Enhancement of Fear Extinction Memory and Resistance to Age-Related Cognitive Decline in Butyrylcholinesterase Knockout Mice and (R)-Bambuterol Treated Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Mouse Genotyping
2.2. Intranasal and Oral Administration of (R)-Bambuterol in WT C57BL/6 Mice
2.3. Animal Experiment Design and Age Description
2.4. BChE Enzymatic Activity Determination
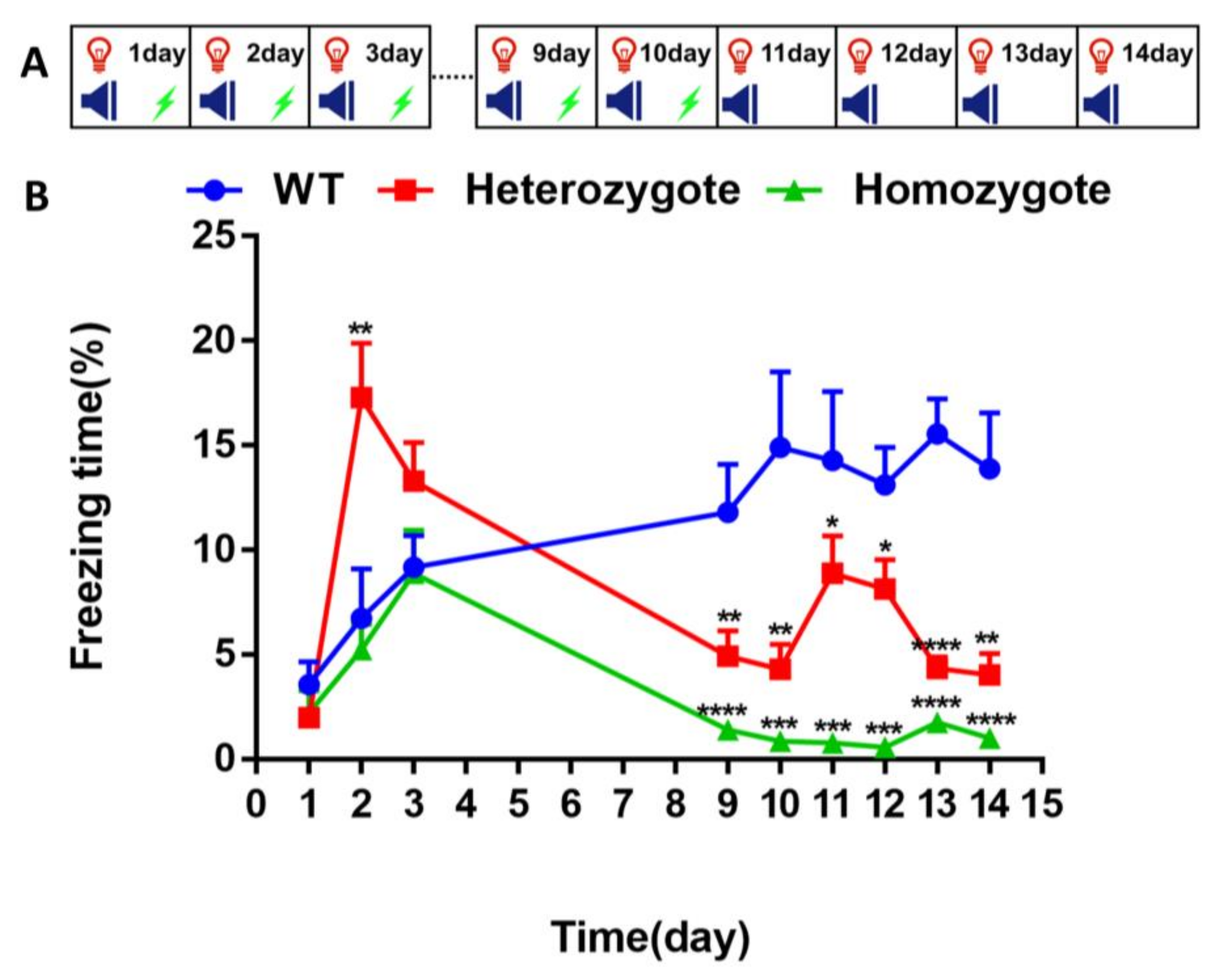
2.5. Fear Conditioning and Fear Extinction Test
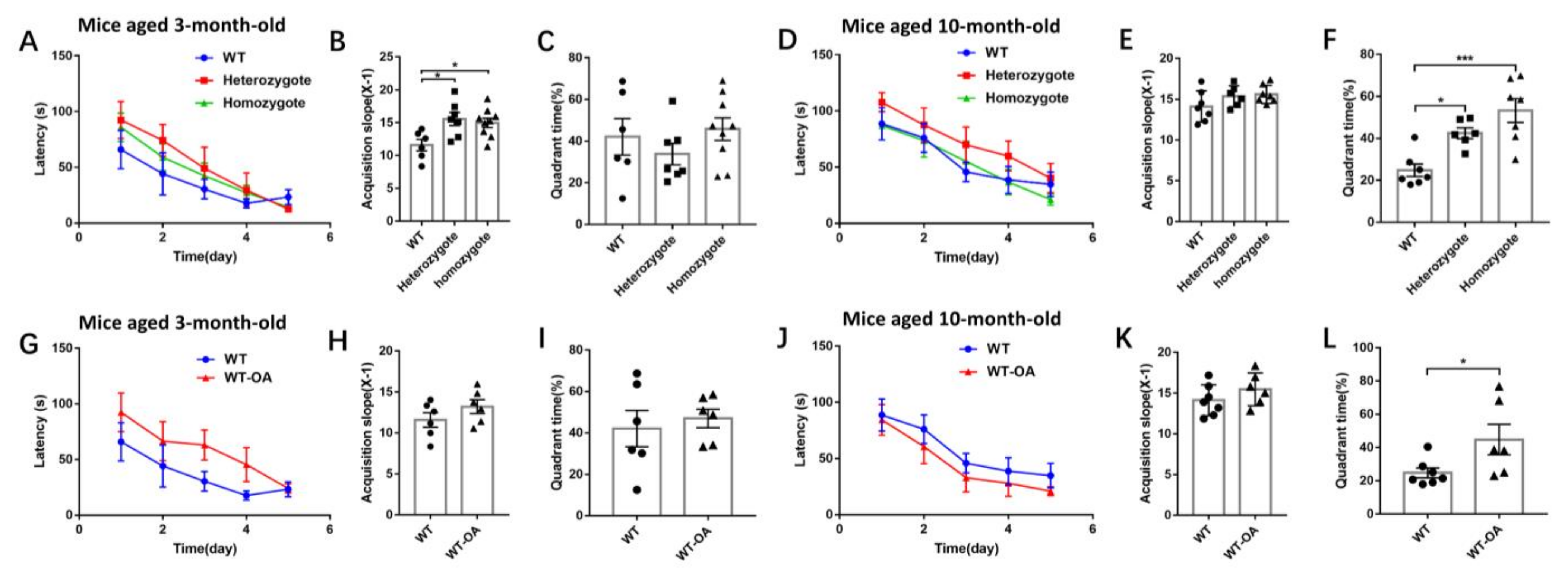
2.6. Morris Water Maze Experiment
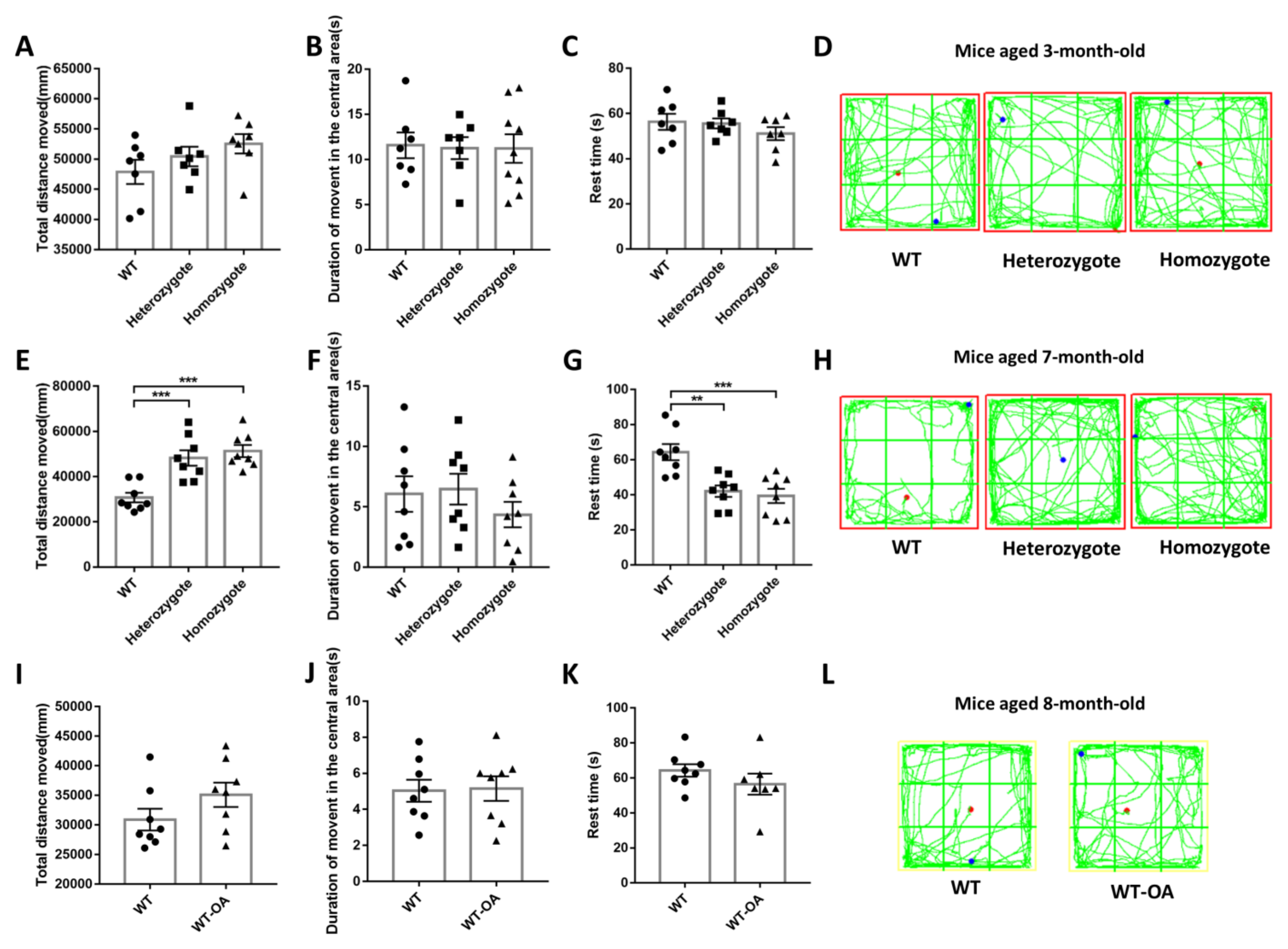
2.7. Open Field Test
2.8. Immunohistochemistry
2.9. DESI-MS Imaging
2.10. Detection of Substances in Serum
2.11. Data Processing
3. Results
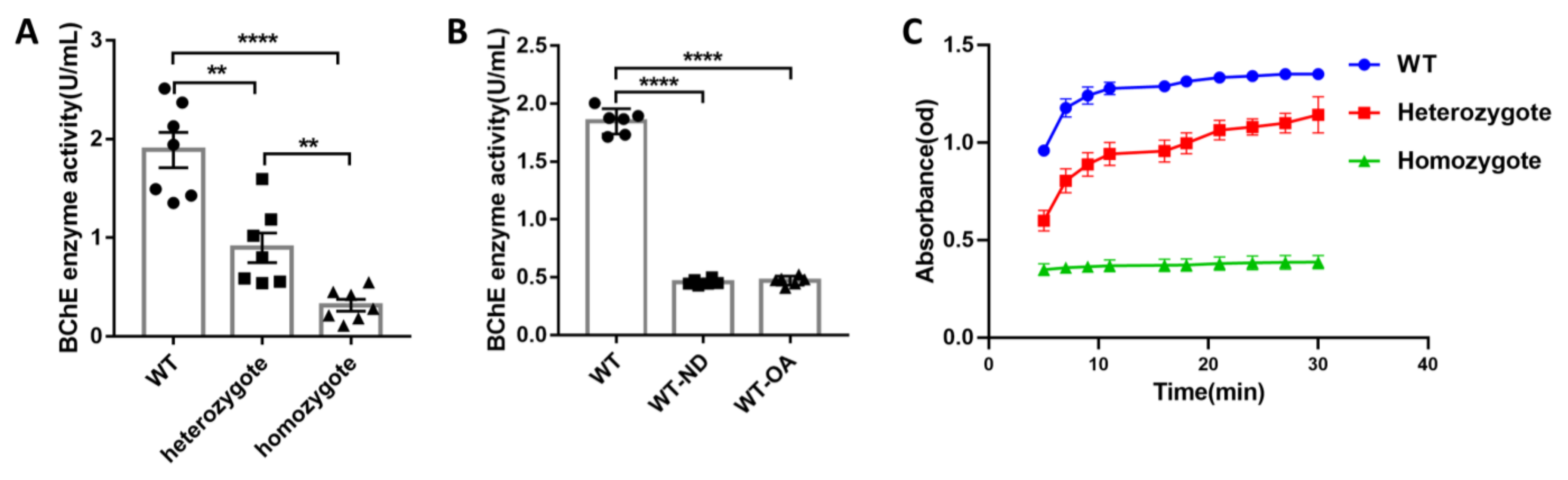
3.1. Both Gene KO and (R)-Bambuterol Administration Significantly Inhibited Serum Enzyme Activity in Mice
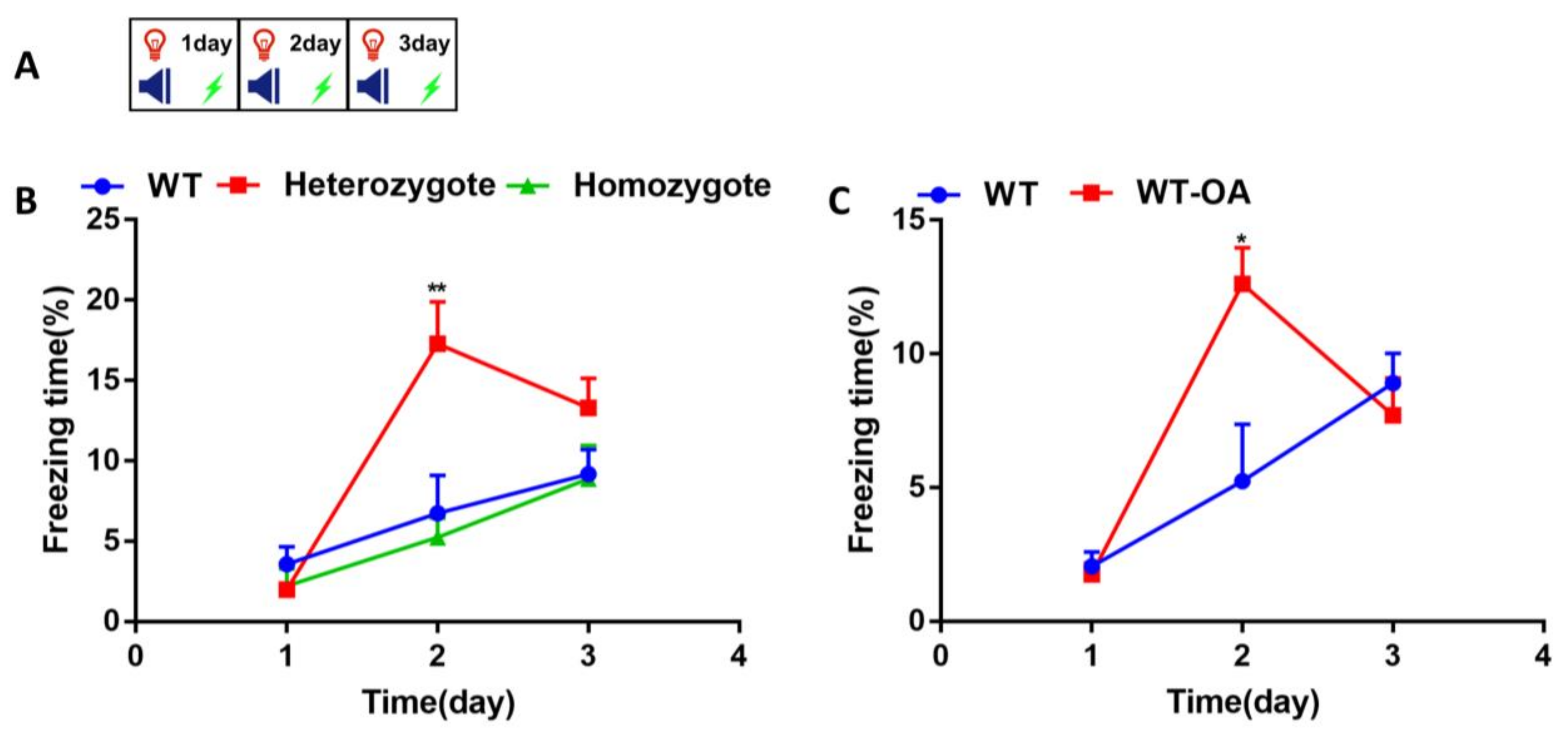
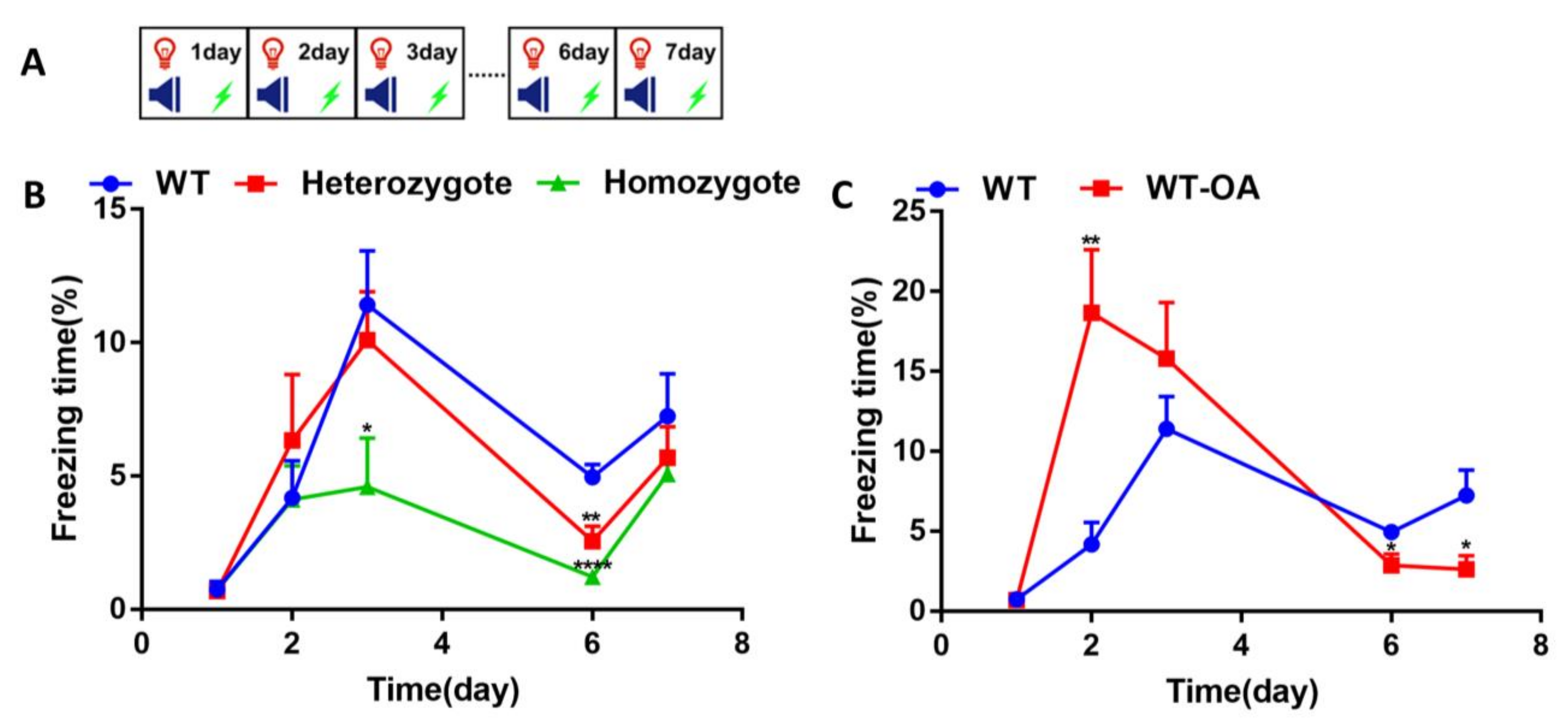
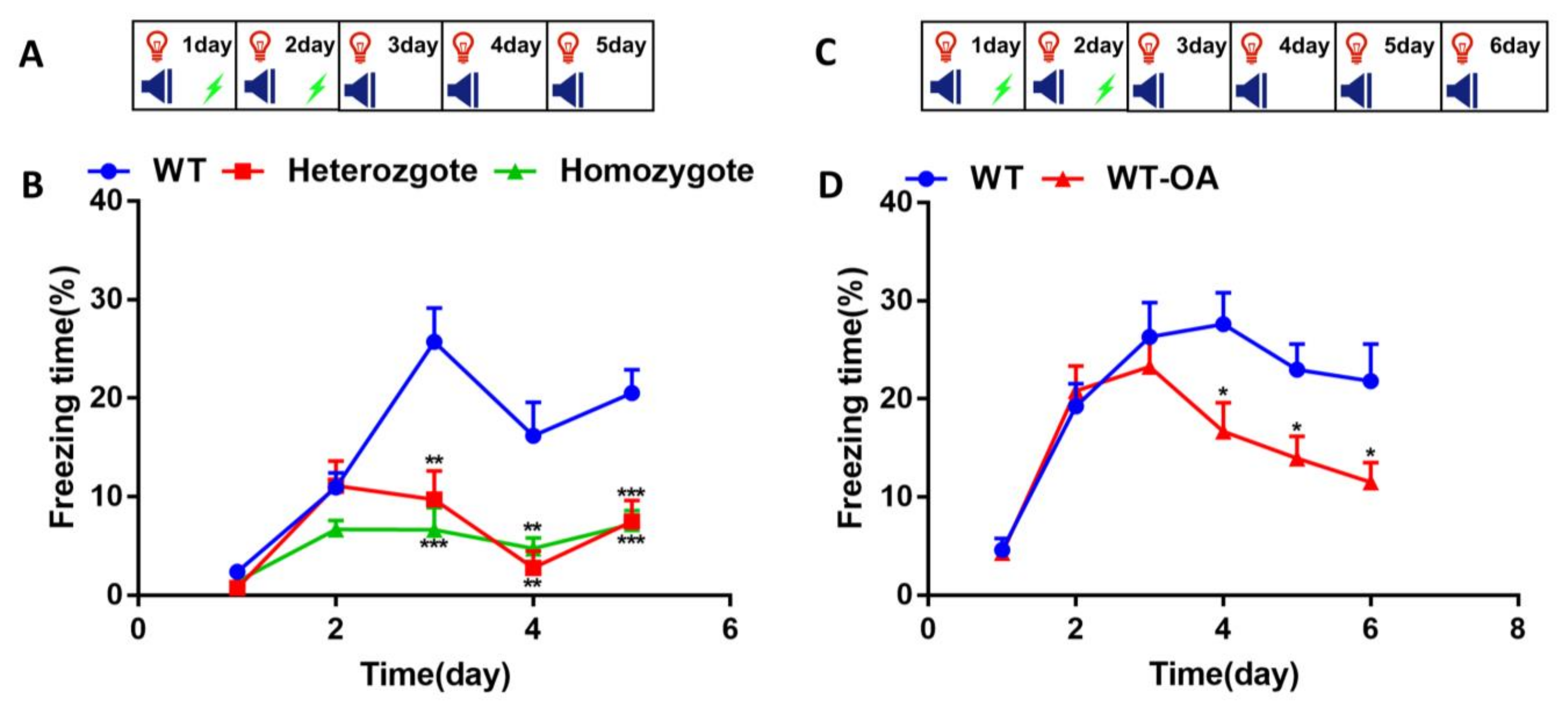
3.2. Inhibition of BChE Enhanced the Fear Memory, Inhibited the Re-Arousal of Fear, and Accelerated the Fear Extinction in Mice
3.3. Inhibition of BChE Resisted Age-Related Spatial Memory Decline in Mice in Morris Water Maze Test
3.4. Inhibition of BChE Rescued the Age-Related General Activity Decline in Mice
3.5. Inhibition of BChE Reduced the Optical Density of the Aβ, GFAP and IBA1 in the Hippocampus and Increased the Ratio of Aβ1-42/Aβ1-40 in Serum of 10-Month-Old Mice
3.6. Inhibited BChE Enzyme Activity Increased Glutamine and N-Acetyl-L-Aspartic Acid Content in Mouse Brain
4. Discussion
4.1. Inhibition of BChE Accelerates Fear Extinction and Enhances the Episodic Memory
4.2. Inhibition of BChE Ameliorates Risk Factors of Neurodegeneration in 10-Month-Old Mice, and Resists the Age-Related Spatial Memory Decline
4.3. Inhibition of BChE by (R)-Bambuterol Accelerates Fear Extinction and Ameliorates Risk Factors for Age-Related Cognitive Decline
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silman, I. The multiple biological roles of the cholinesterases. Prog. Biophys. Mol. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Grandone, I.; Contaldo, F.; Pasanisi, F. Butyrylcholinesterase as a prognostic marker: A review of the literature. J. Cachexia Sarcopenia Muscle 2013, 4, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo-Parmo, S.L.; Bartels, C.F.; Wiersema, B.; van der Spek, A.F.; Innis, J.W.; La Du, B.N. Characterization of 12 silent alleles of the human butyrylcholinesterase (BCHE) gene. Am. J. Hum. Genet. 1996, 58, 52–64. [Google Scholar] [PubMed]
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Tyurenkov, I.N.; Velikorodnaya, Y.I. Debatable Aspects of the Role of Butyrylcholinesterase in the Pathogenesisof Neurodegenerative Diseases. Uspehi Sovremennoj Biologii 2020, 140, 455–463. [Google Scholar] [CrossRef]
- Carmona, G.N.; Schindler, C.W.; Greig, N.H.; Holloway, H.W.; Jufer, R.A.; Cone, E.J.; Gorelick, D.A. Intravenous butyrylcholinesterase administration and plasma and brain levels of cocaine and metabolites in rats. Eur. J. Pharmacol. 2005, 517, 186–190. [Google Scholar] [CrossRef]
- Duysen, E.G.; Li, B.; Lockridge, O. The butyrylcholinesterase knockout mouse a research tool in the study of drug sensitivity, bio-distribution, obesity and Alzheimer’s disease. Expert Opin. Drug. Metab. Toxicol. 2009, 5, 523–528. [Google Scholar] [CrossRef]
- Chen, V.P.; Gao, Y.; Geng, L.; Stout, M.B.; Jensen, M.D.; Brimijoin, S. Butyrylcholinesterase Deficiency Promotes Adipose Tissue Growth and Hepatic Lipid Accumulation in Male Mice on High-Fat Diet. Endocrinology 2016, 157, 3086–3095. [Google Scholar] [CrossRef] [Green Version]
- Kurnutala, L.N.; Rugnath, N. Pseudocholinesterase Deficiency—Is Succinylcholine Still Needed to Facilitate Endotracheal Intubation? Cureus 2020, 12. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [Green Version]
- Nervo, A.; Calas, A.G.; Nachon, F.; Krejci, E. Respiratory failure triggered by cholinesterase inhibitors may involve activation of a reflex sensory pathway by acetylcholine spillover. Toxicology 2019, 424, 12. [Google Scholar] [CrossRef]
- Farooq, M.U.; Min, J.Y.; Goshgarian, C.; Gorelick, P.B. Pharmacotherapy for Vascular Cognitive Impairment. CNS Drugs 2017, 31, 759–776. [Google Scholar] [CrossRef]
- Hamieh, A.M.; Camperos, E.; Hernier, A.M.; Castagné, V. C57BL/6 mice as a preclinical model to study age-related cognitive deficits: Executive functions impairment and inter-individual differences. Brain Res. 2021, 1751, 147173. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Geula, C.; Mesulam, M.M. Cholinesterases and the Pathology of Alzheimer Disease. Alzheimer Dis. Associated Disorders 1995, 9. [Google Scholar] [CrossRef]
- Ha, Z.Y.; Mathew, S.; Yeong, K.Y. Butyrylcholinesterase: A Multifaceted Pharmacological Target and Tool. Curr. Protein Pept. Sci. 2020, 21, 99–109. [Google Scholar] [CrossRef]
- Pierre, A.; Van Schuerbeek, A.; Allaoui, W.; Van Laere, S.; Singewald, N.; Van Eeckhaut, A.; Smolders, I.; De Bundel, D. Effects of ghrelin receptor activation on forebrain dopamine release, conditioned fear and fear extinction in C57BL/6J mice. J. Neurochem。 2020, 154, 389–403. [Google Scholar] [CrossRef]
- Yao, J.; Yuan, Y.; Zheng, F.; Zhan, C.-G. Unexpected Reaction Pathway for butyrylcholinesterase-catalyzed inactivation of “hunger hormone” ghrelin. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Bukalo, O.; Nonaka, M.; Weinholtz, C.A.; Mendez, A.; Taylor, W.W.; Holmes, A. Effects of optogenetic photoexcitation of infralimbic cortex inputs to the basolateral amygdala on conditioned fear and extinction. Behav. Brain Res. 2021, 396. [Google Scholar] [CrossRef]
- Barricklow, J.; Blatnik, M. 2-Arachidonoylglycerol is a substrate for butyrylcholinesterase: A potential mechanism for extracellular endocannabinoid regulation. Arch. Biochem. Biophys. 2013, 536, 1–5. [Google Scholar] [CrossRef]
- Ney, L.J.; Akhurst, J.; Bruno, R.; Laing, P.A.F.; Matthews, A.; Felmingham, K.L. Dopamine, endocannabinoids and their interaction in fear extinction and negative affect in PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110118. [Google Scholar] [CrossRef]
- Davis, E.A.; Wald, H.S.; Suarez, A.N.; Zubcevic, J.; Liu, C.M.; Cortella, A.M.; Kamitakahara, A.K.; Polson, J.W.; Arnold, M.; Grill, H.J.; et al. Ghrelin Signaling Affects Feeding Behavior, Metabolism, and Memory through the Vagus Nerve. Curr. Biol. 2020, 30, 4510–4518.e6. [Google Scholar] [CrossRef]
- Wu, J.; Tian, Y.G.; Wang, S.P.; Pistolozzi, M.; Jin, Y.; Zhou, T.; Roy, G.; Xu, L.; Tan, W. Design, synthesis and biological evaluation of bambuterol analogues as novel inhibitors of butyrylcholinesterase. Eur. J. Med. Chem. 2017, 126, 61–71. [Google Scholar] [CrossRef]
- Bosak, A.; Gazic, I.; Vinkovic, V.; Kovarik, Z. Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers. Chem. Biol. Interact. 2008, 175, 192–195. [Google Scholar] [CrossRef]
- Hu, T.T.; Zhao, H.S.; Zhang, H.; Tan, W. The construction of butyrylcholinesterase knockout mouse model using TALENs technology. Heilongjiang J. Anim. Sci. Veterinar. Med. 2017, 19, 36–38. [Google Scholar] [CrossRef]
- Gülçin, İ.; Tel, A.Z.; Gören, A.C.; Taslimi, P.; Alwasel, S.H. Sage (Salvia pilifera): Determination of its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Meas. Charact. 2019, 13, 2062–2074. [Google Scholar] [CrossRef]
- Takagi, T.; Higashi, Y.; Asai, M.; Ishii, S. Introduction of a de novo Creb-binding protein gene mutation in sperm to produce a Rubinstein-Taybi syndrome model using inbred C57BL/6 mice. Brain Res. 2020, 1749, 147140. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Oitzl, M.S.; Grauer, E. Fear memory for cue and context: Opposite and time-dependent effects of a physiological dose of corticosterone in male BALB/c and C57BL/6J mice. Brain Res. 2012, 1466, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Schulkin, J.; LeDoux, J.E. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav. Brain Res. 2003, 146, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, Z.; Tan, K.-L.; Chen, R.; Su, W.; Zhao, H.; Tan, Q.; Tan, W. Enhanced Contextual Fear Memory and Elevated Astroglial Glutamate Synthase Activity in Hippocampal CA1 BChE shRNA Knockdown Mice. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Z.; Zhang, J.; Cai, X.; Zhong, Z.; Huang, Y.; Qu, S. Electroacupuncture alleviated the depression-like behavior by regulating FGF2 and astrocytes in the hippocampus of rats with chronic unpredictable mild stress. Brain Res. Bull. 2021, 169, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Wang, Q.H.; Liao, Y.J.; Sun, Y.; Yang, R. The effect of sevoflurane on the spatial recall ability and expression of apolipoprotein E and beta amyloid in the hippocampus in rats. Cell. Mol. Biol. 2020, 66, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.; Ferreira, C.R.; Eberlin, L.S.; Jarmusch, A.K.; Pirro, V.; Rodrigues, A.C.B.; Favaron, P.O.; Miglino, M.A.; Cooks, R.G. Metabolites and Lipids Associated with Fetal Swine Anatomy via Desorption Electrospray Ionization-Mass Spectrometry Imaging. Sci. Rep. 2019, 9, 7247. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Duysen, E.G.; Carlson, M.; Lockridge, O. The Butyrylcholinesterase Knockout Mouse as a Model for Human Butyrylcholinesterase Deficiency. J. Pharmacol. Exp. Ther. 2008, 324, 1146–1154. [Google Scholar] [CrossRef]
- Maurice, T.; Strehaiano, M.; Simeon, N.; Bertrand, C.; Chatonnet, A. Learning performances and vulnerability to amyloid toxicity in the butyrylcholinesterase knockout mouse. Behav. Brain Res. 2016, 296, 351–360. [Google Scholar] [CrossRef]
- Song, D.; Wang, D.H.; Yang, Q.H.; Yan, T.Y.; Wang, Z.; Yan, Y.; Zhao, J.; Xie, Z.; Liu, Y.C.; Ke, Z.J.; et al. The lateralization of left hippocampal CA3 during the retrieval of spatial working memory. Nat. Commun. 2020, 11, 13. [Google Scholar] [CrossRef]
- Machado-Santos, A.R.; Alves, N.D.; Araujo, B.; Correia, J.S.; Patricio, P.; Mateus-Pinheiro, A.; Loureiro-Campos, E.; Bessa, J.M.; Sousa, N.; Pinto, L. Astrocytic plasticity at the dorsal dentate gyrus on an animal model of recurrent depression. Neuroscience 2021, 454, 94–104. [Google Scholar] [CrossRef]
- Wiseman, J.M.; Ifa, D.R.; Zhu, Y.; Kissinger, C.B.; Manicke, N.E.; Kissinger, P.T.; Cooks, R.G. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. PNAS 2008, 105, 18120–18125. [Google Scholar] [CrossRef] [Green Version]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Milad, M.R.; Quirk, G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Craske, M.G.; Fanselow, M.; Treanor, M.; Bystritksy, A. Cholinergic Modulation of Exposure Disrupts Hippocampal Processes and Augments Extinction: Proof-of-Concept Study With Social Anxiety Disorder. Biol. Psychiatry 2019, 86, 703–711. [Google Scholar] [CrossRef]
- Gunduz-Cinar, O. The endocannabinoid system in the amygdala and modulation of fear. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110116. [Google Scholar] [CrossRef]
- Shi, L.; Deng, J.H.; Chen, S.J.; Que, J.Y.; Sun, Y.K.; Wang, Z.; Guo, X.J.; Han, Y.; Zhou, Y.X.; Zhang, X.J.; et al. Fasting enhances extinction retention and prevents the return of fear in humans. Transl. Psychiatr. 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhang, F.Y.; Wang, H.; Wang, C.; Zhou, Z.G.; Zhou, Y. Ginsenoside Rg1 fails to rescue PTSD-like behaviors in a mice model of single-prolonged stress. Biochem. Biophys. Res. Commun. 2020, 528, 243–248. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Lacreuse, A.; Parr, L.; Chennareddi, L.; Herndon, J.G. Age-related decline in cognitive flexibility in female chimpanzees. Neurobiol. Aging 2018, 72, 83–88. [Google Scholar] [CrossRef]
- Uddin, M.S.; Stachowiak, A.; Al Mamun, A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 18. [Google Scholar] [CrossRef]
- Kamphuis, W.; Middeldorp, J.; Kooijman, L.; Sluijs, J.A.; Kooi, E.J.; Moeton, M.; Freriks, M.; Mizee, M.R.; Hol, E.M. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 492–510. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Di Rosa, M. CHI3L2 Expression Levels Are Correlated with AIF1, PECAM1, and CALB1 in the Brains of Alzheimer’s Disease Patients. J. Mol. Neurosci. 2020, 70, 1598–1610. [Google Scholar] [CrossRef]
- Zavorotnyy, M.; Zöllner, R.; Rekate, H.; Dietsche, P.; Bopp, M.; Sommer, J.; Meller, T.; Krug, A.; Nenadić, I. Intermittent theta-burst stimulation moderates interaction between increment of N-Acetyl-Aspartate in anterior cingulate and improvement of unipolar depression. Brain Stimul. 2020, 13, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Kwo-On-Yuen, P.F.; Newmark, R.D.; Budinger, T.F.; Kaye, J.A.; Ball, M.J.; Jagust, W.J. Brain N-acetyl-L-aspartic acid in Alzheimer’s disease: a proton magnetic resonance spectroscopy study. Brain Res. 1994, 667, 167–174. [Google Scholar] [CrossRef]
- Luo, L.-L.; Li, Y.-F.; Shan, H.-M.; Wang, L.-P.; Yuan, F.; Ma, Y.-Y.; Li, W.-L.; He, T.-T.; Wang, Y.-Y.; Qu, M.-J.; et al. L-glutamine protects mouse brain from ischemic injury via up-regulating heat shock protein 70. CNS Neurosci. Ther. 2019, 25, 1030–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Deters, B.J.; Saleem, M. The role of glutamine in supporting gut health and neuropsychiatric factors. Food Sci. Hum. Wellness 2021, 10, 149–154. [Google Scholar] [CrossRef]
- Park, S.A.; Han, S.M.; Kim, C.E. New fluid biomarkers tracking non-amyloid-beta and non-tau pathology in Alzheimer’s disease. Exp. Mol. Med. 2020, 52, 556–568. [Google Scholar] [CrossRef] [Green Version]
- Reid, G.A.; Darvesh, S. Butyrylcholinesterase-knockout reduces brain deposition of fibrillar β-amyloid in an Alzheimer mouse model. Neuroscience 2015, 298, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.-S.; Greig, N.H.; Nagai, T.; et al. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-beta peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Seppala, T.T.; Herukka, S.K.; Hanninen, T.; Tervo, S.; Hallikainen, M.; Soininen, H.; Pirttila, T. Plasma A beta 42 and A beta 40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1123–1127. [Google Scholar] [CrossRef]
- Wang, D.; Di, X.; Fu, L.; Li, Y.; Han, X.; Wu, H.; Cai, L.; Meng, X.; Jiang, C.; Kong, W.; et al. Analysis of serum beta-amyloid peptides, alpha 2-macroglobulin, complement factor H, and clusterin levels in APP/PS1 transgenic mice during progression of Alzheimer’s disease. Neuroreport 2016, 27, 1114–1119. [Google Scholar] [CrossRef]


| Sequence (5′–3′) | Template Strand | Length | Start | Stop | Tm | GC% | Self Complementarity | Self 3′ Complementarity | |
|---|---|---|---|---|---|---|---|---|---|
| Forward primer | CTGCTCTGCATGCCTTTTGG | Plus | 20 | 62 | 81 | 60.11 | 55.00 | 6.00 | 0.00 |
| Reverse primer | ATTTCTGACCCCTGGAAGCC | Minus | 20 | 336 | 317 | 59.67 | 55.00 | 3.00 | 1.00 |
| Product primer | 275 |
| Experiments | BChE Knockout Mice | (R)-Bambuterol Treated Mice | |
|---|---|---|---|
| Age | Age | ||
| BChE enzyme activity | 4 months old | 4 months old | |
| Fear conditioning and fear extinction test | Fear conditioning | 4 months old | 4 months old |
| Re-exposure to fear conditioning | 10 months old | 10 months old | |
| Fear extinction | 6 months old | 8 months old | |
| Combination of fear conditioning, re-exposure and extinction training | 4 months old | \ | |
| Morris water maze experiment | 3 months old | 3 months old | |
| 10 months old | 10 months old | ||
| Open field test | 3 months old | \ | |
| 7 months old | 8 months old | ||
| Immunohistochemistry | 10 months old | 10 months old | |
| DESI-MS imaging | 10 months old | \ | |
| Detection of substances in serum | 10 months old | \ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Cao, Y.; Lin, Y.; Tan, K.S.; Zhao, H.; Guo, H.; Tan, W. Enhancement of Fear Extinction Memory and Resistance to Age-Related Cognitive Decline in Butyrylcholinesterase Knockout Mice and (R)-Bambuterol Treated Mice. Biology 2021, 10, 404. https://doi.org/10.3390/biology10050404
Liu W, Cao Y, Lin Y, Tan KS, Zhao H, Guo H, Tan W. Enhancement of Fear Extinction Memory and Resistance to Age-Related Cognitive Decline in Butyrylcholinesterase Knockout Mice and (R)-Bambuterol Treated Mice. Biology. 2021; 10(5):404. https://doi.org/10.3390/biology10050404
Chicago/Turabian StyleLiu, Weiwei, Yan Cao, Yue Lin, Keai Sinn Tan, Haishan Zhao, Haihua Guo, and Wen Tan. 2021. "Enhancement of Fear Extinction Memory and Resistance to Age-Related Cognitive Decline in Butyrylcholinesterase Knockout Mice and (R)-Bambuterol Treated Mice" Biology 10, no. 5: 404. https://doi.org/10.3390/biology10050404
APA StyleLiu, W., Cao, Y., Lin, Y., Tan, K. S., Zhao, H., Guo, H., & Tan, W. (2021). Enhancement of Fear Extinction Memory and Resistance to Age-Related Cognitive Decline in Butyrylcholinesterase Knockout Mice and (R)-Bambuterol Treated Mice. Biology, 10(5), 404. https://doi.org/10.3390/biology10050404







