vitro of fucoidan derivatives from brown alga Saccharina cichorioides. Carbohydr. Polym. 2017, 157, 1503–1510. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Penta, D.; Somashekar, B.S.; Meeran, S.M. Epigenetics of skin cancer: Interventions by selected bioactive phytochemicals. Photodermatol. Photoimmunol. Photomed. 2018, 34, 42–49. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Afzal Shah, S.; Alam, M.M.; et al. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019, 109, 1381–1393. [Google Scholar] [CrossRef]
- Fox, F.E.; Niu, Z.; Tobia, A.; Rook, A.H. Photoactivated Hypericin is an Anti-Proliferative Agent that Induces a High Rate of Apoptotic Death of Normal, Transformed, and Malignant T Lymphocytes: Implications for the Treatment of Cutaneous Lymphoproliferative and Inflammatory Disorders. J. Investig. Dermatol. 1998, 111, 327–332. [Google Scholar] [CrossRef]
- Alecu, M.; Ursaciuc, C.; Hãlãlãu, F.; Coman, G.; Merlevede, W.; Waelkens, E.; De Witte, P. Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res. 1998, 18, 4651–4654. [Google Scholar]
- Kacerovská, D.; Pizinger, K.; Majer, F.; Šmíd, F. Photodynamic therapy of nonmelanoma skin cancer with topical Hypericum perforatum extract—A pilot study. Photochem. Photobiol. 2008, 84, 779–785. [Google Scholar] [CrossRef]
- Head, C.S.; Luu, Q.; Sercarz, J.; Saxton, R. Photodynamic therapy and tumor imaging of hypericin-treated squamous cell carcinoma. World J. Surg. Oncol. 2006, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Eastman, K.L.; McFarland, L.V.; Raugi, G.J. Buyer beware: A black salve caution. J. Am. Acad. Dermatol. 2011, 65, e154–e155. [Google Scholar] [CrossRef]
- Saltzberg, F.; Barron, G.; Fenske, N. Deforming Self-Treatment with Herbal “Black Salve”. Dermatol. Surg. 2009, 35, 1152–1154. [Google Scholar] [CrossRef]
- Osswald, S.S.; Elston, D.M.; Farley, M.F.; Alberti, J.G.; Cordero, S.C.; Kalasinsky, V.F. Self-treatment of a basal cell carcinoma with “black and yellow salve”. J. Am. Acad. Dermatol. 2005, 53, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Affleck, A.G.; Varma, S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br. J. Dermatol. 2007, 157, 1078–1079. [Google Scholar] [CrossRef]
- McDaniel, S.; Goldman, G.D. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch. Dermatol. 2002, 138, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.W.; Goldstein, G.D.; Birkby, C.S. Auto-Mohs.com. Dermatol. Surg. 2001, 27, 975–978. [Google Scholar]
- Es-saady, D.; Simon, A.; Ollier, M.; Maurizis, J.C.; Chulia, A.J.; Delage, C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996, 106, 193–197. [Google Scholar] [CrossRef]
- Junco, J.J.; Cho, J.; Mancha, A.; Malik, G.; Wei, S.J.; Kim, D.J.; Liang, H.; DiGiovanni, J.; Slaga, T.J. Role of AMPK and PPARα in the anti-skin cancer effects of ursolic acid. Mol. Carcinog. 2018, 57, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Liu, Y.; Zhang, L.; Yan, L.; Fan, F.; Shen, C.; Wang, A.; Zheng, S.; Wang, S.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwashita, K.; Kobori, M.; Yamaki, K.; Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in b16 melanoma 4A5 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1813–1820. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakashima, S.; Oda, Y.; Nakamura, S.; Yoshikawa, M. Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg. Med. Chem. 2009, 17. [Google Scholar] [CrossRef]
- Verschooten, L.; Smaers, K.; Van Kelst, S.; Proby, C.; Maes, D.; Declercq, L.; Agostinis, P.; Garmyn, M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J. Investig. Dermatol. 2010, 130, 2277–2285. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G1-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar] [PubMed]
- Hail, N.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292. [Google Scholar] [CrossRef]
- US20100166895A1—Capsicum Extract for Treatment of Skin Cancer—Google Patents’. Available online: https://patents.google.com/patent/US20100166895 (accessed on 15 November 2020).
- Rademan, S.; Anantharaju, P.G.; Madhunapantula, S.V.; Lall, N. The anti-proliferative and antioxidant activity of four indigenous South African plants. Afr. J. Tradit. Complement. Altern. Med. 2019, 16, 13–23. [Google Scholar] [CrossRef]
- Rezadoost, M.H.; Kumleh, H.H.; Ghasempour, A. Cytotoxicity and apoptosis induction in breast cancer, skin cancer and glioblastoma cells by plant extracts. Mol. Biol. Rep. 2019, 46, 5131–5142. [Google Scholar] [CrossRef]
- Twilley, D.; Kishore, N.; Meyer, D.; Kumar, V. Lall The Effect of Helichrysum odoratissimum (L.) Sweet on Cancer Cell Proliferation and Cytokine Production. Int. J. Pharmacogn. Phytochem. Res. 2017, 9. [Google Scholar] [CrossRef]
- Twilley, D.; Langhansová, L.; Palaniswamy, D.; Lall, N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S. Afr. J. Bot. 2017, 112, 494–500. [Google Scholar] [CrossRef]
- Vijaybabu, K.; Punnagai, K. In-vitro anti-proliferative effects of ethanolic extract of vanilla planifolia leaf extract against A431 human epidermoid carcinoma cells. Biomed. Pharmacol. J. 2019, 12, 1141–1146. [Google Scholar]
- Iliescu, I.A.; Peter, S.; Albert, I.; Skalicka-Woźniak, K.; Miron, A.; Luca, S.V.; Wolfram, E. Verbascum nigrum: Cytotoxicity Evaluation in A431 Epidermoid Carcinoma Cells and Untargeted LC-HR-MS/MS Metabolite Profiling. Chem. Biodivers. 2020, 17, e2000644. [Google Scholar] [CrossRef] [PubMed]
- Kessels, J.; Voeten, L.; Nelemans, P.; Cleutjens, J.; Hillen, L.M.; Mosterd, K.; Kelleners-Smeets, N.W.J. Topical sinecatechins, 10%, ointment for superficial basal cell carcinoma: A randomized clinical trial. JAMA Dermatol. 2017, 153, 1061–1063. [Google Scholar] [CrossRef]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar] [CrossRef]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical Curcumin-Based Cream Is Equivalent to Dietary Curcumin in a Skin Cancer Model. J. Skin Cancer 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.P.; Lou, Y.R.; Xie, J.G.; Peng, Q.Y.; Liao, J.; Yang, C.S.; Huang, M.T.; Conney, A.H. Topical applications of caffeine or (-)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef]
- Pflugfelder, A.; Andonov, E.; Weide, B.; Dirschka, T.; Schempp, C.; Stockfleth, E.; Stratigos, A.; Krüger-Krasagakis, S.; Bauer, J.; Garbe, C.; et al. Lack of activity of betulin-based Oleogel-S10 in the treatment of actinic keratoses: A randomized, multicentre, placebo-controlled double-blind phase II trial. Br. J. Dermatol. 2015, 172, 926–932. [Google Scholar] [PubMed]
- Lim, A. Black salve treatment of skin cancer: A review. J. Dermatolog. Treat. 2018, 29, 388–392. [Google Scholar] [PubMed]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Simanek, V.; Liu, L. Carcinogenic potential of sanguinarine, a phytochemical used in ‘therapeutic’ black salve and mouthwash. Mutat. Res. Rev. Mutat. Res. 2017, 774, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Reuter, J.; Rodig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2009, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Camptothecin|C20H16N2O4—PubChem’. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Camptothecine (accessed on 17 November 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).