Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation
Abstract
:1. Introduction
2. The Objectives and Hypothesis of the Work
3. Results and Discussion
3.1. Skin Hydration
3.2. Transepidermal Water Loss (TEWL)
3.3. Skin Elasticity
3.4. Skin Relief
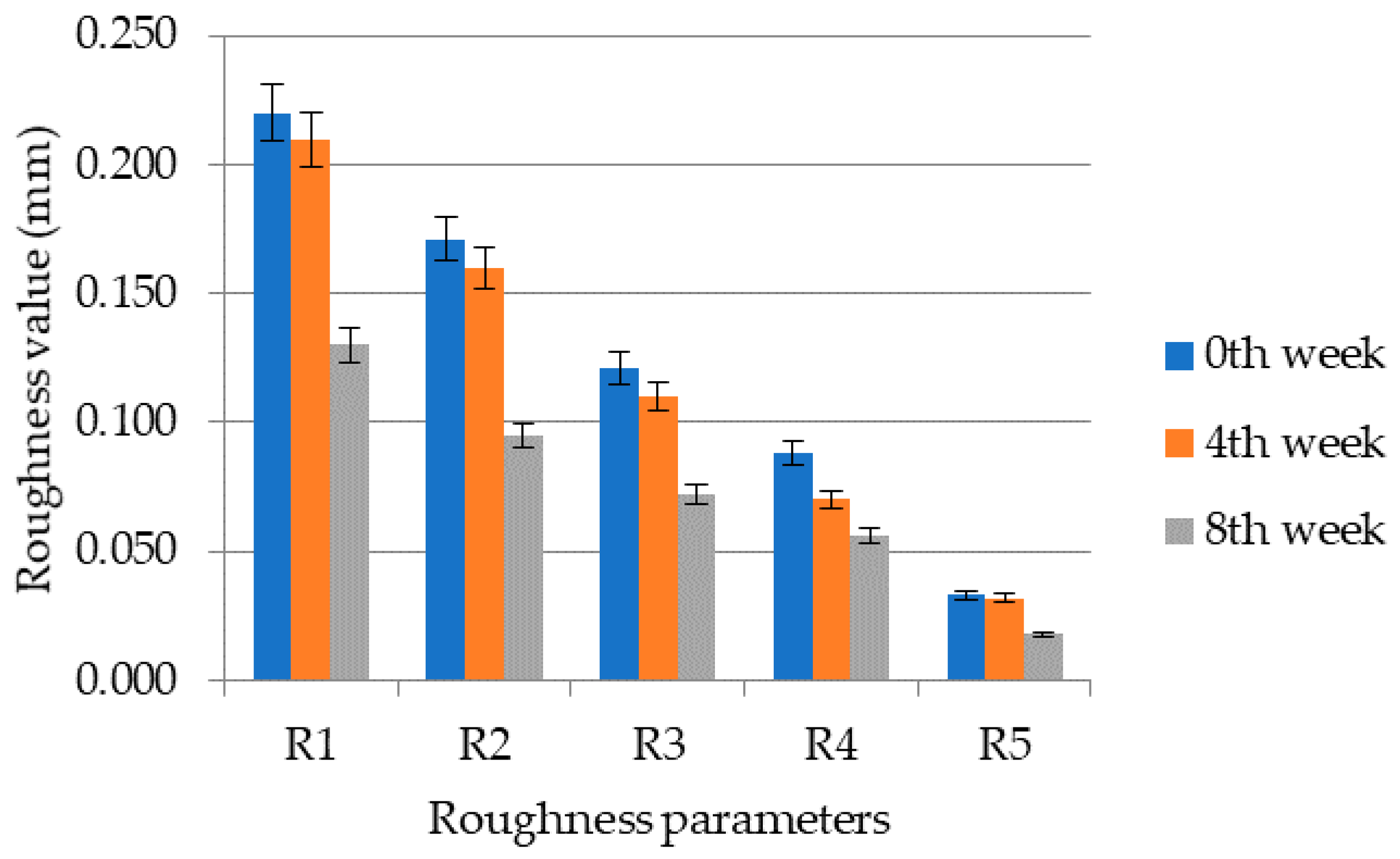
3.4.1. Roughness Parameters
3.4.2. Evaluation of the Skin Replica Image
3.4.3. Evaluation of Skin Anisotropy
3.4.4. Amount of Wrinkles
4. Materials and Methods
4.1. Appliances, Tools and Chemicals
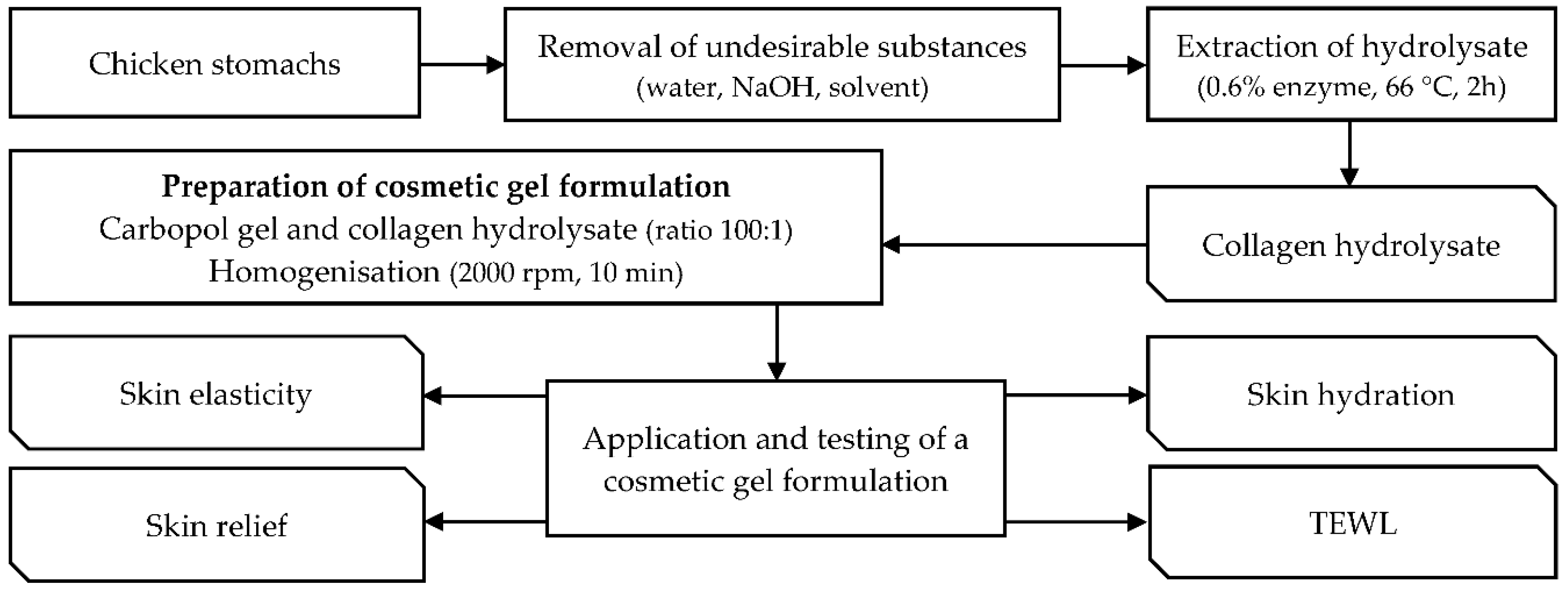
4.2. Preparation of Collagen Hydrolysate and Cosmetic Gel Formulation
4.3. Selection of Volunteers and Application of Cosmetic Gel Formulation
4.4. Diagnosing the Condition of the Skin after the Cosmetic Gel Application
4.4.1. Skin Hydration
4.4.2. Transepidermal Water Loss
4.4.3. Skin Elasticity
4.4.4. Skin Relief
4.4.5. Amount of Wrinkles
4.5. Evaluation of Results and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Poultry Trends 2019: The Statistical Reference for Poultry Executives. Available online: https://www.poultrytrends.com/poultrytrends/poultrytrends2019/MobilePagedReplica.action?pm=2&folio=Cover#pg1 (accessed on 28 November 2020).
- Barbut, S. The Science of Poultry and Meat Processing; Library and Archives Canada Cataloguing in Publication: Ottawa, ON, Canada, 2015; pp. 7–24. [Google Scholar]
- Kosseva, M.R.; Webb, C. Food Industry Wastes, 2nd ed.; Elsevier: New York, NY, USA, 2013; pp. 37–60. [Google Scholar]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Application. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; O’Brien, N.M. Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development. Agriculture 2013, 3, 112–130. [Google Scholar] [CrossRef] [Green Version]
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice, 1st ed.; Wiley–VCH: Weinheim, Germany, 2007; pp. 40–120. [Google Scholar]
- Zarkadas, C.G.; Maloney, S. Assessment of the Protein Quality of the Smooth Muscle Myofibrillar and Connective Tissue Proteins of Chicken Gizzard. Poult. Sci. 1998, 77, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Durand, D.; Chassenieux, C.; Jyotishkumar, P. Handbook of Biopolymer—Based Materials: From Blends and Composites to Gels and Complex Networks; Wiley–VCH: Weinheim, Germany, 2013; pp. 7–36. [Google Scholar]
- Rohrich, J.M.D. The Market of Plastic Surgery: Cosmetic Surgery for Sale—At What Price? Plast. Surg. 2001, 107, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- McCook, J.P. Topical Products for the Aging Face. Clin. Plast. Surg. 2016, 43, 597–604. [Google Scholar] [CrossRef]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology, 4th ed.; Elsevier: New York, NY, USA, 2009; pp. 245–311. [Google Scholar]
- Baki, G.; Durand, D.; Alexander, K.S. Introduction to Cosmetic Formulation and Technology; Wiley–VCH: Weinheim, Germany, 2015; pp. 147–204. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Rahnamaeian, M.; Vilcinskas, A. Short Antimicrobial Peptides as Cosmetic Ingredients to Deter Dermatological Pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef] [Green Version]
- Gorouhi, F.; Maibach, H.I. Role of Topical Peptides in Preventing or Treating Aged Skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti–Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In Vitro Antioxidant, Collagenase Inhibition, and In Vivo Anti–Wrinkle Effects of Combined Formulation Containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba Fruits Extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [Green Version]
- SkinCeuticals: Cosmeceuticals Scientifically Advanced Skincare. Available online: https://www.skinceuticals.com/cosmeceuticals (accessed on 16 November 2020).
- Farris, P.K. Cosmeceutical and Cosmetic Practice, 1st ed.; Elsevier: New York, NY, USA, 2014; pp. 9–44. [Google Scholar]
- Chen, Y.; Guo, Y.S.X.; Zhang, C.; Yang, W.; Ma, M.; Liu, S.; Zhang, M.; Wen, L.P. Transdermal Protein Delivery by a Coadministered Peptide Identified Via Phage Display. Nat. Biotechnol. 2006, 24, 455–460. [Google Scholar] [CrossRef]
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-de-Costa, A.P. Anti–Aging Cosmetics: Facts and Controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, D.; Dong, Y.; Su, W.; Su, H.; Wei, X.; Yang, C.; Jing, L.; Tang, X.; Li, X.; et al. Transdermal Permeation Effect of Collagen Hydrolysates of Deer Sinew on Mouse Skin, ex vitro, and Antioxidant Activity, Increased Type I Collagen Secretion of Percutaneous Proteins in NIH/3T3 Cells. J. Cosmet. Dermatol. 2019, 19, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Secchi, G. Role of Protein in Cosmetics. Clin. Dermatol. 2008, 26, 321–325. [Google Scholar] [CrossRef]
- Berardesca, E.; Abril, E.; Serio, M.; Cameli, N. Effects of Topical Gluco-Oligosaccharide and Collagen Tripeptide F in the Treatment of Sensitive Atopic Skin. Int. J. Cosmet. Sci. 2009, 31, 271–277. [Google Scholar] [CrossRef]
- Scibisz, M.; Arct, J.; Pytkowska, K. Hydrolysed Proteins in Cosmetic Production, Part II. SOFW J. Polish Ed. 2008, 1, 12–16. Available online: https://www.researchgate.net/publication/237078411_Hydrolysed_proteins_in_cosmetic_production_part_II (accessed on 19 March 2021).
- Schunck, M.; Zague, V.; Oesser, S.; Proksch, E. Dietary Supplementation with Specific Collagen Peptides Has a Body Mass Index Dependent Beneficial Effect on Cellulite Morphology. J. Med. Food. 2015, 18, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolke, L.; Schilippe, G.; Gerb, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proksch, E.; Segger, D.; Degwert, J.; Schunsk, M.; Zague, V.; Oesser, S. Oral Supplementation of Specific Collagen Peptides has Beneficial Effects on Human Skin Physiology: A Double–Blind, Placebo–Controlled Study. Ski. Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Schunsk, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral Intake of Specific Bioactive Collagen Peptides Reduces Skin Wrinkles and Increases Dermal Matrix Synthesis. Ski. Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolyse Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Maia Campos, P.; Melo, M.O.; Siqueira Cesar, F.C. Topical Application and Oral Supplementation of Peptides in the Improvement of Skin Viscoelasticity and Density. J. Cosmet. Dermatol. 2019, 18, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological Preparation of Gelatines from Chicken Feet. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polaštíková, A.; Gál, R.; Mokrejš, P.; Krejčí, O. Preparation of Protein Products from Collagen–Rich Poultry Tissues. In Proceedings of the Conference MendelNet, Brno, Czechia, 6–7 November 2019; pp. 392–397. [Google Scholar]
- Bazin, R.; Fancon, C. Equivalence of Face and Volar Forearm for the Testing of Moisturizing and Forming Effect of Cosmetics in Hydration and Biomechanical Studies. Int. J. Cosmet. Sci. 2006, 28, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Rensburg, S.J.; Franken, A.; Plessis, J.L.D. Measurement of Transepidermal Water Loss, Stratum Corneum Hydration and Skin Surface pH in Occupational Settings: A review. Ski. Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Mac–Mary, S.; Creidi, P.; Marsaut, D.; Courderot–Masuyer, C.; Cochet, V.; Gharbi, T.; Guidicelli–Arranz, D.; Tondu, F.; Humbert, P. Assessment of Effects and Additional Dietary Natural Mineral Water Uptake on Skin Hydration in Healthy Subjects by Dynamic Barrier Function Measurements and Clinic Scoring. Ski. Res. Technol. 2006, 12, 199–205. [Google Scholar] [CrossRef]
- Samad, N.; Sikarwar, A. Collagen: New Dimension in Cosmetic and Healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Aguirre Cruz, G.; León López, A.; Cruz Gómez, V.; Jiménez Alvarado, R.; Aguirre Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Ruvolo, E.C.; Stamatas, G.N.; Kollias, N. Skin Viscoelasticity Displays Site and Age Dependent Angular Anisotropy. Ski. Pharmacol. Physiol. 2007, 20, 313–321. [Google Scholar] [CrossRef]
- Cartigliani, C.; Bonfigli, A.; Brancato, S.; Rogano, L. The Agar Factor in the Cosmetic Management of Biophysical Skin Parameters. Cosmetics 2014, 1, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Ryu, D.J.; Jung, J.Y.; Chung, K.Y.; Suh, H. The Efficacy and Safety of Succinylated Atelocollagen and Adenosine for the Treatment of Periorbital Wrinkles. J. Chem. Dermatol. Sci. Appl. 2013, 3, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Tadini, K.A.; Mercurio, D.G.; Campos, P. M Acetyl hexapeptide–3 in a Cosmetic Formulation Acts on Skin Mechanical Properties—Clinical Study. Braz. J. Pharm. Sci. 2015, 51, 2175–9790. [Google Scholar] [CrossRef] [Green Version]
- BioSolutions, Novozymes: Protease, Granulate Protamex. Available online: https://biosolutions.novozymes.com/en/animal-protein/products/protamex (accessed on 21 April 2020).
- SpecialChem: The Material Selection Platform. Carbomer. Available online: https://cosmetics.specialchem.com/inci/carbomer (accessed on 19 March 2021).
- SpecialChem: The Material Selection Platform. Collagen Hydrolysate Powder. Available online: https://cosmetics.specialchem.com/product/i-dsm-collagen-hydrolysate-powder (accessed on 19 March 2021).
- SpecialChem: The Material Selection Platform. Hydrolyzed Collagen. Available online: https://cosmetics.specialchem.com/inci/hydrolyzed-collagen (accessed on 19 March 2021).
- Safety Assessment of Tissue-Derived Proteins and Peptides as Used in Cosmetics. Available online: http://www.cir-safety.org/sites/default/files/tsupep042017slr.pdf (accessed on 19 March 2021).
- CIOMS: Council for International Organization of Medical Sciences. Available online: https://cioms.ch/ (accessed on 12 January 2020).
- Polaskova, J.; Pavlackova, J.; Egner, P. Effect of Vehicle on the Performance of Active Moisturizing Substances. Ski. Res. Technol. 2015, 21, 403–412. [Google Scholar] [CrossRef]
- Courage & Khazaka Electronic GmbH: Corneometer® CM 825. Available online: https://www.courage-khazaka.de/en/16-wissenschaftliche-produkte/alle-produkte/183-corneometer-e (accessed on 3 July 2020).
- Zghaibi, N.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Harun, R. Kinetics Study of Microwave–Assisted Brine Extraction of Lipid from the Microalgae. Molecules 2020, 25, 784. [Google Scholar] [CrossRef] [Green Version]
- Courage & Khazaka Electronic GmbH: Tewameter® TM 300. Available online: https://www.courage-khazaka.de/en/16-wissenschaftliche-produkte/alle-produkte/172-tewameter-e (accessed on 26 July 2020).
- Paye, M.; Mac Mary, S.; Elkhyat, A.; Tarrit, C. Use of the Reviscometer® for Measuring Cosmetics–Induced Skin Surface Effects. Ski. Res. Technol. 2007, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.O.; Henau, K.; Clarys, P. In vitro Calibration and Validation of the Reviscometer Using Silicone Polymers as Simple Skin Model Systems. Ski. Res. Technol. 2005, 11, 294. [Google Scholar]
- Clarys, P.; Henau, K.; Barel, A.O. Investigation of Intrinsic and Photoaging of Human Skin Using the Reviscometer and the Cutometer. Ski. Res. Technol. 2005, 11, 295. [Google Scholar]
- DIN. DIN 4768: Determination of Values of Surface Roughness Parameters Ra. Rz, Rmax Using Electrical Contact (Stylus) Instruments Concepts and Measuring Conditions; Deutsches Institut für Normung E.V. (DIN): Berlin, Germany, 1990. [Google Scholar]
- Sakai, S.; Yamanari, M.; Lim, Y.; Nakagawa, N.; Yasuno, Y. In vivo Evaluation of Human Skin Anisotropy by Polarization–Sensitive Optical Coherence Tomography. Biomed. Opt. Express 2011, 2, 2623–2631. [Google Scholar] [CrossRef] [Green Version]
- Rosado, C.; Antunes, F.; Barbosa, R.; Fernando, R.; Estudante, M.; Silva, H.N.; Rodrigues, L.M. About the in vivo Quantitation of Skin Anisotropy. Ski. Res. Technol. 2017, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]










| Angle (°) | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
| Week | RRT ± SD (a.u.) | ||||||
| 0 | 235 ± 50 | 218 ± 50 | 216 ± 40 | 251 ± 60 | 325 ± 60 | 364 ± 50 | 296 ± 50 |
| 4 | 147 ± 20 | 285 ± 80 | 227 ± 40 | 162 ± 16 | 175 ± 30 | 192 ± 40 | 188 ± 30 |
| 8 | 171 ± 30 | 284 ± 90 | 167 ± 30 | 104 ± 60 | 264 ± 70 | 266 ± 70 | 238 ± 60 |
| Angle (°) | 210 | 240 | 270 | 300 | 330 | 360 | |
| Week | RRT ± SD (a.u.) | ||||||
| 0 | 276 ± 40 | 260 ± 50 | 254 ± 60 | 238 ± 60 | 198 ± 30 | 213 ± 30 | |
| 4 | 187 ± 30 | 230 ± 70 | 206 ± 60 | 142 ± 15 | 158 ± 30 | 185 ± 40 | |
| 8 | 197 ± 60 | 236 ± 90 | 291 ± 90 | 200 ± 50 | 135 ± 16 | 176 ± 40 | |
| Angle (°) | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
| Week | RRT ± SD (a.u.) | ||||||
| 0 | 228 ± 50 | 234 ± 50 | 230 ± 30 | 262 ± 50 | 321 ± 60 | 344 ± 40 | 309 ± 50 |
| 4 | 156 ± 18 | 276 ± 80 | 242 ± 40 | 164 ± 15 | 183 ± 30 | 200 ± 40 | 197 ± 30 |
| 8 | 167 ± 20 | 288 ± 90 | 173 ± 30 | 200 ± 60 | 266 ± 70 | 265 ± 70 | 245 ± 60 |
| Angle (°) | 210 | 240 | 270 | 300 | 330 | 360 | |
| Week | RRT ± SD (a.u.) | ||||||
| 0 | 262 ± 40 | 267 ± 50 | 245 ± 60 | 231 ± 60 | 211 ± 30 | 213 ± 30 | |
| 4 | 185 ± 19 | 234 ± 70 | 208 ± 50 | 145 ± 16 | 165 ± 30 | 186 ± 30 | |
| 8 | 195 ± 60 | 240 ± 80 | 301 ± 90 | 196 ± 50 | 137 ± 16 | 179 ± 40 | |
| Week | Right Side | Left Side | ||
|---|---|---|---|---|
| Skin Anisotropy Index ± SD (a.u.) | Change in Skin Anisotropy Index (a.u.) | Skin Anisotropy Index ± SD (a.u.) | Change in Skin Anisotropy Index (a.u.) | |
| 0 | 23.9 ± 0.9 | ---1 | 25.7 ± 0.5 | ---1 |
| 4 | 20.8 ± 0.6 | –3.1 | 18.6 ± 0.7 | –7.1 |
| 8 | 17.1 ± 0.6 | –3.7 | 15.3 ± 0.8 | –3.3 |
| Week | Right Side | Left Side | ||
|---|---|---|---|---|
| Amount of Wrinkles ± SD (%) | Overall Reduction of Wrinkles (%) ---2 | Amount of Wrinkles ± SD (%) | Overall Reduction of Wrinkles (%) ---2 | |
| 0 | 15.6 ± 0.3 | ---1 | 15.2 ± 0.3 | ---1 |
| 4 | 14.4 ± 0.6 | 7.7 | 14.5 ± 0.5 | 4.6 |
| 8 | 12.2 ± 0.1 | 21.8 | 12.7 ± 0.2 | 16.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopová, A.; Pavlačková, J.; Mokrejš, P.; Gál, R. Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation. Molecules 2021, 26, 2021. https://doi.org/10.3390/molecules26072021
Prokopová A, Pavlačková J, Mokrejš P, Gál R. Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation. Molecules. 2021; 26(7):2021. https://doi.org/10.3390/molecules26072021
Chicago/Turabian StyleProkopová, Aneta, Jana Pavlačková, Pavel Mokrejš, and Robert Gál. 2021. "Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation" Molecules 26, no. 7: 2021. https://doi.org/10.3390/molecules26072021
APA StyleProkopová, A., Pavlačková, J., Mokrejš, P., & Gál, R. (2021). Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation. Molecules, 26(7), 2021. https://doi.org/10.3390/molecules26072021








