Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Cellulose
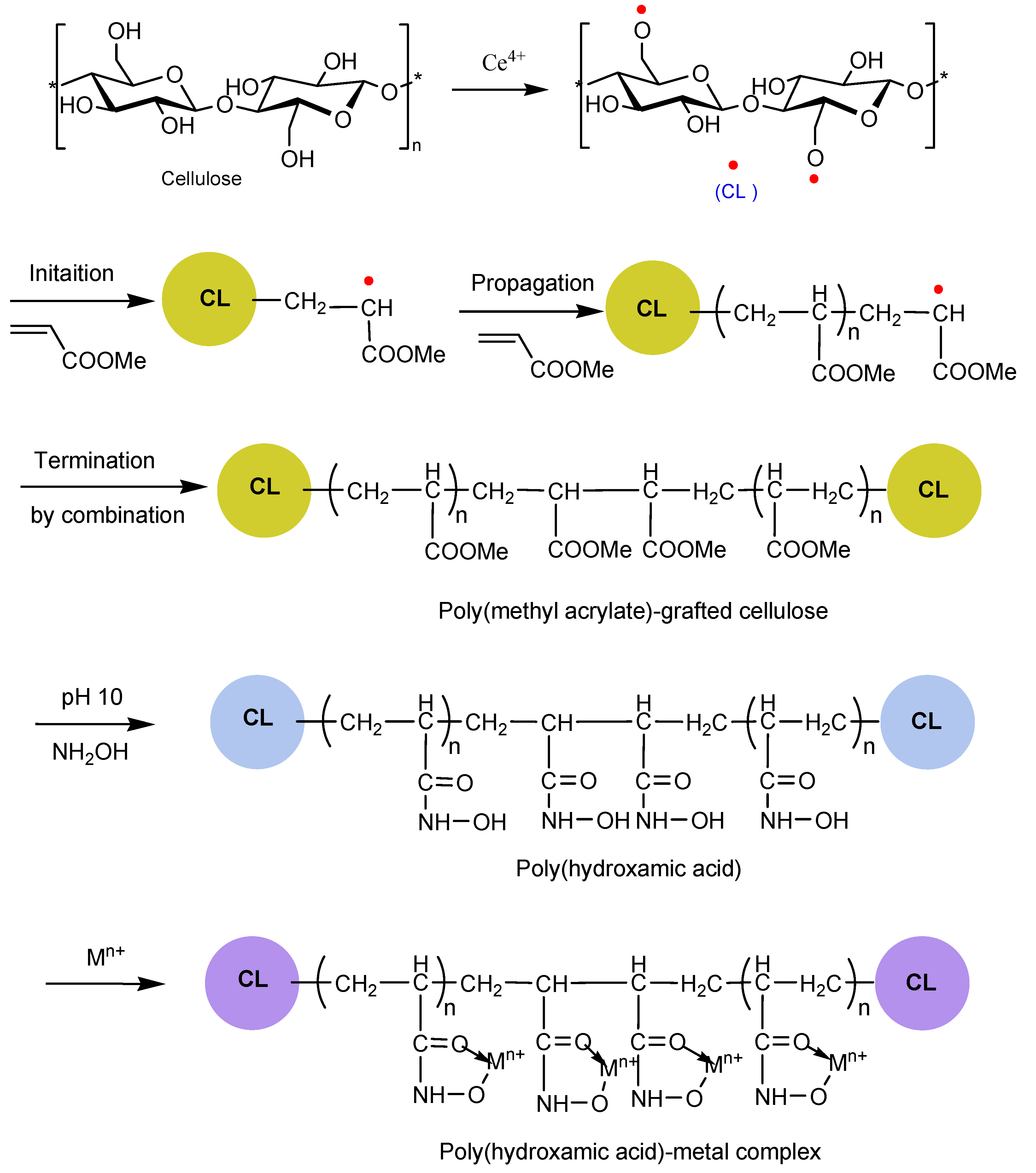
2.2. Graft Copolymerization
2.3. Synthesis of Poly(hydroxamic acid) Ligand
2.4. Batch Adsorption Studies
2.5. Desorption and Reusability Studies
3. Results and Discussion
3.1. Synthesis of Poly(hydroxamic acid) Ligand
3.2. FT-IR Analysis
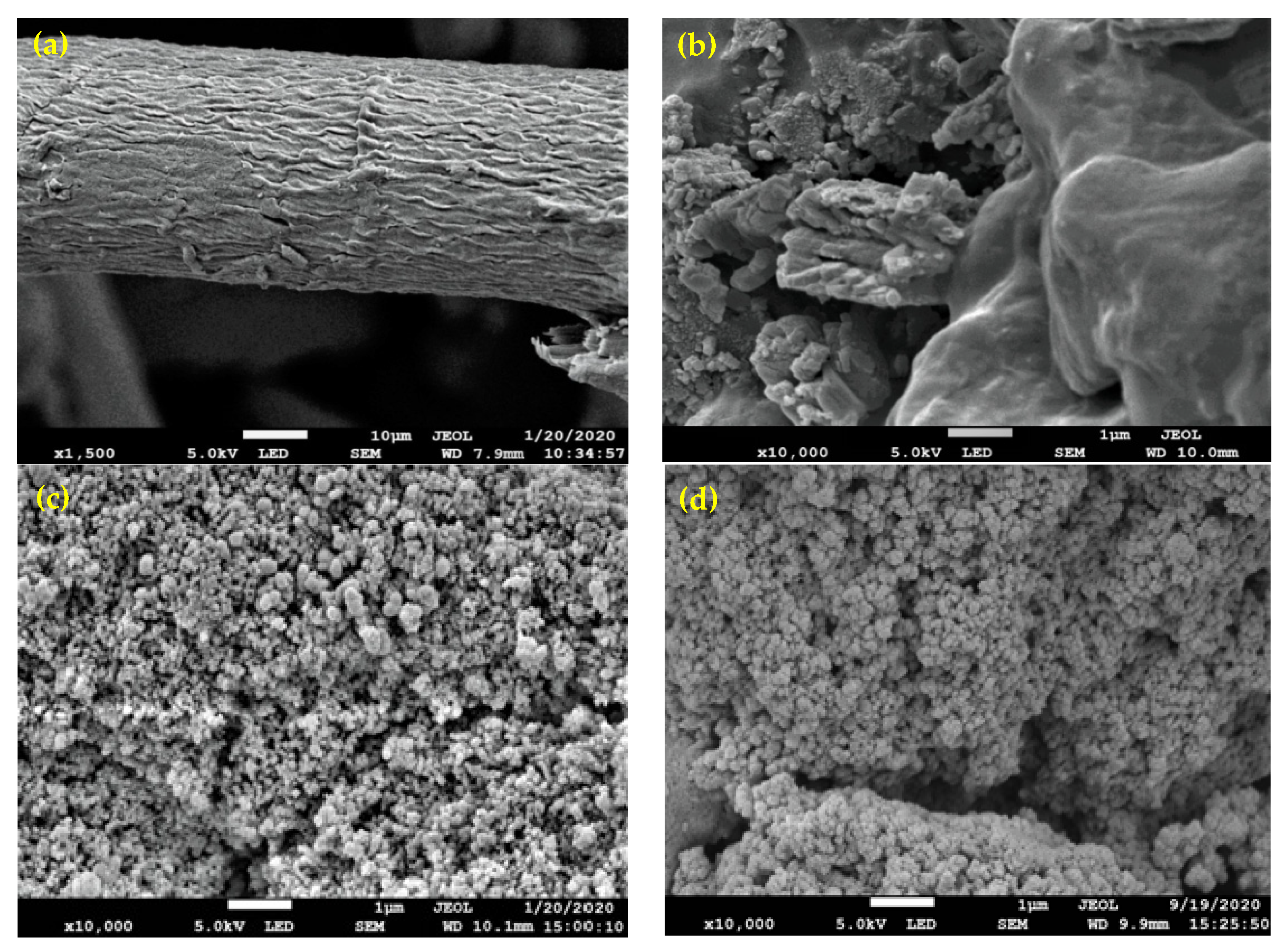
3.3. FE-SEM Analysis
3.4. TEM and EDX Analysis
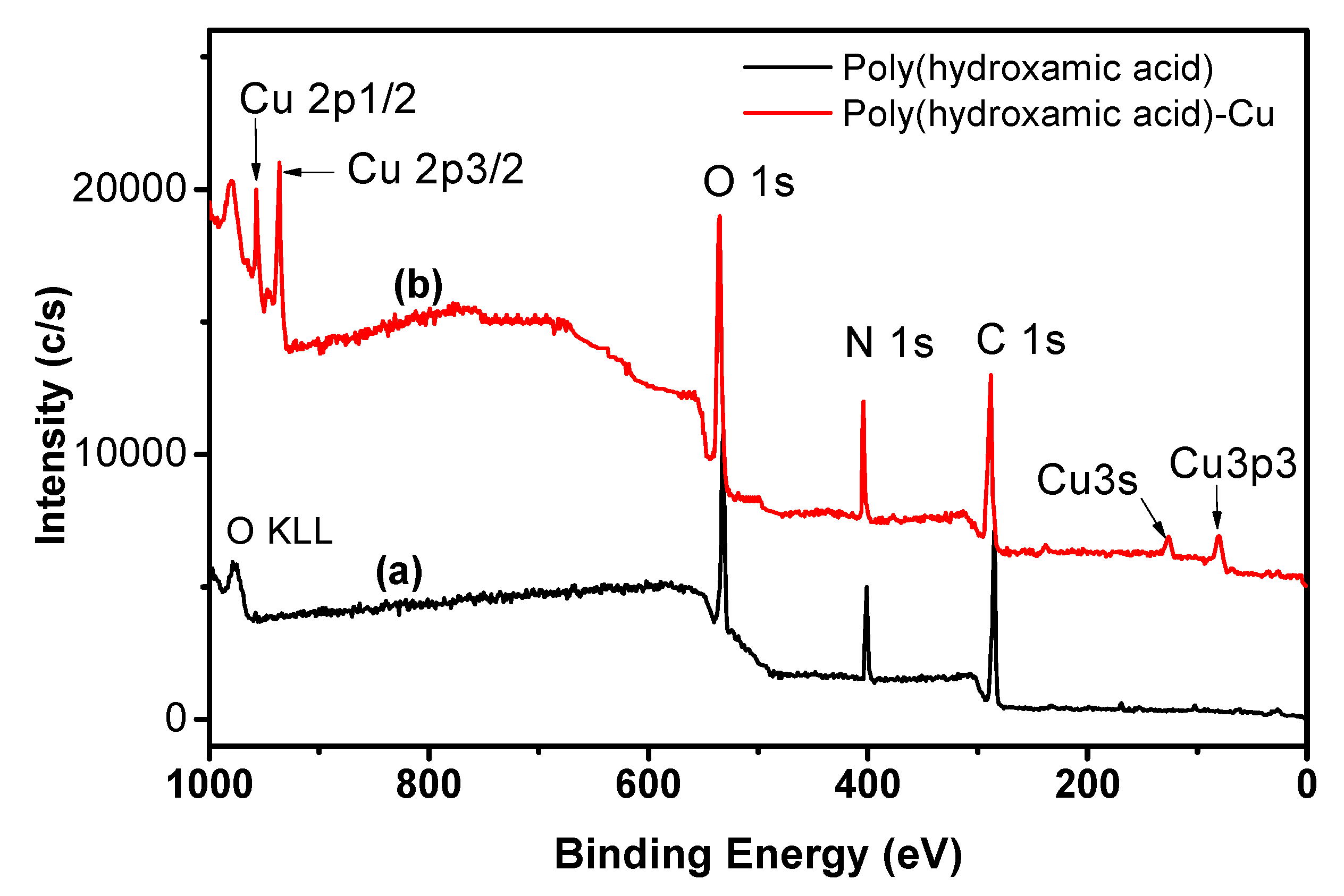
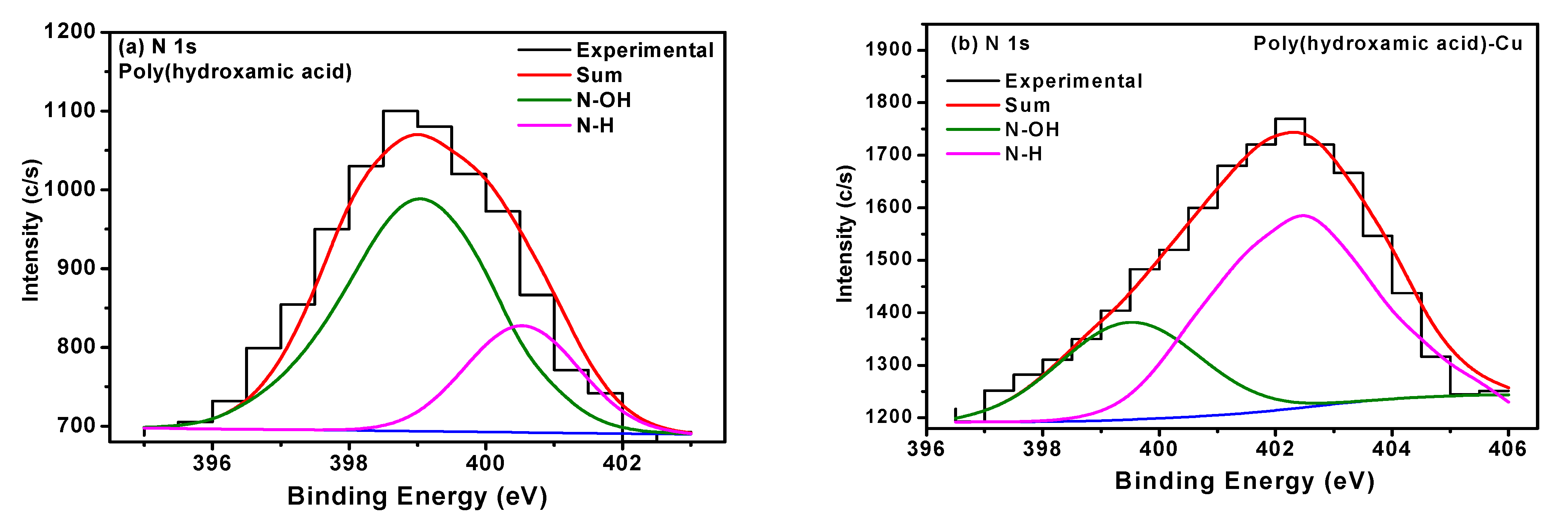
3.5. X-ray Photoelectron Spectroscopy Analysis (XPS)
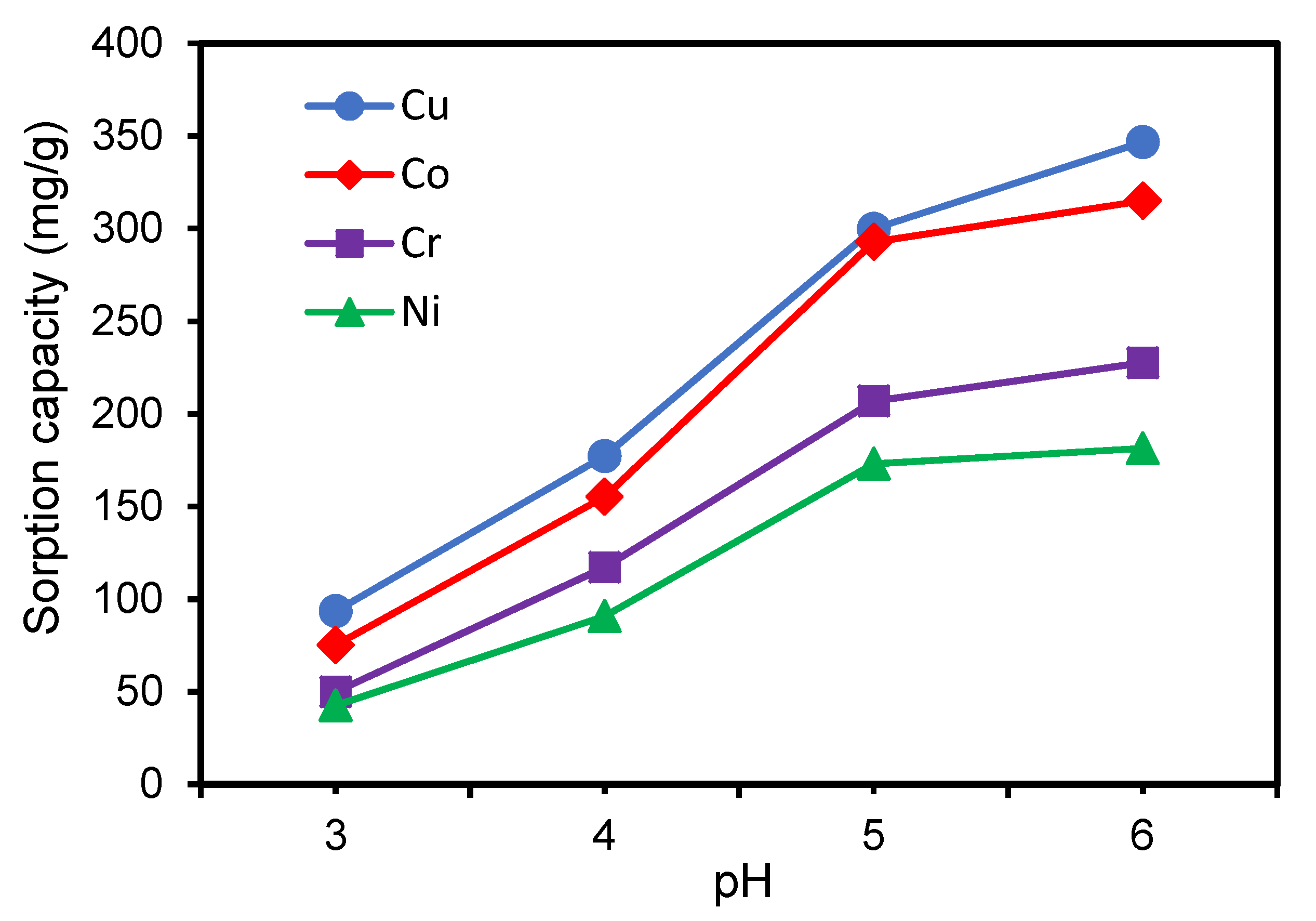
3.6. Adsorption of Heavy Metal Ions
Effect of pH on the Adsorption
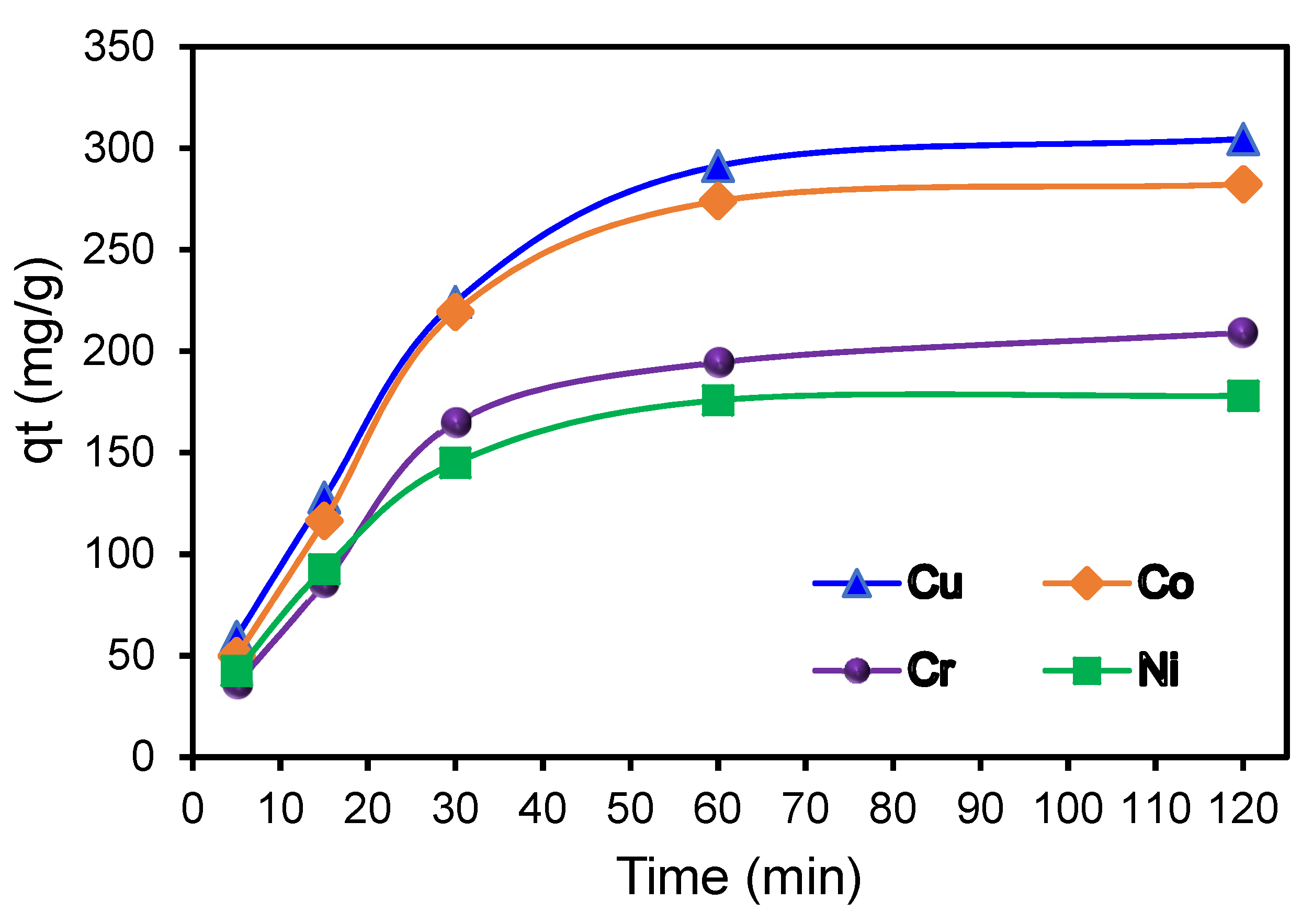
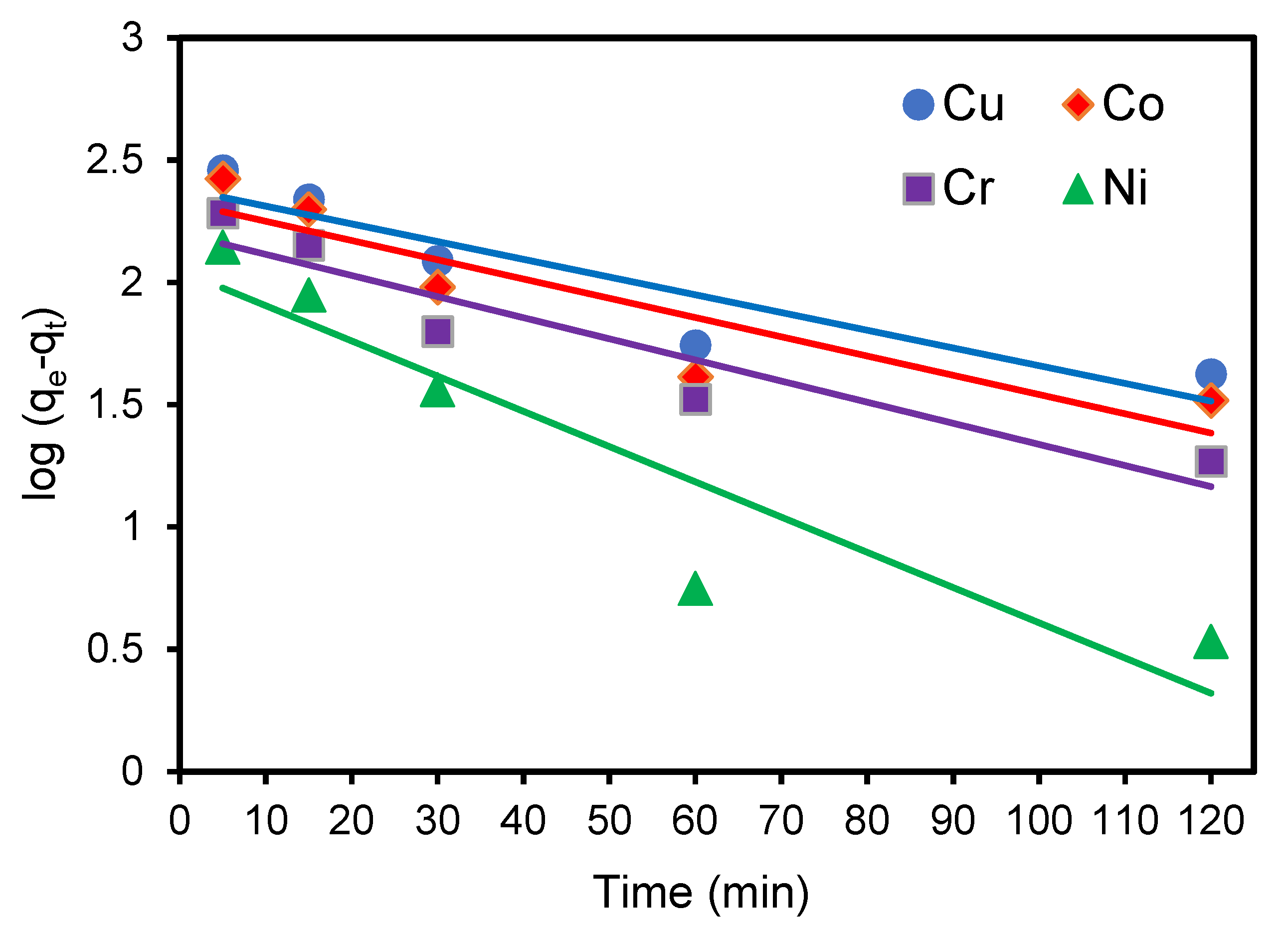
3.7. Adsorption Kinetic Studies
3.7.1. Pseudo-First-Order Rate of Reaction
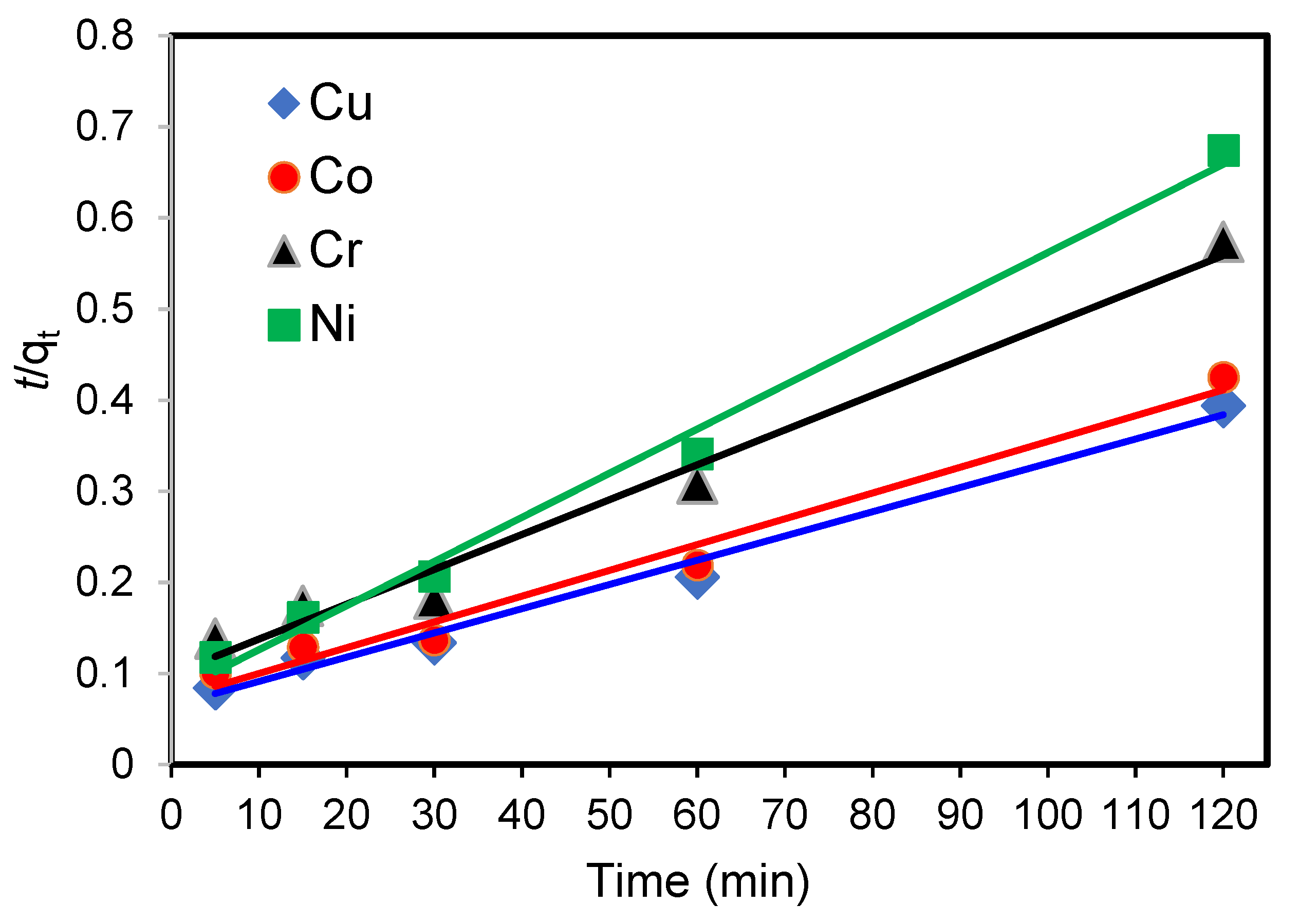
3.7.2. Pseudo-Second-Order Rate of Reaction
3.8. Adsorption Isothermal Studies
3.8.1. Linear Langmuir Adsorption Isotherm
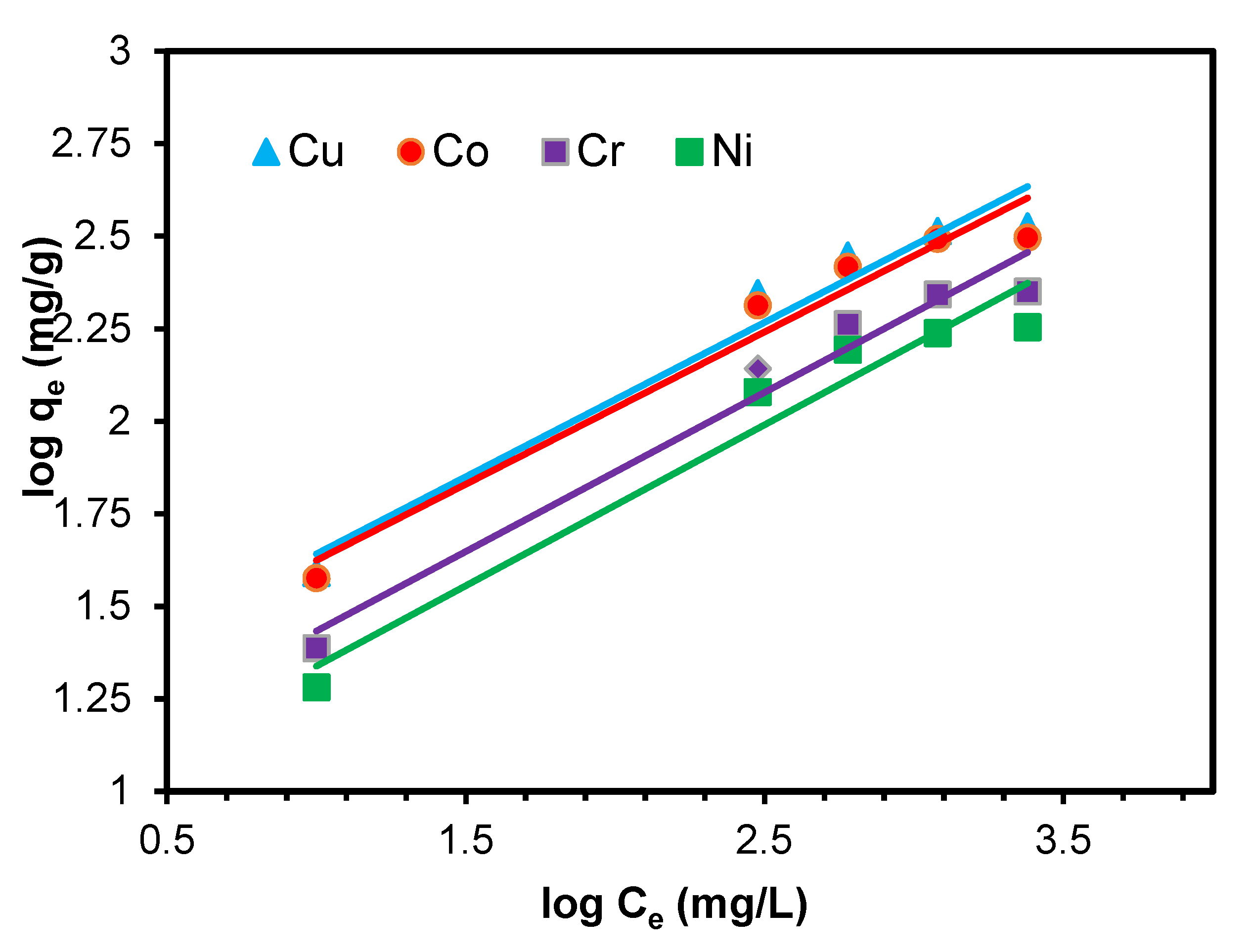
3.8.2. Linear Freundlich Adsorption Isotherm
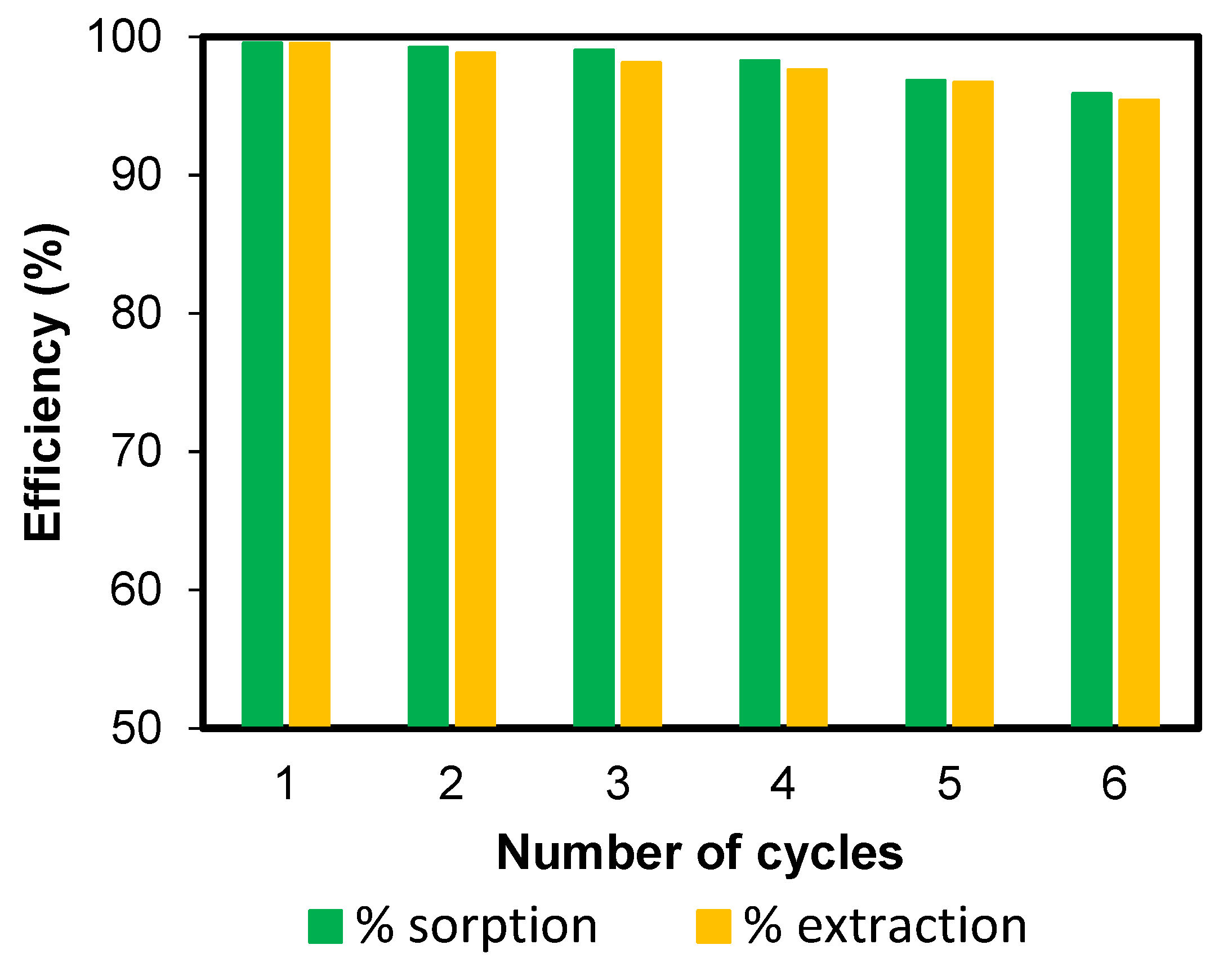
3.9. Reusability Study of Poly(hydroxamic acid) Ligand
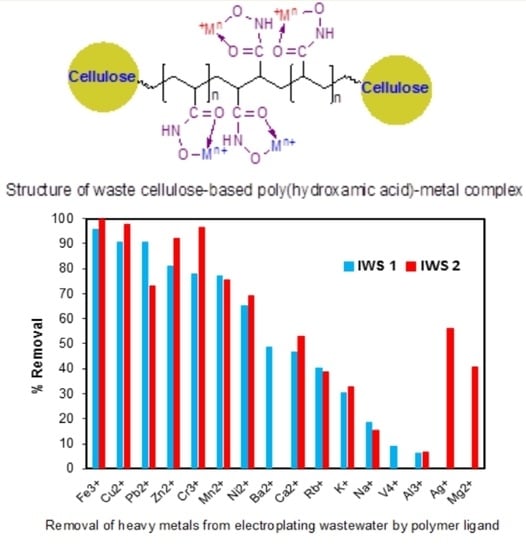
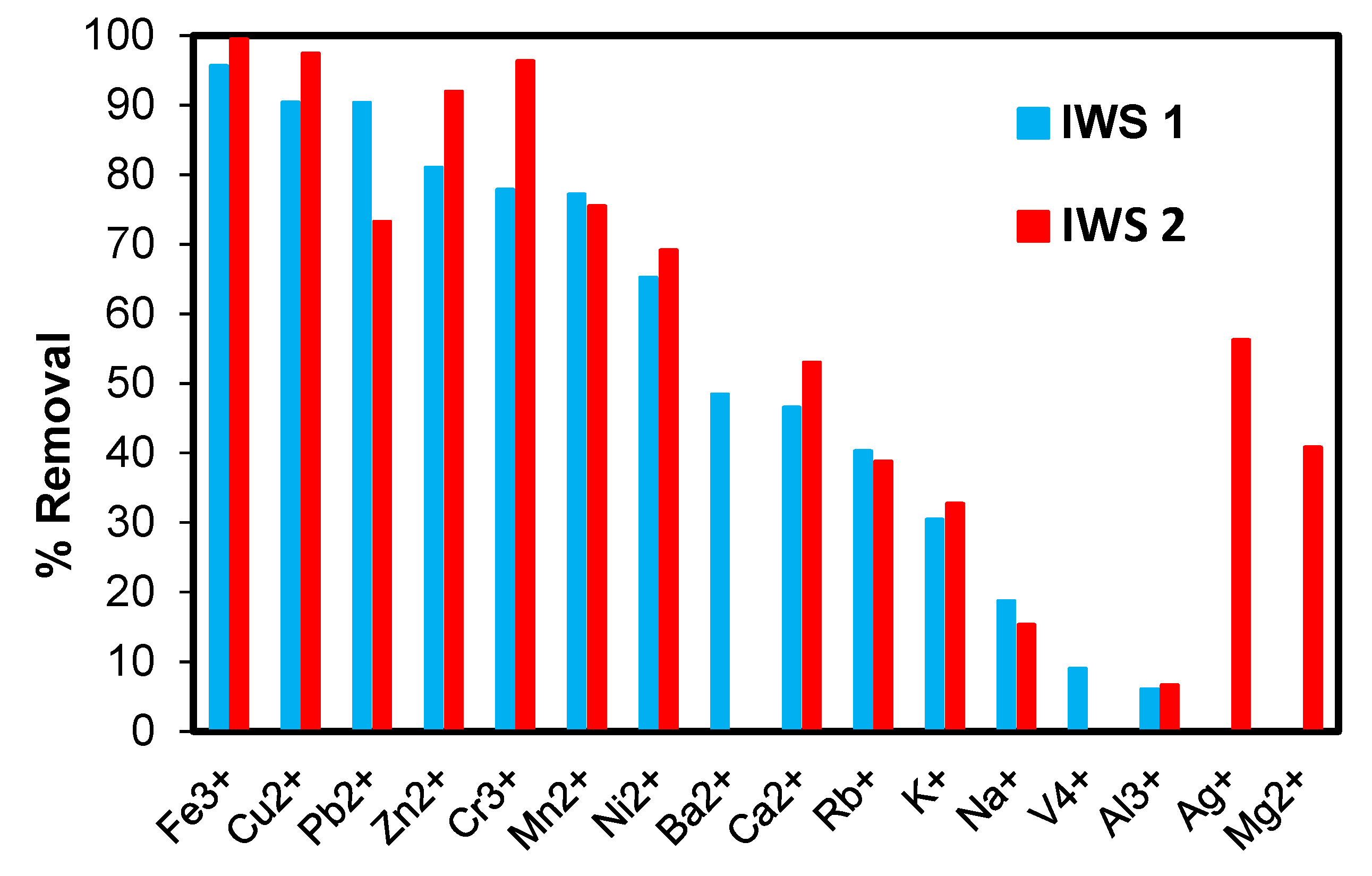
3.10. Practical Application of Poly(hydroxamic acid) Ligand
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Sulaymon, I.D.; Ugya, A.Y.; Wang, H.C.; Wang, A. Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of Removing Toxic Metals for Industrial Wastewater. J. Multi. Eng. Sci. Stud. 2015, 1, 12–18. [Google Scholar]
- Rahman, M.L.; Sarkar, S.M.; Farida, E.M.; Arshad, S.E.; Sarjadi, M.S.; Wid, N. Synthesis of tapioca cellulose-based poly(amidoxime) ligand for removal of heavy metal ions. J. Macromol. Sci. Part B Phys. 2018, 57, 83–99. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Luo, Y.; Liu, C.; Kyzas, G.Z.; Luo, Y.; Zhao, D.; An, S.; Zhu, H. Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J. Hazard. Mater. 2014, 270, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Rohani, N.N.M.; Yusoff, M.M. Synthesis of polyamidoxime chelating ligand from polymer-grafted corn-cob cellulose for metal extraction. J. Appl. Polym. Sci. 2014, 131, 40833. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, X.; Cai, P.; Guo, T.; Tong, Z.; Xiao, H. Dye removal from single and binary systems using gel-like bioadsorbent based on functional-modified cellulose. Cellulose 2018, 25, 2559–2575. [Google Scholar] [CrossRef]
- Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Peng, J.; Cheng, S.; Fu, L. Selective recovery of Au (III) from aqueous solutions using 2-aminothiazole functionalized corn bract as low-cost bioadsorbent. J. Clean. Prod. 2018, 196, 1007–1015. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Luo, Z.; Shen, J.; Ni, Q.; Yao, J. Facile preparation of a cellulose-based bioadsorbent modified modified by hPEI in heterogenous system for high-efficiency removal of multiple types of dyes. React. Funct. Polym. 2018, 125, 77–83. [Google Scholar] [CrossRef]
- Dos Santos Silva, L.; de Oliveira Carvalho, J.; de Sousa Bezerra, R.D.; Da Silva, M.S.; Ferreira, F.J.L.; Osajima, J.A.; da Silva Filho, E.C. Potential of cellulose functionalized with carboxylic acid as biosorbent for the removal of cationic dyes in aqueous solution. Molecules 2018, 23, 743. [Google Scholar] [CrossRef] [Green Version]
- Lakherwal, D. Adsorption of Toxic Metals: A Review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Lin, L.; Jin, P.X.; Yu, J.L.; Qin, Z.; Ju, M.Y. Three-dimensional macroporous cellulose-based bioadsorbents for efficient removal of nickel ions from aqueous solution. Cellulose 2016, 23, 723–736. [Google Scholar]
- Wang, F.; Zhu, Y.; Wang, A. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/attapulgite porous monolith with an eco-friendly pickering-mipe template for Ce(III) and Gd(III) adsorption. Front. Chem. 2020, 8, 398. [Google Scholar] [CrossRef]
- Hajeeth, T.; Vijayalakshmi, K.; Gomathi, T.; Sudha, P.N. Removal of Cu (II) and Ni (II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int. J. Biol. Macromol. 2013, 62, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of toxic metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of toxic metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W. Chemical coagulation process for the removal of toxic metals from water: A review. Desalin. Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- Bashkim, S.T.; Salih, T.G. Reverse osmosis removal of toxic metals from wastewater effluents using biowaste materials pretreatment. Pol. J. Environ. Stud. 2019, 28, 337–341. [Google Scholar]
- Khulbe, K.C.; Matsuura, T. Removal of toxic metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2019, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Carrott, P.J.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066. [Google Scholar]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymer 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Abdullah, M.H. Optical detection and efficient removal of transition metal ions from water using poly (hydroxamic acid) ligand. Sens. Actuators B Chem. 2017, 242, 595–608. [Google Scholar] [CrossRef]
- Rahman, M.L.; Fui, C.J.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Abdullah, M.H.; Sarkar, S.M.; O’Reilly, E.J. Poly (amidoxime) ligand derived from waste palm fiber for the removal of toxic metals from electroplating wastewater. Environ. Sci. Pollut. Res. 2020, 27, 34541–34556. [Google Scholar] [CrossRef]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Abdullah, M.H.; Heffernan, M.A.; Sarkar, M.S.; O’Reilly, E. Poly (hydroxamic acid) ligand from palm-based waste materials for removal of toxic metals from electroplating wastewater. J. Appl. Polym. Sci. 2020, 182, 49671. [Google Scholar]
- Dmitrii, S.B.; Nadezhda, A.B.; Vadim, Y.K. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar]
- Agrawal, Y.K. Hydroxamic acids and their metal complexes. Russ. Chem. Rev. 1979, 48, 948–963. [Google Scholar] [CrossRef]
- Rachel, C. Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord. Chem. Rev. 2008, 252, 1387–1408. [Google Scholar]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Abdullah, M.H. Efficient removal of transition metal ions using poly(amidoxime) ligand from polymer grafted kenaf cellulose. RSC Adv. 2016, 6, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.L.; Mandal, B.H.; Sarkar, S.M.; Yusoff, M.M.; Arshad, S.; Musta, B. Synthesis of poly(hydroxamic acid) ligand from polymer grafted corn-cob cellulose for transition metals extraction. Polym. Adv. Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.L.; Biswas, T.K.; Sarkar, S.M.; Yusoff, M.M.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Adsorption of rare earth metals from water using a kenaf cellulose-based poly(hydroxamic acid) ligand. J. Mol. Liq. 2017, 243, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.L.; Mandal, B.H.; Sarkar, S.M.; Wahab, N.A.A.; Yusoff, M.M.; Arshad, S.E.; Musta, B. Synthesis of poly(hydroxamic acid) ligand from polymer grafted khaya cellulose for transition metals extraction. Fibers Polym. 2016, 17, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef] [Green Version]
- Appel, J. Freundlich’s Adsorption Isotherm. Surf. Sci. 1973, 39, 237–244. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, F.; Wei, T.; Zhang, C.; Xiao, H. Hydrophobic modification of bagasse cellulose fibers with cationic latex: Adsorption kinetics and mechanism. Chem. Eng. J. 2016, 302, 33–43. [Google Scholar] [CrossRef]
- Zheng, L.; Dang, Z.; Yi, X.; Zhang, H. Equilibrium and kinetic studies of adsorption of Cd (II) from aqueous solution using modified corn stalk. J. Hazard. Mater. 2010, 176, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Yaita, T.; El-Safty, S.A.; Shiwaku, H.; Suzuki, H.; Okamoto, Y. Copper (II) ions capturing from water using ligand modified a new type mesoporous adsorbent. Chem. Eng. J. 2013, 221, 322–330. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectra-Structure Correlations: Characteristics Group Frequencies. In IR and Raman Spectroscopy Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–115. [Google Scholar]
- Wu, C.-K.; Yin, M.; O’Brien, S.; Koberstein, J.T. Quantitative Analysis of Copper Oxide Nanoparticle Composition and Structure 693 by X-ray Photoelectron Spectroscopy. Chem. Mater. 2006, 18, 6054–6058. [Google Scholar] [CrossRef]
- Rahman, M.L.; Fui, C.J.; Tang, X.T.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Polymer ligands derived from jute fiber for heavy metal removal from electroplating wastewater. Polymers 2020, 12, 2521. [Google Scholar] [CrossRef] [PubMed]






| Adsorbate | Pseudo-First-Order | Experimental | Differences | ||
|---|---|---|---|---|---|
| qt (mg·g−1) | Kads (g·mg−1 min) | R2 | qe (mg·g−1) | (mg·g−1) | |
| Cu | 242.4 | 0.01658 | 0.8538 | 346.7 | 104.3 |
| Co | 213.1 | 0.01819 | 0.8223 | 315.0 | 101.9 |
| Cr | 158.9 | 0.01981 | 0.8896 | 227.6 | 68.7 |
| Ni | 111.9 | 0.03316 | 0.8643 | 181.4 | 69.5 |
| Adsorbate | Pseudo-Second Order | Experimental | Differences | ||
|---|---|---|---|---|---|
| qt (mg·g−1) | k2 (g·mg−1 min) × 10−4 | R2 | qe (mg·g−1) | (mg·g−1) | |
| Cu | 370.4 | 1.1266 | 0.9877 | 346.7 | 23.7 |
| Co | 357.1 | 1.0892 | 0.9783 | 315.0 | 42.1 |
| Cr | 263.2 | 1.4493 | 0.9812 | 227.6 | 35.6 |
| Ni | 208.3 | 2.9662 | 0.9920 | 181.4 | 26.9 |
| Adsorbent | Langmuir | Difference with qm (mg·g−1) | ||
|---|---|---|---|---|
| qe (mg·g−1) | KL (L·g−1) | R2 | ||
| Cu2+ | 357.1 | 0.008112 | 0.999 | 10.4 |
| Co2+ | 333.3 | 0.007797 | 0.997 | 18.3 |
| Cr3+ | 238.1 | 0.007348 | 0.996 | 10.5 |
| Ni2+ | 185.2 | 0.009878 | 0.998 | 3.8 |
| Adsorbent | Freundlich | ||
|---|---|---|---|
| n | KF (L·mg−1) | R2 | |
| Cu2+ | 2.6853 | 22.8034 | 0.958 |
| Co2+ | 2.4307 | 16.323 | 0.959 |
| Cr3+ | 2.7563 | 16.0029 | 0.965 |
| Ni2+ | 3.3944 | 21.0039 | 0.950 |
| Adsorbent | Langmuir | ||||
|---|---|---|---|---|---|
| qe (mg·g−1) | KL (L·mg−1) | R2 | HYBRID | MPSD | |
| Cu | 357.1 | 0.008112 | 0.999 | 0.1759 | 20.0614 |
| Co | 333.3 | 0.007797 | 0.997 | 0.1961 | 21.5535 |
| Cr | 238.1 | 0.007348 | 0.996 | 0.2022 | 22.1535 |
| Ni | 185.2 | 0.009878 | 0.998 | 0.1067 | 11.6378 |
| Metal Ions | IWS 1 | IWS 2 | ||||
|---|---|---|---|---|---|---|
| Before Treatment (ppm) | After Treatment (ppm) | % Removal | Before Treatment (ppm) | After Treatment (ppm) | % Removal | |
| Fe3+ | 32.0095 | 1.3896 | 95.66 | 1.4637 | 0.0076 | 99.48 |
| Cu2+ | 23.0493 | 2.2051 | 90.43 | 85.7627 | 2.1787 | 97.46 |
| Pb2+ | 0.0207 | 0.0021 | 90.34 | 0.1331 | 0.0356 | 73.25 |
| Zn2+ | 0.0421 | 0.0081 | 81.01 | 0.1037 | 0.0084 | 91.93 |
| Cr3+ | 0.0268 | 0.0059 | 77.85 | 0.0857 | 0.0031 | 96.34 |
| Mn2+ | 0.1296 | 0.0296 | 77.17 | 0.0055 | 0.0013 | 75.47 |
| Ni2+ | 0.01828 | 0.0064 | 65.23 | 0.4421 | 0.1364 | 69.14 |
| Ba2+ | 0.0013 | 0.0007 | 48.42 | - | - | - |
| Ca2+ | 0.5517 | 0.2948 | 46.56 | 0.8918 | 0.4191 | 53.01 |
| Rb+ | 0.0305 | 0.0180 | 40.30 | 0.0109 | 0.0066 | 38.78 |
| K+ | 0.0783 | 0.0544 | 30.45 | 0.2791 | 0.1877 | 32.72 |
| Na+ | 15.23 | 12.38 | 18.70 | 9.7411 | 8.2496 | 15.31 |
| V4+ | 0.00006 | 0.000055 | 9.01 | - | - | - |
| Al3+ | 0.0505 | 0.0474 | 6.09 | 0.0063 | 0.0059 | 6.64 |
| Ag+ | - | - | - | 0.0322 | 0.0141 | 56.25 |
| Mg2+ | - | - | - | 0.0879 | 0.0520 | 40.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.L.; Wong, Z.-J.; Sarjadi, M.S.; Joseph, C.G.; Arshad, S.E.; Musta, B.; Abdullah, M.H. Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater. Polymers 2021, 13, 1486. https://doi.org/10.3390/polym13091486
Rahman ML, Wong Z-J, Sarjadi MS, Joseph CG, Arshad SE, Musta B, Abdullah MH. Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater. Polymers. 2021; 13(9):1486. https://doi.org/10.3390/polym13091486
Chicago/Turabian StyleRahman, Md. Lutfor, Zhi-Jian Wong, Mohd Sani Sarjadi, Collin G. Joseph, Sazmal E. Arshad, Baba Musta, and Mohd Harun Abdullah. 2021. "Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater" Polymers 13, no. 9: 1486. https://doi.org/10.3390/polym13091486
APA StyleRahman, M. L., Wong, Z.-J., Sarjadi, M. S., Joseph, C. G., Arshad, S. E., Musta, B., & Abdullah, M. H. (2021). Waste Fiber-Based Poly(hydroxamic acid) Ligand for Toxic Metals Removal from Industrial Wastewater. Polymers, 13(9), 1486. https://doi.org/10.3390/polym13091486