Plasma Mediated Chlorhexidine Immobilization onto Polylactic Acid Surface via Carbodiimide Chemistry: Antibacterial and Cytocompatibility Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification
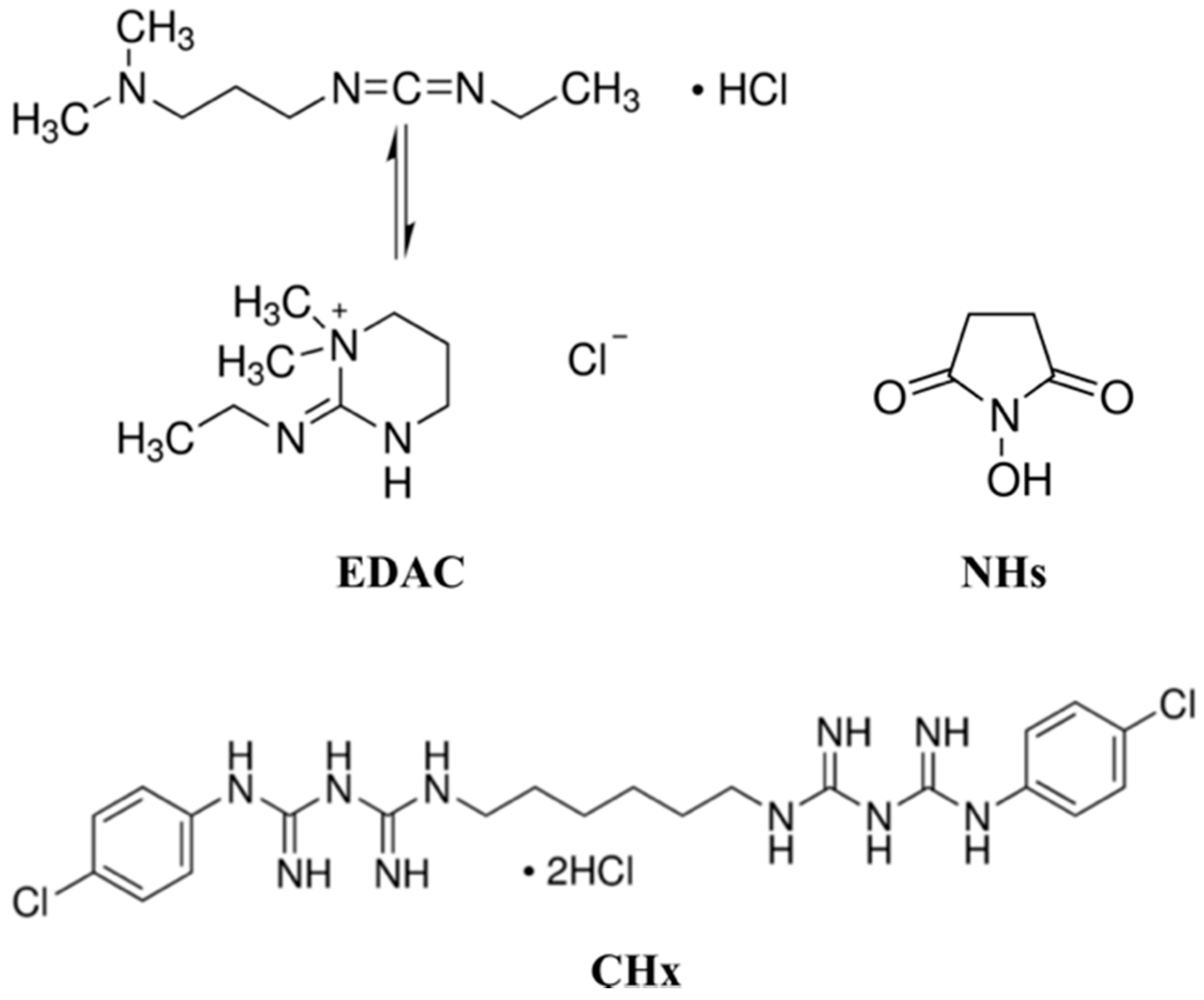
2.3. Immobilization of the Chlorhexidine
2.4. Surface Wettability Essay
2.5. Surface Morphology Characterization by Scanning Electron Microscope
2.6. Surface Chemical Analysis by X-ray Photoelectron Spectroscopy
2.7. Antibacterial Test
2.8. Cytocompatibility Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Surface Wettability Results
3.2. Surface Chemical Analysis Results
3.3. Surface Morphology Results by Scanning Electron Microscopy (SEM)
3.4. Antibacterial Activity Results
3.5. Cytocompatibility Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carrasco, F.; Pages, P.; Gamez-Perez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Lehocky, M.; Stahel, P.; Koutny, M.; Cech, J.; Institoris, J.; Mracek, A. Adhesion of Rhodococcus sp. S3E2 and Rhodococcus sp. S3E3 to plasma prepared Teflon-like and organosilicon surfaces. J. Mater. Process. Technol. 2009, 209, 2871–2875. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Szyk-Warszynska, L.; Zembala, M.; Lehocky, M. In situ studies of particle deposition on non-transparent substrates. Colloids. Surf. A Physicochem. Eng. Asp. 2004, 235, 65–72. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Lehocky, M.; Saha, P.; Mozetic, M. Recent Progress in Surface Modification of Polyvinyl Chloride. Materials 2012, 5, 2937–2959. [Google Scholar] [CrossRef] [Green Version]
- Bilek, F.; Sulovska, K.; Lehocky, M.; Saha, P.; Humpolicek, P.; Mozetic, M.; Junkar, I. Preparation of active antibacterial LDPE surface through multistep physicochemical approach II: Graft type effect on antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.D.; Gorade, V.G.; Madye, N.; Chaudhary, B.; Bangde, P.S.; Dandekar, P.P. Preparation and characterization of biocomposite packaging film from poly(lactic acid) and acylated microcrystalline cellulose using rice bran oil. Int. J. Biol. Macromol. 2018, 118, 1090–1102. [Google Scholar] [CrossRef]
- Lehocky, M.; Amaral, P.F.F.; Coelho, M.A.Z.; Stahel, P.; Barros-Timmons, A.M.; Coutinho, J.A.P. Attachment/detachment of Saccharomyces cerevisiae on plasma deposited organosilicon thin films. Czechoslov. J. Phys. 2006, 56, 1256–1262. [Google Scholar] [CrossRef]
- Bilek, F.; Krizova, T.; Lehocky, M. Preparation of active antibacterial LDPE surface through multistep physicochemical approach: I. Allylamine grafting, attachment of antibacterial agent and antibacterial activity assessment. Colloids Surf. B Biointerfaces 2011, 88, 440–447. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Sedlarik, V.; Vesel, A.; Junkar, I.; Saha, P.; Chodak, I. An in vitro bacterial adhesion assessment of surface-modified medical-grade PVC. Colloids Surf. B Biointerfaces 2010, 77, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Junkar, I.; Cvelbar, U.; Lehocky, M. Plasma treatment of biomedical materials. Mater. Tehnol. 2011, 45, 221–226. [Google Scholar]
- Cao, H.; Liu, X. Silver nanoparticles-modified films versus biomedical device-associated infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Lehocky, M.; Amaral, P.F.F.; Stahel, P.; Coelho, M.A.Z.; Barros-Timmons, A.M.; Coutinho, J.A.P. Deposition of Yarrowia lipolytica on plasma prepared teflonlike thin films. Surf. Eng. 2008, 24, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.; Ferguson, J. Phenotypic chlorhexidine and triclosan susceptibility in clinical Staphylococcus aureus isolates in Australia. Pathology 2017, 49, 633–637. [Google Scholar] [CrossRef]
- Greenhalgh, R.; Dempsey-Hibbert, N.C.; Whitehead, K.A. Antimicrobial strategies to reduce polymer biomaterial infections and their economic implications and considerations. Int. Biodeterior. Biodegrad. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Lobato-Aguilar, H.; Uribe-Calderon, J.A.; Herrera-Kao, W.; Duarte-Aranda, S.; Baas-Lopez, J.M.; Escobar-Morales, B.; Cauich-Rodriguez, J.V.; Cervantes-Uc, J.M. Synthesis, characterization and chlorhexidine release from either montmorillonite or palygorskite modified organoclays for antibacterial applications. J. Drug Deliv. Sci. Technol. 2018, 46, 452–460. [Google Scholar] [CrossRef]
- Scheibler, E.; da-Silva, R.M.; Leite, C.E.; Campos, M.M.; Figueiredo, M.A.; Salum, F.G.; Cherubini, K. Stability and efficacy of combined nystatin and chlorhexidine against suspensions and biofilms of Candida albicans. Arch. Oral. Biol. 2018, 89, 70–76. [Google Scholar] [CrossRef]
- Kao, H.F.; Chen, I.C.; Hsu, C.; Chang, S.Y.; Chien, S.F.; Chen, Y.C.; Hu, F.C.; Yang, J.C.H.; Cheng, A.L.; Yeh, K.H. Chlorhexidine for the prevention of bloodstream infection associated with totally implantable venous ports in patients with solid cancers. Support. Care Cancer 2014, 22, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Keerthisinghe, T.P.; Nguyen, L.N.; Kwon, E.E.; Oh, S. Antiseptic chlorhexidine in activated sludge: Biosorption, antimicrobial susceptibility, and alteration of community structure. J. Environ. Manag. 2019, 237, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Xu, C.; Zhang, X.; Li, J.; Dong, G.; Cao, J.; Zhou, T. Chlorhexidine exposure of clinical Klebsiella pneumoniae strains leads to acquired resistance to this disinfectant and to colistin. Int. J. Antimicrob. Agents 2019, 53, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Bonez, P.C.; Alves, C.F.D.S.; Dalmolin, T.V.; Agertt, V.A.; Mizdal, C.R.; Flores, V.D.C.; Marques, J.B.; Santos, R.C.V.; Campos, M.M.A.D. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control. 2013, 41, 119–122. [Google Scholar] [CrossRef]
- Popelka, A.; Novak, I.; Lehocky, M.; Chodak, I.; Sedliacik, J.; Gajtanska, M.; Sedliacikova, M.; Vesel, A.; Junkar, I.; Kleinova, A.; et al. Anti-bacterial treatment of polyethylene by cold plasma for medical purposes. Molecules 2012, 17, 762–785. [Google Scholar] [CrossRef] [Green Version]
- Vesel, A.; Mozetic, M. Surface modification and ageing of PMMA polymer by oxygen plasma treatment. Vacuum 2012, 86, 634–637. [Google Scholar] [CrossRef]
- Lehocky, M.; Lapcik, L.; Neves, M.C.; Trindade, T.; Szyk-Warszynska, L.; Warszynski, P.; Hui, D. Deposition/Detachment of Particles on Plasma Treated Polymer Surfaces. Mater. Sci. Forum 2003, 426, 2533–2538. [Google Scholar] [CrossRef]
- Lehocky, M.; Lapcik, L.; Dlabaja, R.; Rachunek, L.; Stoch, J. Influence of artificially accelerated ageing on the adhesive joint of plasma treated polymer materials. Czech. J. Phys. 2004, 54, 533–538. [Google Scholar] [CrossRef]
- Patel, D.; Wu, J.; Chan, P.; Upreti, S.; Turcotte, G.; Ye, T. Surface modification of low density polyethylene films by homogeneous catalytic ozonation. Chem. Eng. Res. Des. 2012, 90, 1800–1806. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Bilek, F.; Lehocky, M.; Junkar, I.; Mozetic, M.; Sowe, M. Enhanced printability of polyethylene through air plasma treatment. Vacuum 2013, 95, 43–49. [Google Scholar] [CrossRef]
- Mozetic, M. Plasma-Stimulated Super-Hydrophilic Surface Finish of Polymers. Polymers 2020, 12, 2498. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Arkadiusz Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Wieland, F.; Bruch, R.; Bergmann, M.; Partel, S.; Urban, G.A.; Dincer, C. Enhanced Protein Immobilization on Polymers—A Plasma Surface Activation Study. Polymers 2020, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Slepicka, P.; Kasalkova, N.S.; Stranska, E.; Bacakova, L.; Svorcik, V. Surface characterization of plasma treated polymers for applications as biocompatible carriers. Express Polym. Lett. 2013, 7, 535–545. [Google Scholar] [CrossRef]
- Vidaurre, E.F.C.; Achete, C.A.; Gallo, F.; Garcia, D.; Simao, R.; Habert, A.C. Surface Modification of Polymeric Materials by Plasma Treatment. Mater. Res. 2002, 5, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Sedlarik, V.; Vesel, A.; Junkar, I.; Saha, P.; Chodak, I. A physicochemical approach to render antibacterial surfaces on plasma-treated medical-grade PVC: Irgasan coating. Plasma Process. Polym. 2010, 7, 504–514. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Bilek, F.; Vesel, A.; Junkar, I.; Saha, P.; Popelka, A. Polysaccharides Coatings on Medical-Grade PVC: A Probe into Surface Characteristics and the Extent of Bacterial Adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.A.; Clapes, P.; Infante, M.R.; Comas, J.; Manresa, A. Comparative study of the antimicrobial activity of bis(Nα-caproyl-L-arginine)-1,3-propanediamine dihydrochloride and chlorhexidine dihydrochloride against Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Ozaltin, K.; Lehocky, M.; Kucekova, Z.; Humpolicek, P.; Saha, P. A novel multistep method for chondroitin sulphate immobilization and its interaction with fibroblast cells. Mater. Sci. Eng. C 2017, 70, 94–100. [Google Scholar] [CrossRef]
- Esmail, A.; Pereira, J.R.; Zoio, P.; Silvestre, S.; Menda, U.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Henriques, C.; et al. Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion. Polymers 2021, 13, 1056. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Vesela, D.; Lehocky, M.; Humpolicek, P.; Mozetic, M. Antibacterial Activity and Cytotoxicity ofImmobilized Glucosamine/Chondroitin Sulfate on Polylactic Acid Films. Polymers 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaltin, K.; Vargun, E.; Martino, A.D.; Capakova, Z.; Lehocky, M.; Humpolicek, P.; Kazantseva, N.; Saha, P. Cell response to PLA scaffolds functionalized with various seaweedpolysaccharides. Int. J. Polym. Mater. Polym. Biomater. 2020. [Google Scholar] [CrossRef]
- Bernal-Ballen, A.; Lopez-Garcia, J.A.; Ozaltin, K. (PVA/Chitosan/Fucoidan)-Ampicillin: A BioartificialPolymeric Material with Combined Properties in CellRegeneration and Potential Antibacterial Features. Polymers 2020, 11, 1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Samples | C1s% * | O1s% * | N1s% * | Cl2p% * | Qw ° |
|---|---|---|---|---|---|
| PLA | 73.7 | 25.3 | 1 | 0 | 78.9 ± 2.3 |
| PLA_DC | 69.8 | 30.2 | 0 | 0 | 51.6 ± 1.0 |
| PLA_DC_EDAC | 68.9 | 29.6 | 1.4 | 0.2 | 66.2 ± 1.8 |
| PLA_DC_NHs | 66.8 | 32.6 | 0.5 | 0.1 | 67.3 ± 2.4 |
| PLA_DC_EDAC_CHx | 69 | 16.4 | 10.8 | 3.8 | 60.5 ± 3.7 |
| PLA_DC_NHs_CHx | 66.3 | 28.4 | 4.3 | 1 | 60.3 ± 2.8 |
| Samples | S. aureus, N (CFU/cm2) | E. coli, N (CFU/cm2) | Cell Viability (%) |
|---|---|---|---|
| PLA | 8.8 × 104 | 2.2 × 106 | 100 ± 1.7 |
| PLA_DC | 4.1 × 105 | 1.3 × 106 | 132.2 ± 2.1 |
| PLA_DC_EDAC | 8.3 × 104 | 1.1 × 106 | 132.7 ± 4.2 |
| PLA_DC_NHs | 1.1 × 105 | 1.9 × 106 | 118.6 ± 1.2 |
| PLA_DC_EDAC_CHx | 1.2 × 104 | 2.5 × 106 | 103.4 ± 1.9 |
| PLA_DC_NHs_CHx | 6.2 × 103 | 6.0 × 101 | 117.6 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaltin, K.; Di Martino, A.; Capakova, Z.; Lehocky, M.; Humpolicek, P.; Saha, T.; Vesela, D.; Mozetic, M.; Saha, P. Plasma Mediated Chlorhexidine Immobilization onto Polylactic Acid Surface via Carbodiimide Chemistry: Antibacterial and Cytocompatibility Assessment. Polymers 2021, 13, 1201. https://doi.org/10.3390/polym13081201
Ozaltin K, Di Martino A, Capakova Z, Lehocky M, Humpolicek P, Saha T, Vesela D, Mozetic M, Saha P. Plasma Mediated Chlorhexidine Immobilization onto Polylactic Acid Surface via Carbodiimide Chemistry: Antibacterial and Cytocompatibility Assessment. Polymers. 2021; 13(8):1201. https://doi.org/10.3390/polym13081201
Chicago/Turabian StyleOzaltin, Kadir, Antonio Di Martino, Zdenka Capakova, Marian Lehocky, Petr Humpolicek, Tomas Saha, Daniela Vesela, Miran Mozetic, and Petr Saha. 2021. "Plasma Mediated Chlorhexidine Immobilization onto Polylactic Acid Surface via Carbodiimide Chemistry: Antibacterial and Cytocompatibility Assessment" Polymers 13, no. 8: 1201. https://doi.org/10.3390/polym13081201
APA StyleOzaltin, K., Di Martino, A., Capakova, Z., Lehocky, M., Humpolicek, P., Saha, T., Vesela, D., Mozetic, M., & Saha, P. (2021). Plasma Mediated Chlorhexidine Immobilization onto Polylactic Acid Surface via Carbodiimide Chemistry: Antibacterial and Cytocompatibility Assessment. Polymers, 13(8), 1201. https://doi.org/10.3390/polym13081201










