Acupuncture Alleviates Anxiety and 22-kHz Ultrasonic Vocalizations in Rats Subjected to Repeated Alcohol Administration by Modulating the Brain-Derived Neurotrophic Factor/Corticotropin-Releasing Hormone Signaling Pathway
Abstract
1. Introduction
2. Results
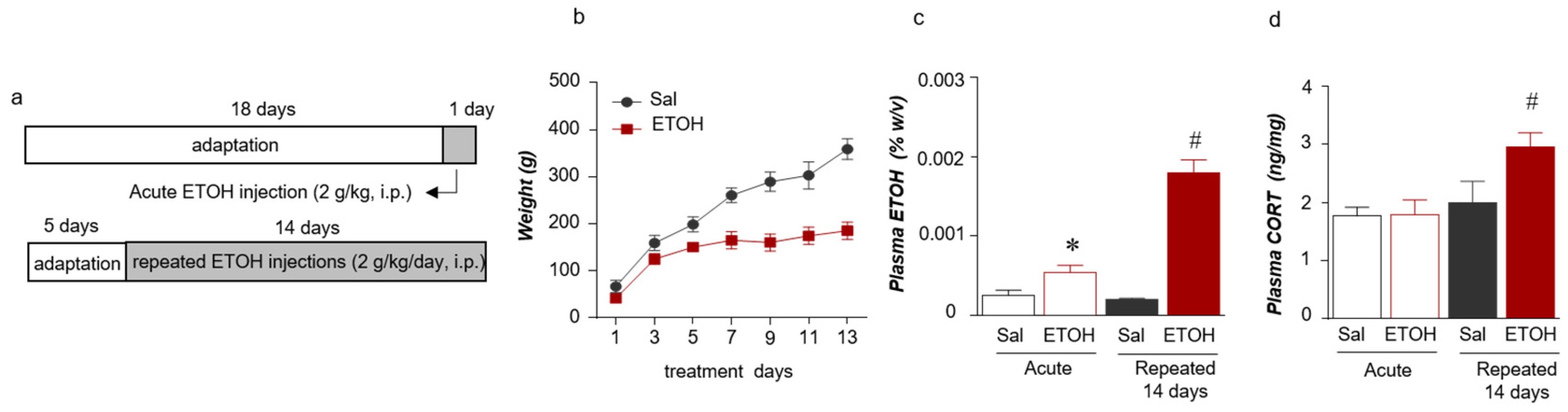
2.1. Acute and Repeated ETOH Exposures Increased the Plasma Stress Hormone and ETOH Concentrations
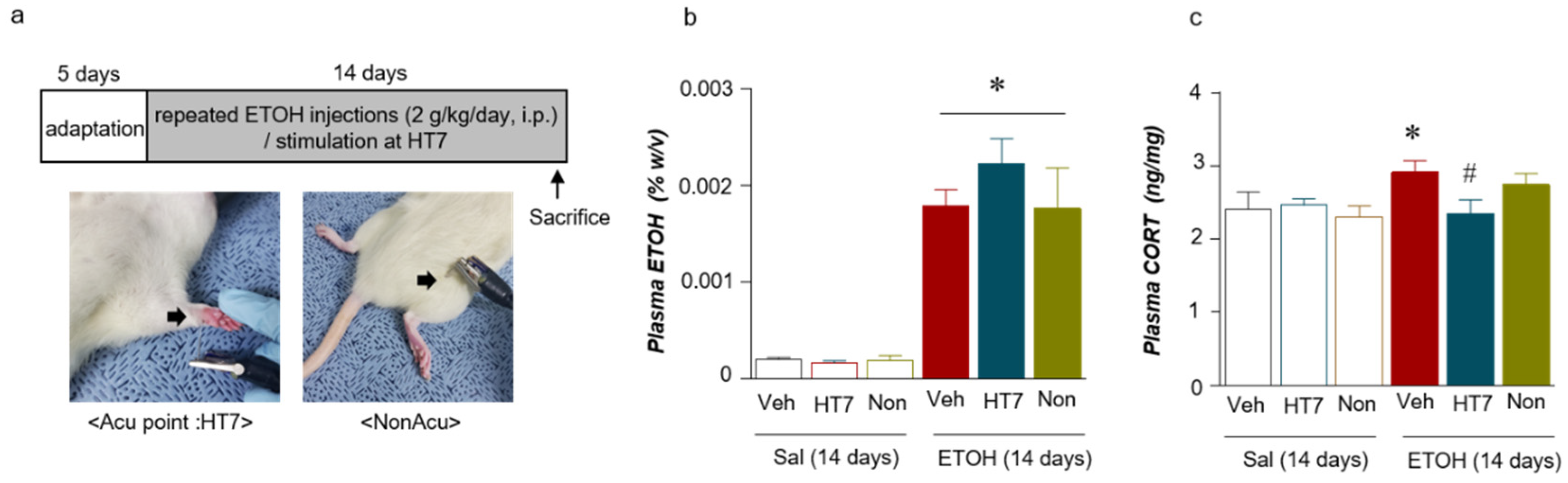
2.2. Stimulation at HT7 Reduced the Plasma CORT Levels in the Repeated ETOH-Administered-Rats
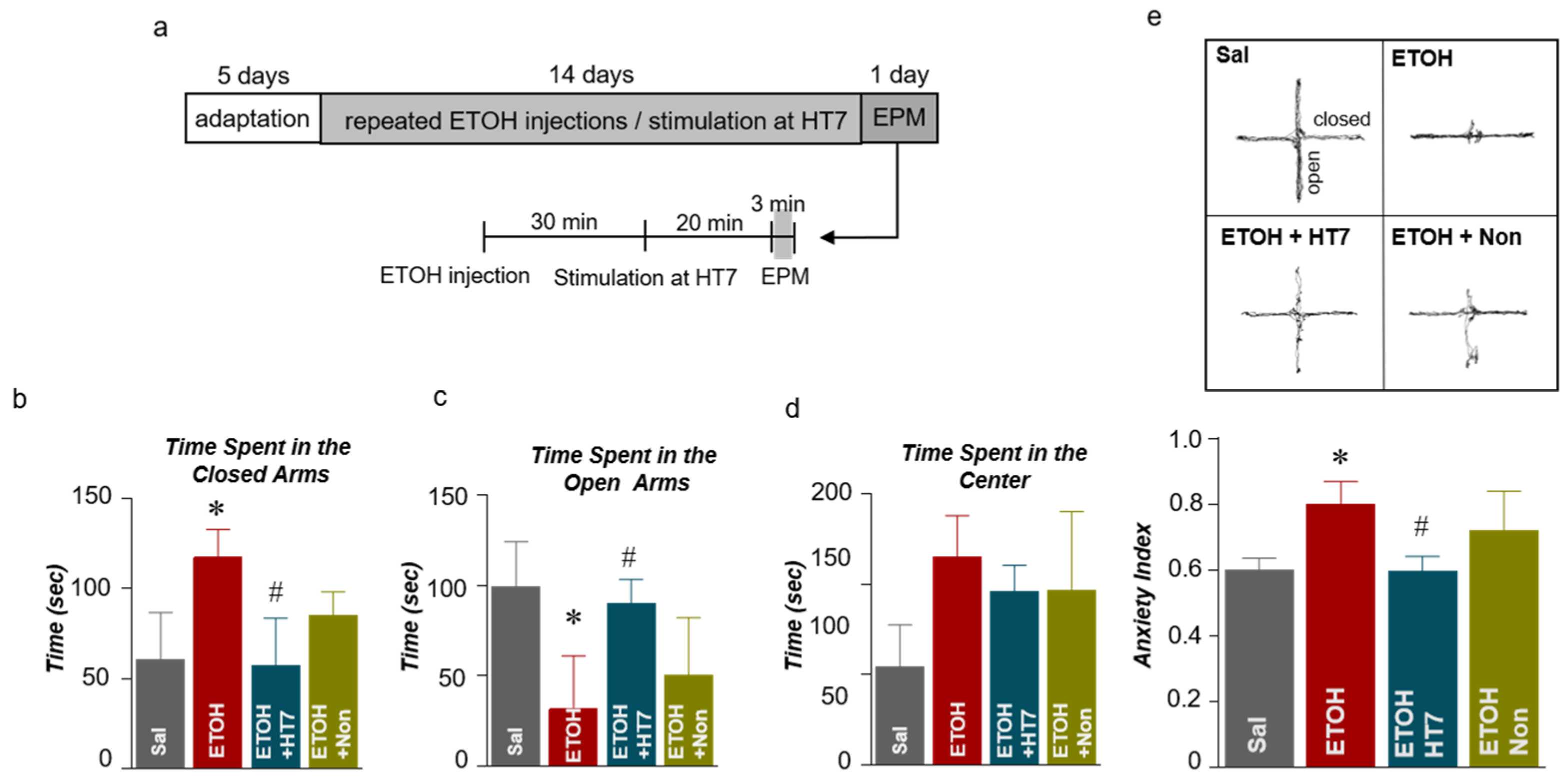
2.3. Stimulation at HT7 Alleviated Anxiety-Like Behaviors in Rats Subjected to Repeated ETOH Administration
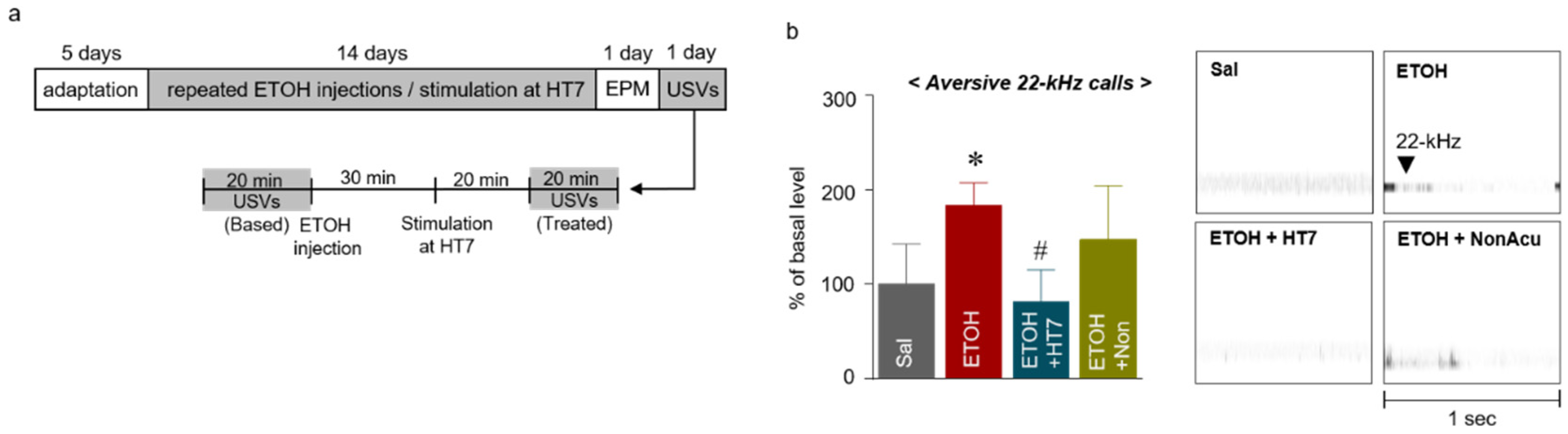
2.4. Stimulation at HT7 Reduced ETOH-Induced 22-kHz USVs
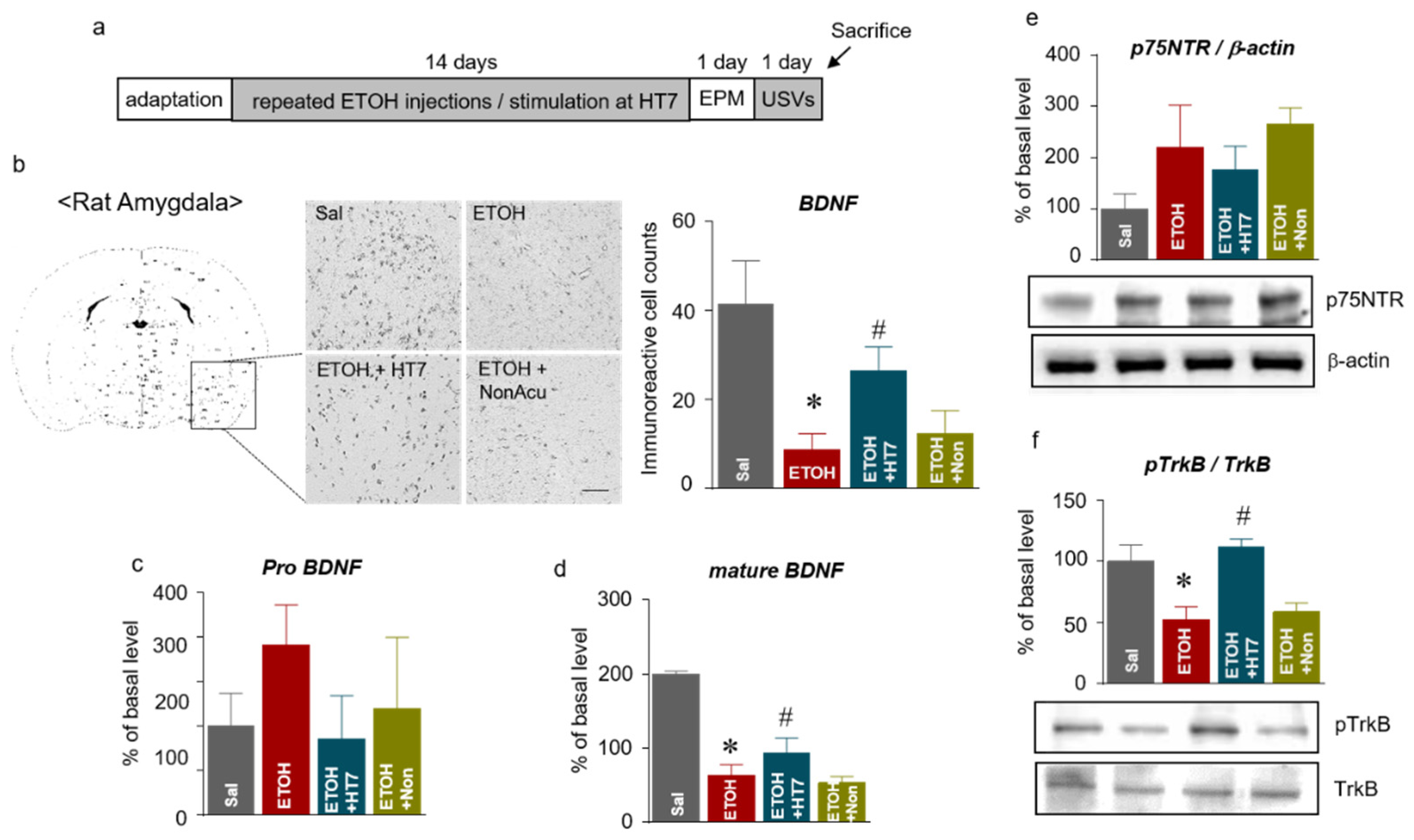
2.5. Stimulation at HT7 Increased the Levels of mBDNF and Phosphorylated TrkB Receptors in the Amygdala of Rats Subjected to Repeated ETOH Administration
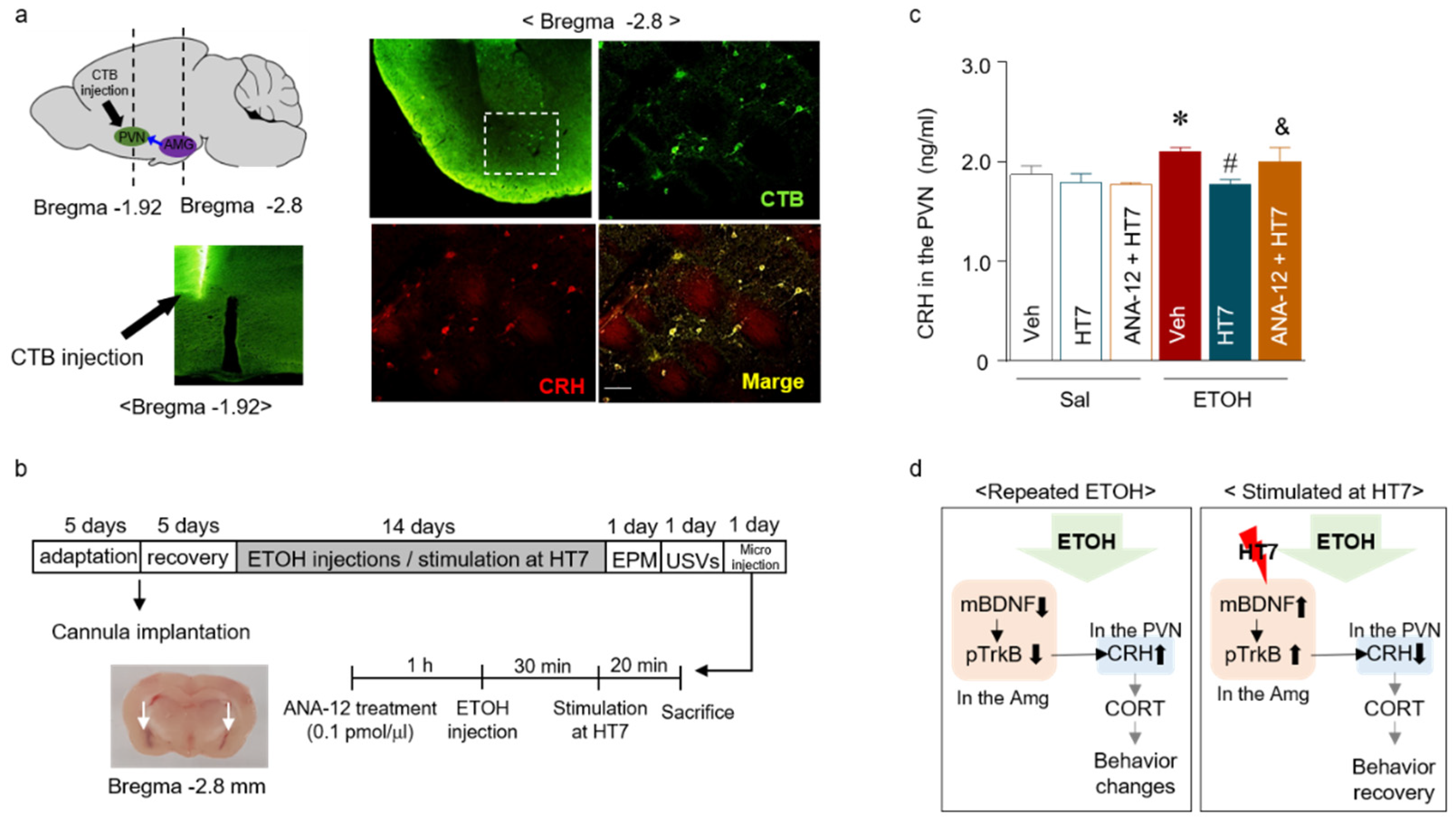
2.6. Stimulation at HT7 Decreased the CRH Levels in the PVN of Repeated ETOH-Administered Rats
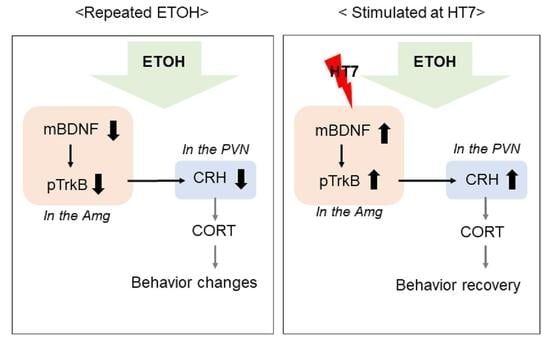
2.7. The Mechanism by Which Stimulation at HT7 Mitigates the Secretion of CRH in the PVN Is Mediated by BDNF Expression in the Amygdala
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Elevated Plus Maze (EPM) Test
4.3. Ultrasonic Vocalization
4.4. Blood ETOH Analysis
4.5. ELISA and Alcohol Assay
4.6. Immunohistochemistry
4.7. Western Blotting
4.8. Intra-Amygdala Infusion
4.9. Injection of a Retrograde Neuronal Tracer
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kosten, T.R.; O’Connor, P.G. Management of drug and alcohol withdrawal. N. Engl. J. Med. 2003, 348, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.C.; Hale, R.L. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: An animal model of alcohol withdrawal “kindling”. Alcohol. Clin. Exp. Res. 1993, 17, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.; Liu, X.; Nunes, E.; McCloud, S.; Samet, S.; Endicott, J. Effects of major depression on remission and relapse of substance dependence. Arch. Gen. Psychiatry 2002, 59, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kyzar, E.J.; Pandey, S.C. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci. Lett. 2015, 601, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gianoulakis, C.; Dai, X.; Brown, T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol. Clin. Exp. Res. 2003, 27, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Koob, G.F. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004, 311, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Schuckit, M.A.; Gold, E.; Risch, C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch. Gen. Psychiatry 1987, 44, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, M.N.; Lazar-Wesley, E.; Grant, K.A.; Kunos, G. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol Clin. Exp. Res. 1992, 16, 1147–1151. [Google Scholar] [CrossRef]
- Stephens MA, C.; McCaul, M.E.; Wand, G.S. The potential role of glucocorticoids and the HPA axis in alcohol dependence. In Neurobiology of Alcohol Dependence; Noronha, A., Cui, C., Harris, R.A., Crabbe, J.C., Eds.; Elsevier Inc.: London, UK, 2014; pp. 429–450. [Google Scholar]
- George, O.; Sanders, C.; Freiling, J.; Grigoryan, E.; Vu, S.; Allen, C.D.; Crawford, E.; Mandyam, C.D.; Koob, G.F. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl. Acad. Sci. USA 2012, 109, 18156–18161. [Google Scholar] [CrossRef] [PubMed]
- Wrase, J.; Makris, N.; Braus, D.F.; Mann, K.; Smolka, M.N.; Kennedy, D.N.; Caviness, V.S.; Hodge, S.M.; Tang, L.; Albaugh, M.; et al. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry 2008, 165, 1179–1184. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, H.; Damasio, A.R.; Lee, G.P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999, 19, 5473–5481. [Google Scholar] [CrossRef] [PubMed]
- Logrip, M.L.; Barak, S.; Warnault, V.; Ron, D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015, 1628, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.C.; Zhang, H.; Roy, A.; Misra, K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J. Neurosci. 2006, 26, 8320–8331. [Google Scholar] [CrossRef] [PubMed]
- Briones, T.L.; Woods, J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience 2013, 254, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, F.E.; Ibrahim, F.; Rashid, R.A.; Seghatoleslam, T.; Habil, H. Acupuncture therapy for drug addiction. J. Chin. Med. 2016, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Han, S.M.; Shim, I. Acupuncture attenuates cocaine-induced expression of behavioral sensitization in rats: Possible involvement of the dopaminergic system in the ventral tegmental area. Neurosci. Lett. 2009, 449, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Yang, E.J.; Lee, B.H.; Jang, E.Y.; Kim, H.Y.; Choi, S.M.; Steffensen, S.C.; Yang, C.H. Effects of acupuncture on stress-induced relapse to cocaine-seeking in rats. Psychopharmacology 2012, 222, 303–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.H.; Yoon, S.S.; Hansen, D.M.; Wilcox, J.D.; Blumell, B.R.; Park, J.J.; Steffensen, S.C. Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin. Exp. Res. 2010, 34, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Kwon, O.S.; Moon, J.-Y.; Cho, S.J.; Choi, K.-H.; Kim, J.; Ahn, S.-H.; Ryu, Y. Mechanical stimulation of the HT7 acupuncture point to reduce ethanol self-administration in rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 6578621. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Kim, S.P.; Bang, S.K.; Kang, S.Y.; Cho, S.J.; Choi, K.-H.; Ryu, Y. The effect of acupuncture stimulation on alleviating emotional changes due to acute alcohol administration and the possibility of sigma1 receptor involvement. Integr. Med. Res. 2021, 10, 100497. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kim, D.H.; Jang, E.Y.; Yoon, S.S.; Gwak, Y.S.; Yi, Y.J.; Lee, J.Y.; Ahn, S.H.; Kim, J.M.; Ryu, Y.-H. Acupuncture attenuates alcohol dependence through activation of endorphinergic input to the nucleus accumbens from the arcuate nucleus. Sci. Adv. 2019, 5, eaax1342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jin, X.; Wu, Y.; Yang, X.; Xu, Y.; Jiang, J.Z.; Kim, S.C.; Lee, B.H.; Yang, C.H.; Zhao, R. Amygdaloid corticotropin-releasing factor is involved in the anxiolytic effect of acupuncture during ethanol withdrawal in rats. J. Acupunct. Meridian Stud. 2013, 6, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Zhao, G.W.; Li, H.Z.; Yang, X.D.; Wu, Y.Y.; Lin, F.; Guan, L.X.; Zhai, F.G.; Liu, J.Q.; Yang, C.H. Acupuncture attenuates anxiety-like behavior by normalizing amygdaloid catecholamines during ethanol withdrawal in rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 429843. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Liu, J.; Chen, J.; Liu, X.; Nie, G.; Byun, J.-S.; Liang, Y.; Park, J.; Huang, R. Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. Neuroimage Clin. 2016, 12, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A.C.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 2012, 34, 468–483. [Google Scholar] [PubMed]
- Brudzynski, S.M. Ethotransmission: Communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Brown, T.H. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J. Neurosci. 2003, 23, 8713–8721. [Google Scholar] [CrossRef]
- Yee, N.; Schwarting, R.K.; Fuchs, E.; Wöhr, M. Juvenile stress potentiates aversive 22-kHz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats. Stress 2012, 15, 533–544. [Google Scholar] [CrossRef]
- Besheer, J.; Fisher, K.R.; Lindsay, T.G.; Cannady, R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology 2013, 72, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Brymer, K.J.; Fenton, E.Y.; Kalynchuk, L.E.; Caruncho, H.J. Peripheral etanercept administration normalizes behavior, hippocampal neurogenesis, and hippocampal reelin and GABAA receptor expression in a preclinical model of depression. Front. Pharmacol. 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.J.; Kwon, S.; Jung, E.; Shim, I.; Lee, H.; Hahm, D.H. Acupuncture stimulation alleviates corticosterone-induced impairments of spatial memory and cholinergic neurons in rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 670536. [Google Scholar] [CrossRef] [PubMed]
- Blaine, S.K.; Sinha, R. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017, 122, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Panksepp, J.; Moskal, J.R. Frequency-modulated 50 kHz ultrasonic vocalizations: A tool for uncovering the molecular substrates of positive affect. Neurosci. Biobehav. Rev. 2011, 35, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Ociepa, D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol. Behav. 1992, 52, 655–660. [Google Scholar] [CrossRef]
- Kim, N.J.; Ryu, Y.; Lee, B.H.; Chang, S.; Fan, Y.; Gwak, Y.S.; Yang, C.H.; Bills, K.B.; Steffensen, S.C.; Koo, J.S. Acupuncture inhibition of methamphetamine-induced behaviors, dopamine release and hyperthermia in the nucleus accumbens: Mediation of group II mGluR. Addict. Biol. 2019, 24, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, M.; Hansson, A.C.; Spanagel, R.; Bilbao, A. Chronic intermittent ethanol exposure in mice leads to an up-regulation of CRH/CRHR 1 signaling. Alcohol. Clin. Exp. Res. 2015, 39, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.G.; Liu, H.R.; Zhang, Z.A.; Zhou, E.H.; Wang, X.M.; Jiang, B.; Shi, Z.; Zhou, C.L.; Qi, L.; Ma, X.P. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci. Lett. 2009, 465, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhang, J.J.; Qie, L.L. Acupuncture relieves the excessive excitation of hypothalamic-pituitary-adrenal cortex axis function and correlates with the regulatory mechanism of GR, CRH, and ACTHR. Evid. Based Complement. Alternat. Med. 2014, 2014, 495379. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R.; Thomas, T.L. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In Handbook of Psychophysiology; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: New York, NY, USA, 2000; pp. 342–367. [Google Scholar]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Mashayekh, R.; Motevalian, M.; Safari, S. The possible role of CREB-BDNF signaling pathway in neuroprotective effects of minocycline against alcohol-induced neurodegeneration: Molecular and behavioral evidences. Fundam. Clin. Pharmacol. 2021, 35, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Morais-Silva, G.; Alves, G.C.; Marin, M.T. N-acetylcysteine treatment blocks the development of ethanol-induced behavioural sensitization and related ΔFosB alterations. Neuropharmacology 2016, 110, 135–142. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, B.H.; Bae, J.H.; Kim, K.J.; Steffensen, S.C.; Ryu, Y.-H.; Leem, J.W.; Yang, C.H.; Kim, H.Y. Peripheral afferent mechanisms underlying acupuncture inhibition of cocaine behavioral effects in rats. PLoS ONE 2013, 8, e81018. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Buskila, D.; Kaplan, Z.; Zohar, J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol. Psychiatry 2008, 64, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002, 128, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine-dopamine interaction and acoustic coding. Behav. Brain Res. 2007, 182, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Leonard, K.C.; Brudzynski, S.M. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behav. Brain Res. 2006, 168, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Yoshida, M.; Yokoo, H.; Nishi, M.; Tanaka, M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci. Lett. 1993, 162, 81–84. [Google Scholar] [CrossRef]
- Seo, S.Y.; Moon, J.Y.; Kang, S.Y.; Kwon, O.S.; Kwon, S.; Bang, S.K.; Kim, S.P.; Choi, K.H.; Ryu, Y. An estradiol-independent BDNF-NPY cascade is involved in the antidepressant effect of mechanical acupuncture instruments in ovariectomized rats. Sci. Rep. 2018, 8, 5849. [Google Scholar] [CrossRef]
- Conte, W.L.; Kamishina, H.; Reep, R.L. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat. Protoc. 2009, 4, 1157–1166. [Google Scholar] [CrossRef]
- Pan, L.; Gilbert, F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology 1992, 56, 797–802. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.Y.; Bang, S.K.; Kang, S.Y.; Cho, S.J.; Choi, K.H.; Ryu, Y.H. Acupuncture Alleviates Anxiety and 22-kHz Ultrasonic Vocalizations in Rats Subjected to Repeated Alcohol Administration by Modulating the Brain-Derived Neurotrophic Factor/Corticotropin-Releasing Hormone Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4037. https://doi.org/10.3390/ijms22084037
Seo SY, Bang SK, Kang SY, Cho SJ, Choi KH, Ryu YH. Acupuncture Alleviates Anxiety and 22-kHz Ultrasonic Vocalizations in Rats Subjected to Repeated Alcohol Administration by Modulating the Brain-Derived Neurotrophic Factor/Corticotropin-Releasing Hormone Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(8):4037. https://doi.org/10.3390/ijms22084037
Chicago/Turabian StyleSeo, Su Yeon, Se Kyun Bang, Suk Yun Kang, Seong Jin Cho, Kwang Ho Choi, and Yeon Hee Ryu. 2021. "Acupuncture Alleviates Anxiety and 22-kHz Ultrasonic Vocalizations in Rats Subjected to Repeated Alcohol Administration by Modulating the Brain-Derived Neurotrophic Factor/Corticotropin-Releasing Hormone Signaling Pathway" International Journal of Molecular Sciences 22, no. 8: 4037. https://doi.org/10.3390/ijms22084037
APA StyleSeo, S. Y., Bang, S. K., Kang, S. Y., Cho, S. J., Choi, K. H., & Ryu, Y. H. (2021). Acupuncture Alleviates Anxiety and 22-kHz Ultrasonic Vocalizations in Rats Subjected to Repeated Alcohol Administration by Modulating the Brain-Derived Neurotrophic Factor/Corticotropin-Releasing Hormone Signaling Pathway. International Journal of Molecular Sciences, 22(8), 4037. https://doi.org/10.3390/ijms22084037