Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest
Abstract
:1. Introduction
2. Results
2.1. In Vitro Inhibition of Digestive Enzymes
2.2. In Vitro Inhibition of Human Aldose Reductase
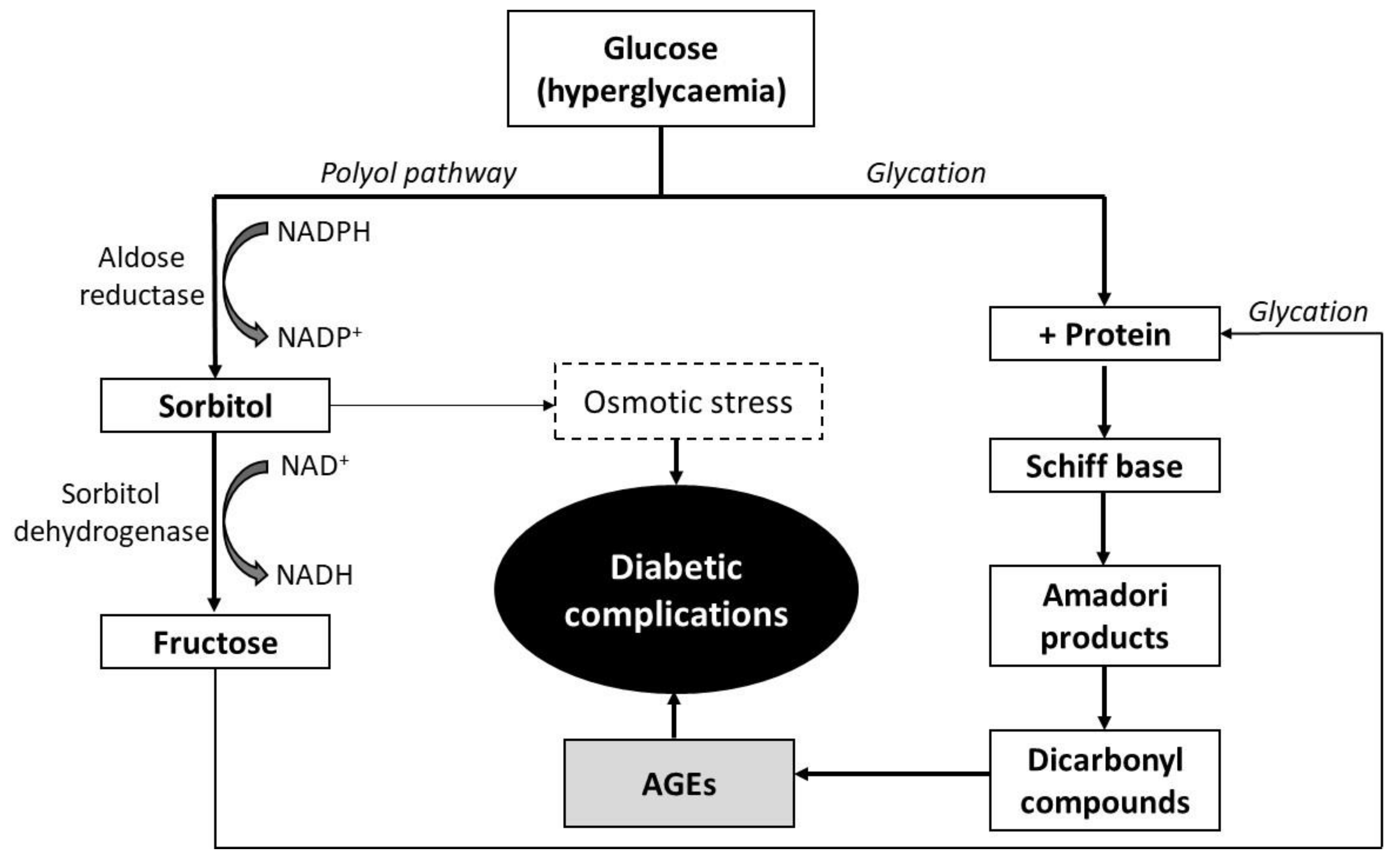
2.3. In Vitro Inhibition of Ribose Mediated BSA-Glycation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation and Extraction of Phenolic Compounds
3.3. In Vitro Anti-Diabetic and Anti-Obesity Assays
3.3.1. α-Amylase Inhibition Assay
3.3.2. Rat α-Glucosidase Inhibition Assay
3.3.3. Pancreatic Lipase Inhibition Assay
3.3.4. Aldose Reductase Inhibition Assay
3.3.5. BSA Glycation Inhibition Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Indrianingsih, A.W.; Tachibana, S.; Itoh, K. In vitro evaluation of antioxidant and α-glucosidase inhibitory assay of several tropical and subtropical plants. Procedia Environ. Sci. 2015, 28, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Tsai, S.F.; Lee, S.S. Flavonoid glycosides from the leaves of Machilus philippinensis. J. Chin. Chem. Soc. 2011, 58, 555–562. [Google Scholar] [CrossRef]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 210–218. [Google Scholar]
- Li, T.; Kongstad, K.T.; Staerk, D. Identification of α-glucosidase inhibitors in Machilus litseifolia by combined use of high-resolution α-glucosidase inhibition profiling and HPLC-PDA-HRMS-SPE-NMR. J. Nat. Prod. 2019, 82, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-S.; Lin, H.-C.; Chen, C.-K. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry 2008, 69, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Balaji, M.; Ganjayi, M.S.; Hanuma Kumar, G.E.N.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef]
- Khedr, N.F.; Ebeid, A.M.; Khalil, R.M. New insights into weight management by orlistat in comparison with cinnamon as a natural lipase inhibitor. Endocrine 2020, 67, 109–116. [Google Scholar] [CrossRef]
- Sellami, M.; Louati, H.; Kamoun, J.; Kchaou, A.; Damak, M.; Gargouri, Y. Inhibition of pancreatic lipase and amylase by extracts of different spices and plants. Int. J. Food Sci. Nutr. 2017, 68, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kouamé, N.M.; Koffi, C.; N’zoué, K.S.; Yao, N.A.R.; Doukouré, B.; Kamagaté, M. Comparative antidiabetic activity of aqueous, ethanol, and methanol leaf extracts of Persea americana and their effectiveness in type 2 diabetic rats. Evidence-Based Complement. Altern. Med. 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Duletić-Laušević, S.; Oalđe, M.; Alimpić-Aradski, A. In vitro evaluation of antioxidant, antineurodegenerative and antidiabetic activities of Ocimum basilicum L., Laurus nobilis L. leaves and Citrus reticulata Blanco peel extracts. Lek. Sirovine 2019, 39, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ervina, M.; Lie, H.S.; Diva, J.; Caroline, S.; Tewfik, I. Optimization of water extract of Cinnamomum burmannii bark to ascertain its in vitro antidiabetic and antioxidant activities. Biocatal. Agric. Biotechnol. 2019, 19, 101152. [Google Scholar] [CrossRef]
- Arachchige, S.P.G.; Abeysekera, W.P.K.M.; Ratnasooriya, W.D. Antiamylase, anticholinesterases, antiglycation, and glycation reversing potential of bark and leaf of Ceylon cinnamon (Cinnamomum zeylanicum Blume) in vitro. Evid.-Based Complement. Altern. Med. 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, J.L.; Bowden, R.G.; Willoughby, D.S. Cassia cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. J. Diet. Suppl. 2016, 13, 461–471. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chao, J.; Sun, Z.; Chang, R.C.C.; Tse, I.; Li, E.T.S.; Chen, F.; Wang, M. Beneficial effects of cinnamon proanthocyanidins on the formation of specific advanced glycation endproducts and methylglyoxal-induced impairment on glucose consumption. J. Agric. Food Chem. 2010, 58, 6692–6696. [Google Scholar] [CrossRef]
- Lin, G.M.; Chen, Y.H.; Yen, P.L.; Chang, S.T. Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J. Tradit. Complement. Med. 2016, 6, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Ogawa, S.; Akihiro, T.; Yokota, K. Structural analysis of A-type or B-type highly polymeric proanthocyanidins by thiolytic degradation and the implication in their inhibitory effects on pancreatic lipase. J. Chromatogr. A 2011, 1218, 7704–7712. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.M.; Lin, H.Y.; Hsu, C.Y.; Chang, S.T. Structural characterization and bioactivity of proanthocyanidins from indigenous cinnamon (Cinnamomum osmophloeum). J. Sci. Food Agric. 2016, 96, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Spínola, V.; Castilho, P.C. Phenolic profiles of Lauraceae plant species endemic to Laurisilva forest: A chemotaxonomic survey. Ind. Crops Prod. 2017, 107, 1–12. [Google Scholar] [CrossRef]
- Ogundajo, A.L.; Adeniran, L.A.; Ashafa, A.O. Medicinal properties of Ocotea bullata stem bark extracts: Phytochemical constituents, antioxidant, anti-inflammatory activity, cytotoxicity and inhibition of carbohydrate-metabolizaing enzymes. J. Integr. Med. 2018, 16, 132–140. [Google Scholar] [CrossRef]
- Mahadeva, R.U. Phytochemical screening and in vitro antioxidant and anti-diabetic potentials of Persea americana Mill. (Lauraceae) fruit extract. Univ. J. Pharm. Res. 2018, 3, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Alhassan, A.; Sule, M.; El-ta’alu, A.; Lawal, A. In vitro inhibitory activities of Persea americana seed extracts on α-amylase and α-glucosidase. Bayero J. Pure Appl. Sci. 2017, 10, 546–552. [Google Scholar] [CrossRef]
- Njateng, G.S.S.; Zaib, S.; Chimi, L.Y.; Feudjio, C.; Mouokeu, R.S.; Gatsing, D.; Kuiate, J.-R.; Adewole, E.; Iqbal, J. Antidiabetic potential of methanol extracts from leaves of Piper umbellatum L. and Persea americana Mill. Asian Pac. J. Trop. Biomed. 2018, 8, 160–165. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohshima, T.; Kan, C.; Mimaki, Y. Chemical constituents of the leaves of Tussilago farfara and their aldose reductase inhibitory activity. Nat. Prod. Commun. 2016, 11, 1661–1664. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, M.I.; Ashafa, A.O.T.; Nafiu, M.O. Biological activities of three nigerian spices—Laurus nobilis linn, Murraya koenigii (L) spreng and Thymus vulgaris Linn. Trop. J. Pharm. Res. 2015, 14, 2255–2261. [Google Scholar] [CrossRef] [Green Version]
- Ramdan, B.; Ramdan, R.; El Karbane, M.; El Maadoudi, M.; Mrid, B.R.; Nhiri, M. Anti-glycation study on hydro-alcohol and aqueous extracts of Moroccan plant species. Int. J. Res. Pharm. Sci. 2018, 10, 826–837. [Google Scholar] [CrossRef]
- Khan, A.; Zaman, G.; Anderson, R.A. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J. Clin. Biochem. Nutr. 2009, 44, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Okafor, S.C.; Gyang, S.S.; Maiha, B.B.; Eze, E.D.; Yakubu, M.I.; Chindo, B.A. Aqueous extract of Persea americana leaves ameliorates alloxan-induced hyperglycaemia and hyperlipidaemia in rats. J. Med. Plants Res. 2017, 11, 755–762. [Google Scholar] [CrossRef]
- Khan, I.; Shah, S.; Ahmad, J.; Abdullah, A.; Johnson, S.K. Effect of incorporating Bay leaves in cookies on postprandial glycemia, appetite, palatability, and gastrointestinal well-being. J. Am. Coll. Nutr. 2017, 36, 514–519. [Google Scholar] [CrossRef]
- Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. α-Amylase and lipase inhibitory activity and structural characterization of Acacia bark proanthocyanidins. J. Nat. Prod. 2011, 74, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, Y.J.; Jung, S.H.; Kim, J.H.; Kim, J.S. Flavonoids from Litsea japonica inhibit AGEs formation and rat lense aldose reductase in vitro and vessel dilation in zebrafish. Planta Med. 2017, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Iwao, T.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, W.; Khoo, C.; Taylor, J.; Gu, L. Cranberry phytochemicals inhibit glycation of human hemoglobin and serum albumin by scavenging reactive carbonyls. Food Funct. 2011, 2, 475–482. [Google Scholar] [CrossRef]
- Dearlove, R.P.; Greenspan, P.; Hartle, D.K.; Swanson, R.B.; Hargrove, J.L. Inhibition of protein glycation by extracts of culinary herbs and spices. J. Med. Food 2008, 11, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yagiz, Y.; Buran, T.J.; do Nunes, C.; Gu, L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Harris, C.S.; Beaulieu, L.-P.; Fraser, M.-H.; McIntyre, K.L.; Owen, P.L.; Martineau, L.C.; Cuerrier, A.; Johns, T.; Haddad, P.S.; Bennett, S.A.L.; et al. Inhibitory effect of the Cree traditional medicine Wiishichimanaanh (Vaccinium vitisidaea) on advanced glycation endproduct formation: Identification of active principles. Planta Med. 2011, 77, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Ju, E.; Choi, I.; Ahmad, S. Quercetin as a finer substitute to aminoguanidine in the inhibition of glycation products. Int. J. Biol. Macromol. 2015, 77, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein g. LWT 2020, 118. [Google Scholar] [CrossRef]
| Phenolic Acids | Flavones | Flavan-3-ols | Flavones | Flavanones | TIPC | Extraction Yield (%) | |
|---|---|---|---|---|---|---|---|
| A. barbujana | Not detected | 15.56 c | 72.82 d | 0.60 d | Not detected | 88.98 d | 4.62 |
| L. novocanariensis | 0.5 a | 2.69 a | 64.59 c | 0.23 c | Not detected | 68.01 c | 4.68 |
| O. foetens | Not detected | Not detected | 18.35 b | 0.09 a | Not detected | 18.44 a | 4.48 |
| P. indica | 5.6 b | 7.36 b | 6.36 a | 0.15 b | 2.28 | 21.75 b | 9.84 |
| α-Amylase | α-Glucosidase | Lipase | Aldose Reductase | BSA Glycation | |
|---|---|---|---|---|---|
| A. barbujana | 1.15 ± 0.05 d | 5.54 ± 0.20 f | 2.60 ± 0.12 b | 0.38 ± 0.01 c | 1.15 ± 0.03 d |
| L. novocanariensis | 0.57 ± 0.02 c | 4.08 ± 0.20 e | 2.40 ± 0.10 b | 0.37 ± 0.02 c | 1.06 ± 0.04 c |
| O. foetens | 1.52 ± 0.03 e | 3.59 ± 0.12 d | 3.69 ± 0.08 c | 1.02 ± 0.05 e | 2.42 ± 0.16 e |
| P. indica | 0.50 ± 0.02 b | 2.83 ± 0.09 c | 4.70 ± 0.12 d | 0.28 ± 0.01 b | 0.96 ± 0.04 c |
| Acarbose | 0.02 ± 0.001 a | 0.12 ± 0.01 b | − | − | − |
| 1-Deoxynojirimycin | − | 0.01 ± 0.01 a | − | − | − |
| Orlistat | − | − | 0.47 ± 0.02 a | − | − |
| Quercetin | − | − | − | 0.10 ± 0.01 a | 0.11 ± 0.01 a |
| (+)-Catechin | − | − | − | 0.76 ± 0.04 d | 0.24 ± 0.02 b |
| Aminoguanidine | − | − | − | − | 9.56 ± 0.36 f |
| Parameters | α-Amylase | α-Glucosidase | Lipase | Aldose Reductase | BSA Glycation |
|---|---|---|---|---|---|
| TIPC | −0.657 | −0.621 | −0.269 | −0.482 | −0.497 |
| Phenolic acids | −0.020 | −0.310 | +0.839 | −0.492 | −0.467 |
| Flavonols | −0.792 | −0.756 | −0.740 | −0.761 | −0.735 |
| Flavan-3-ols/PACs | −0.773 | −0.705 | −0.874 | −0.325 | −0.350 |
| Flavones | +0.097 | +0.917 | −0.367 | −0.433 | −0.424 |
| Flavanones | −0.296 | +0.464 | +0.870 | −0.222 | −0.254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. https://doi.org/10.3390/molecules26072023
Spínola V, Castilho PC. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules. 2021; 26(7):2023. https://doi.org/10.3390/molecules26072023
Chicago/Turabian StyleSpínola, Vítor, and Paula C. Castilho. 2021. "Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest" Molecules 26, no. 7: 2023. https://doi.org/10.3390/molecules26072023
APA StyleSpínola, V., & Castilho, P. C. (2021). Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules, 26(7), 2023. https://doi.org/10.3390/molecules26072023







