Abstract
The impact of organic species which are present in the Earth’s atmosphere on the burst of new particles is critically important for the understanding of the molecular nature of atmospheric nucleation phenomena. Amines have recently been proposed as possible stabilizers of binary pre-nucleation clusters. In order to advance the understanding of atmospheric nucleation phenomena, a quantum-chemical study of hydrogen-bonded complexes of binary sulfuric acid-water clusters with methyl-, dimethyl- and trimethylamines representing common atmospheric organic species, vegetation products and laboratory impurities has been carried out. The thermochemical stability of the sulfuric acid-amines-water complexes was found to be higher than that of the sulfuric acid-ammonia-water complexes, in qualitative agreement with the previous studies. However, the enhancement in stability due to amines appears to not be large enough to overcome the difference in typical atmospheric concentrations of ammonia and amines. Further research is needed in order to address the existing uncertainties and to reach a final conclusion about the importance of amines for the atmospheric nucleation.
1. Introduction
Aerosol particles formed in the Earth’s atmosphere via nucleation [1,2] influence the Earth’s climate by affecting cloud properties and precipitation. They play an important role in global climate changes [3,4] and are responsible for the adverse public health impacts of airborne ultrafine particles, including various cardiovascular deceases, lung cancer and enhanced mortality rates [5,6,7]. The atmospheric nucleation process can be described schematically as nucleation of H2SO4-H2O-X. In other words, atmospheric nucleation involves sulfuric acid, the key atmospheric nucleation precursor, water, the dominant constituent of the mixture of condensable vapours in the Earth’s atmosphere, and something else [8,9,10,11,12,13,14,15]. A perfectly logical question “What else is involved in the atmospheric nucleation?” has yet to be answered and a consensus on the dominant nucleation mechanism/ mechanisms in the Earth’s atmosphere is yet to be achieved. Since the 1990s ammonia, the most common base in the Earth’s atmosphere, has been considered as a principle stabilizer of H2SO4-H2O clusters. The somewhat excessive initial enthusiasm about the ternary homogeneous H2SO4-H2O-NH3 nucleation theory (THN) disappeared, when it was discovered that the widely used THN model [11] grossly overestimates nucleation rates [12]. Kulmala et al. have revised the original THN model [13], but the revised model THN predicts negligible THN rates under typical atmospheric conditions. Other candidate nucleation mechanisms include ion mediated nucleation of H2SO4-H2O-ion (IMN) [10], nucleation of iodine-containing vapours [14] and organics-enhanced nucleation [15].
It is well-known that atmospheric organic species may be involved in nucleation. The presence of common organic species in the aerosol particles has been corroborated in a number of observations [16,17,18,19,20,21,22,23] However, the role of organics in the atmospheric nucleation has long been underestimated or neglected due to the complexity of laboratory experiments and the absence of theoretical instruments, which would be able to account for the complex intermolecular interactions occurring in nucleating vapours. The importance of organic species has been pointed out in the pioneering experiments of Zhang et al. [15], in which a considerable enhancement in nucleation rates due to organic species has been observed. The ability of computational quantum chemical methods to provide an adequate description of molecular interactions in nucleating vapours has been pointed out in Nadykto et al. [24], in which the classical problem of the ion sign preference established in back 1897 by Wilson [1] has been solved. Recently, computational chemical studies of atmospheric species have become a focus of intense activity [24,25,26,27,28,29,30,31,32,33,34,35,36]. Although a large number of the computational quantum studies considering atmospheric clusters has been published within the last five years, only a few are dedicated to the interaction of common organics with atmospheric nucleation precursors [32,34,35]. The ability of common low-molecular carboxylic formic and acetic acids to stabilize H2SO4-H2O clusters has been pointed in our recent work [34]. The conclusion about the importance of organic species for the stabilization of atmospheric pre-nucleation clusters has been confirmed in independent studies [32,35].
Amines are common atmospheric species originating mainly from vegetation and common laboratory impurities. The activity of amines as potential nucleation agents has been indicated in a number of experiments and observations [37,38,39,40,41,42,43,44,45]. Typically, the atmospheric concentrations of amines are much lower than those of ammonia; however, a number of sites with high amine concentration exist in the boundary layer. Angelino et al. [43] have concluded, based on smog chamber experiments and field measurements, that amine chemistry plays “a significant role in particle formation in regions with high amine concentrations”. Laboratory experiments and quantum chemical calculations of crystal structures of Murphy et al. [44] showed that diethylamine is more efficient in catalyzing the nucleation of nitric acid than ammonia. Observations of Makela et al. [45] have indicated that dimethylammonium ion, the ionic form of dimethylamine, was present in aerosol particles formed during nucleation events and/or subsequent particle growth process in Hyytiala, Southern Finland. Although the potential relevance of amines to nucleation in the Earth’s atmosphere is well-established, their roles in the gas-to-particle conversion and the extent at which they can affect atmospheric nucleation rates are unknown. More recently, several experimental studies and reviews concerning the role of amines in atmospheric nucleation have been published [37,38,39,40,41,42].
Recently, amines have been proposed as potential stabilizers of sulfuric acid-water clusters [32]. The conclusion about the potential importance of amines for atmospheric nucleation made in Kurten et al. [32] is based solely on the RI-MP2/CC2 formation free energies of H2SO4-amine complexes, which appeared to be more stable than the ammonia bisulfate formed via the NH3 + H2SO4 ⇔ (NH3)(H2SO4) reaction. Although the enhancement in dimerization free energies may be an important indicator of the stability in simple unitary systems, the stability of complex, essentially multicomponent, H2SO4-H2O-amine complexes is controlled by three somewhat competing factors: attachment of the sulfuric acid, affinity of amines to clusters being formed and hydration. Therefore, no meaningful conclusion can be reached without a thorough study of the interactions of all three species.
In the present paper, the hydrogen bonded complexes of common amines, methylamine (CH3)NH2, dimethylamine (CH3)2NH and trimethylamine (CH3)3N, with sulfuric acid and water have been studied using the Density Functional Theory (DFT). The properties of mixed dimers, trimers, tetramers and pentamers have been investigated and a comprehensive thermochemical analysis has been carried out. The impact of amines on the stability of binary pre-nucleation clusters has been investigated and possible involvement of amines in the atmospheric nucleation has been discussed.
2. Methods
At the present time, DFT is the only option for studying large clusters due to the enormously large computational expenses associated with the application of ab initio MP2 and higher level methods [46]. However, the relative importance of different nucleation pathways is primarily related to the difference in stepwise free energy changes associated with the formation of different types of clusters, which is predicted by different methods in good agreement with each other. For example, it has been shown that the difference in binding energies of (H2SO4)(NH3) and (H2SO4)(H2O) complexes given by different ab initio and DFT methods, with and without counterpoise corrections, is several times lower that the difference in the absolute values of the binding energy of (H2SO4)(NH3) or (H2SO4)(H2O) clusters [47]. In the present study, the initial/generated geometries have been treated using the semi-empirical PM3 [46] method and then optimized at the PW91PW91/6-31+G* level of theory. The most stable isomers (within 2 kcal mole−1 of the most stable isomer/global minimum) obtained at the PW91PW91/6-31+G* level have been optimized using the PW91PW91/6-311+G(3df,3pd) method to obtain the final results. The choice of the computational method is based on the satisfactory performance of the PW91PW91 [46] on atmospheric clusters, including predicting the Gibbs free energies, structural characteristics and vibrational spectra in a very good agreement with experiments and ab initio studies, and availability of large amount of data for different atmospheric species/clusters computed at PW91PW91/6-311+G(3df,3pd) level of theory for comparison [24,25,26,27,28,29,30,34]. The availability of data for ternary sulfuric acid-water-ammonia clusters computed at the same level of theory is a very important factor because the assessment of different nucleation pathways, which is based on the analysis of the reaction free energies computed using the same method, is clearly more legitimate than that based on the comparison of results obtained at different levels of theory [47].
3. Results and Discussion
Figure 1 and Figure 2 present the equilibrium geometries of the most stable isomers of (CH3NH2)m(H2SO4)n (H2O)k and [(CH3)2NH]m(H2SO4)n(H2O)k complexes. The equilibrium geometries of all the (CH3NH2)2, (CH3NH2)3, [(CH3)2NH]2, [(CH3)2NH]3, (CH3NH2)(H2SO4), [(CH3)2NH](H2SO4), and [(CH3)3N](H2SO4) are in good agreement with the previous ab initio MP2 studies [32,48,49,50]. A comparison of geometries of CH3NH2-H2SO4-H2O, (CH3)2NH-H2SO4-H2O and (CH3)3N-H2SO4-H2O complexes presented in Figure 1 and Figure 2 reveals a number of similarities. For example, the attachment of water molecules to both CH3NH2 and (CH3)2NH occurs without the proton transfer, which is likely a sign of weak or moderately weak hydration. The interaction of both amines with both the free and hydrated H2SO4 leads to the deprotonation of the sulfuric acid and transfer of the detached proton towards NH2 and NH groups of CH3NH2 and (CH3)2NH, respectively. Both amines and sulfuric acid are present in the binary amine-sulfuric acid and ternary amine-sulfuric acid-water clusters in the ionic form. The proton transfer and number of ionic structures in the aforementioned clusters depend strongly on the cluster size and composition. All the mixed dimers, trimers and tetramers at n = 1; 2 and m = 1; 2, which contain less than two amines and water, include single ion pairs CH3NH3+ and HSO4− or (CH3)2NH2+ and HSO4−, while tetramers and pentamers with n ≥ 2 and m ≥ 2 contain two protonoted amine- HSO4− ion pairs.
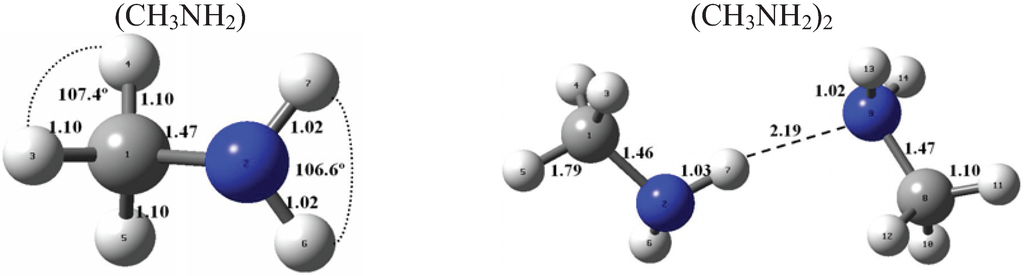
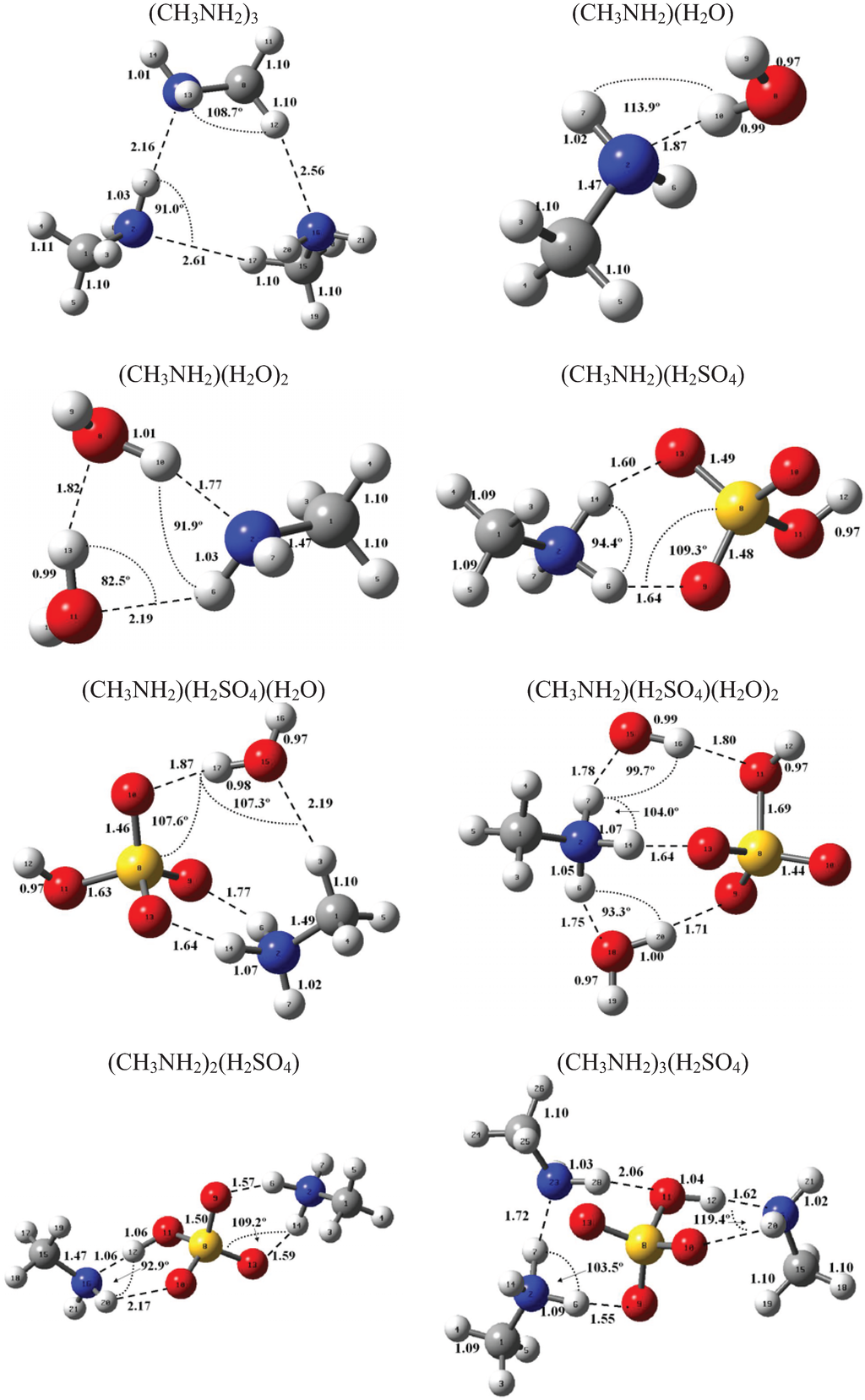
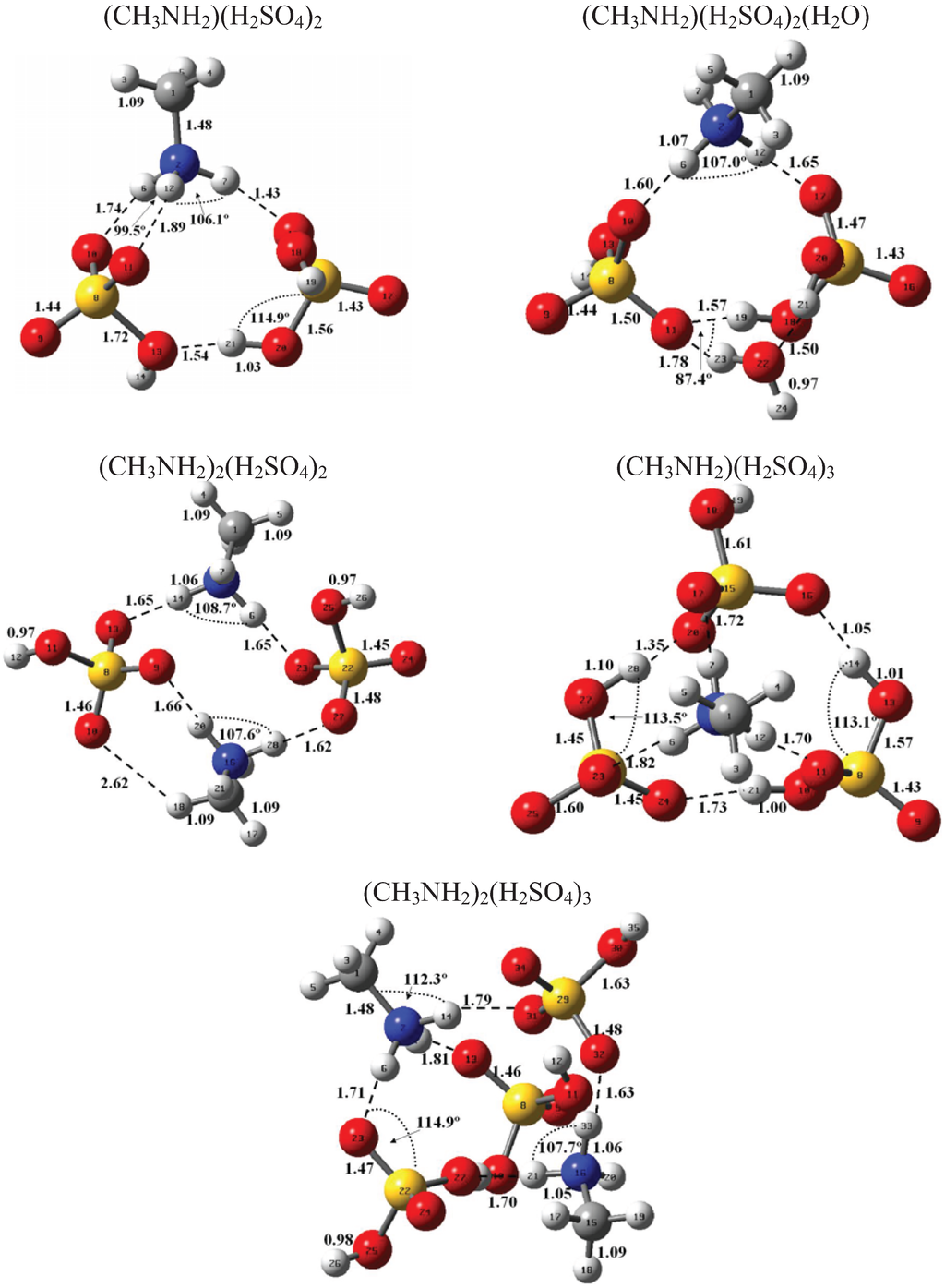
Figure 1.
Equilibrium geometries of the most stable isomers of (CH3NH2)m(H2SO4)n(H2O)k complexes. Distances and angles are given in angstroms and degrees, respectively.
Figure 1.
Equilibrium geometries of the most stable isomers of (CH3NH2)m(H2SO4)n(H2O)k complexes. Distances and angles are given in angstroms and degrees, respectively.
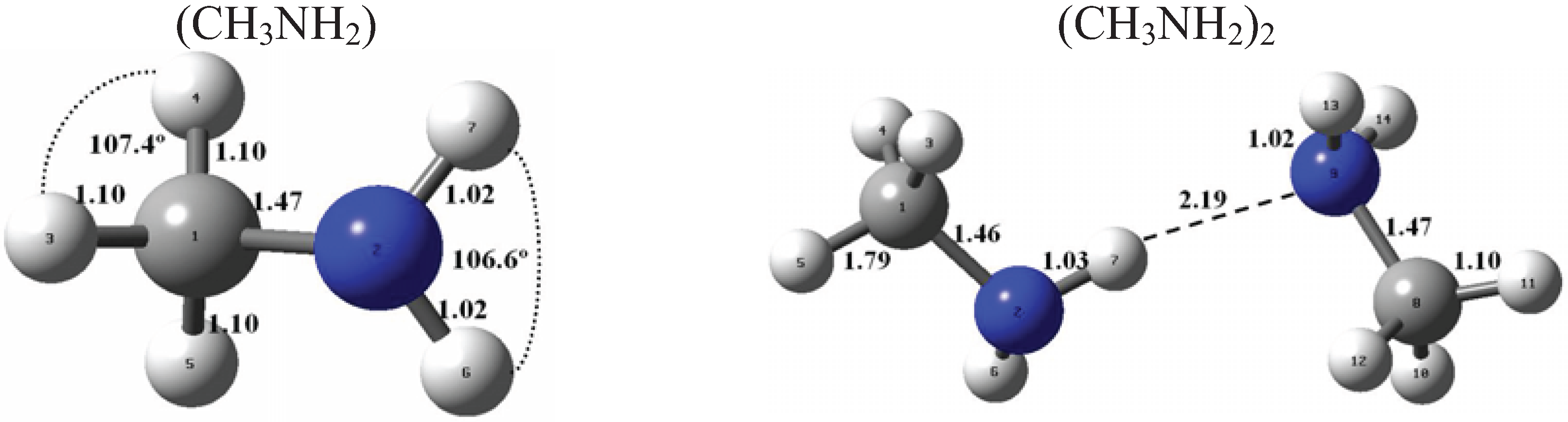
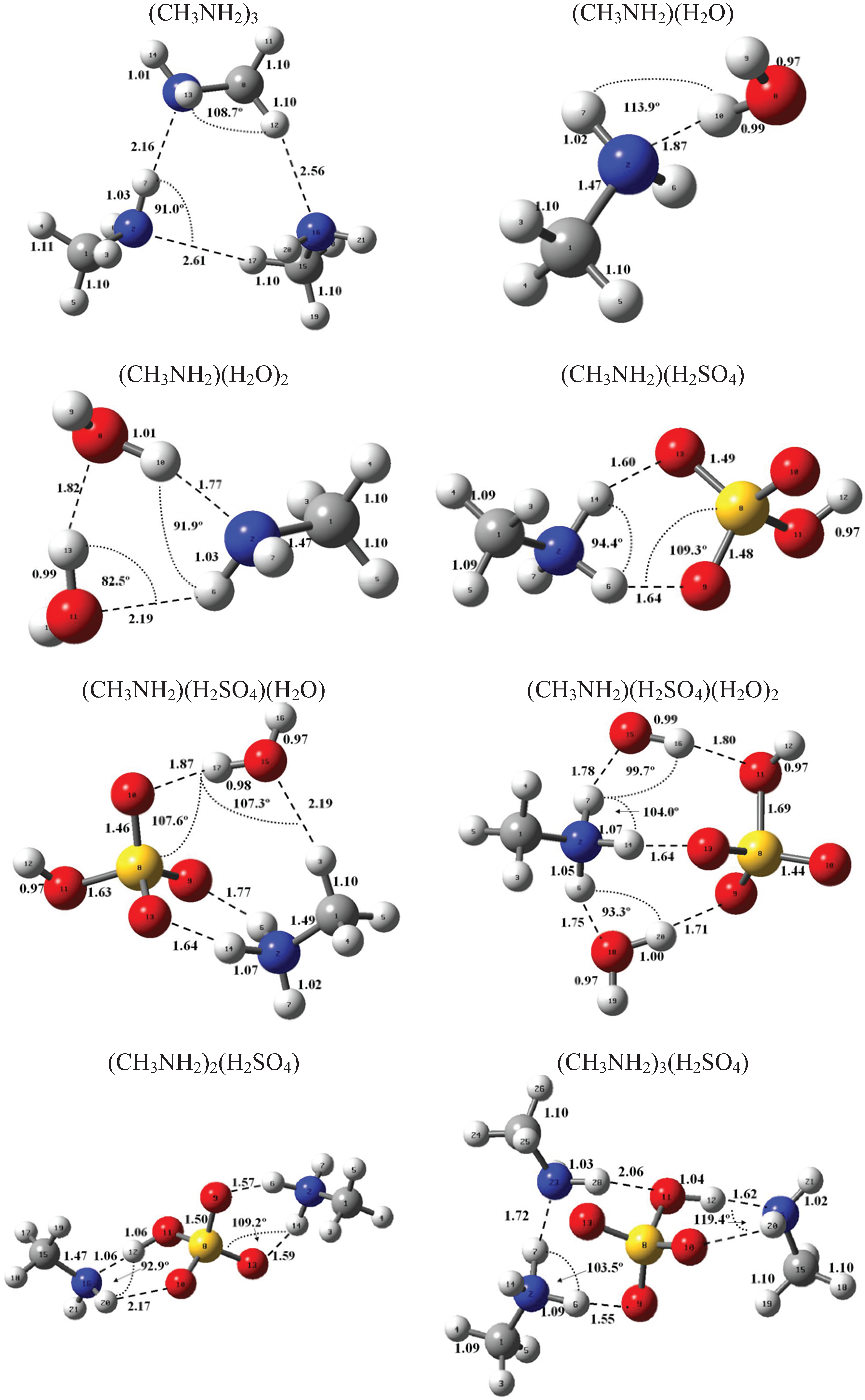
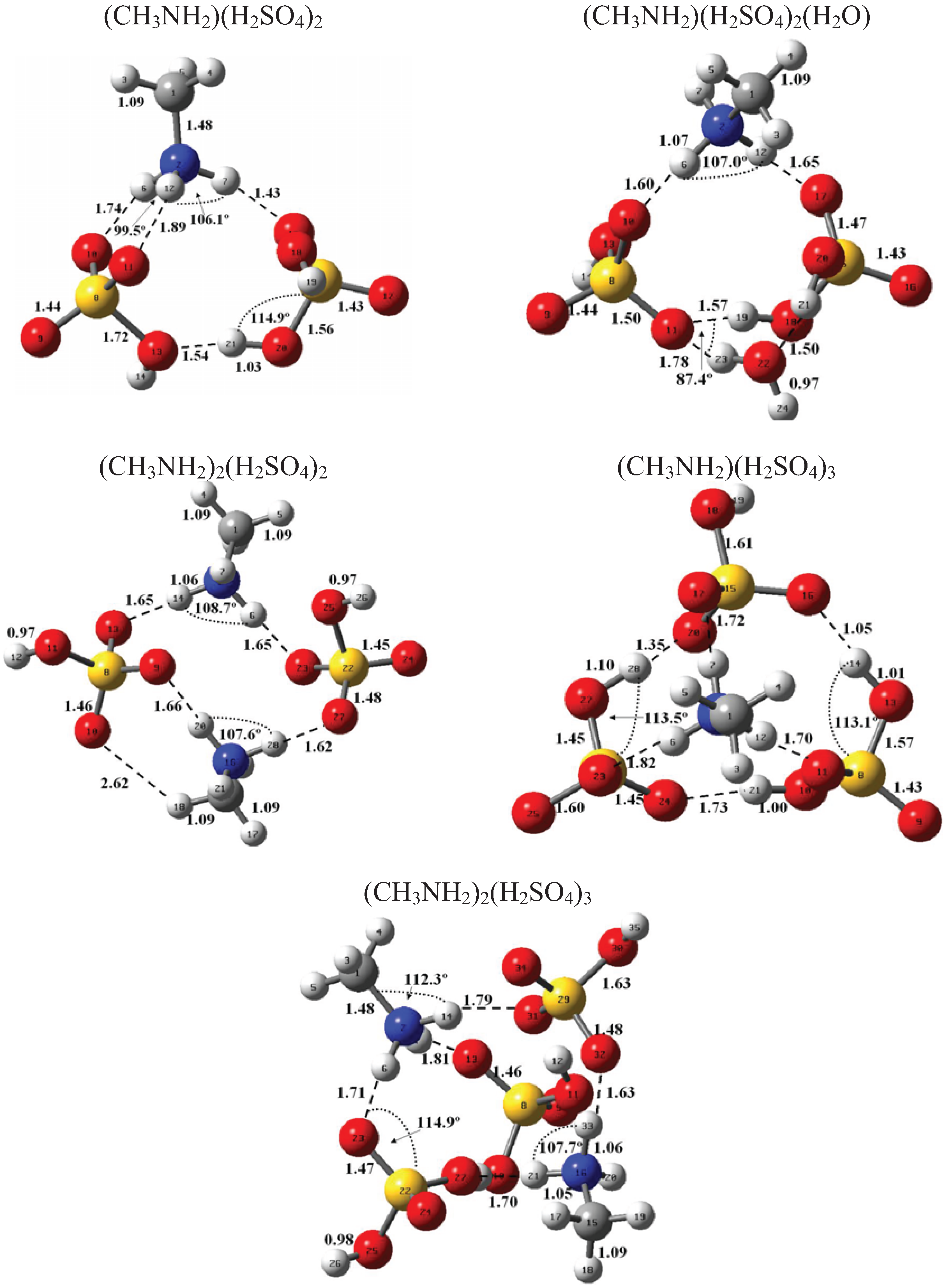
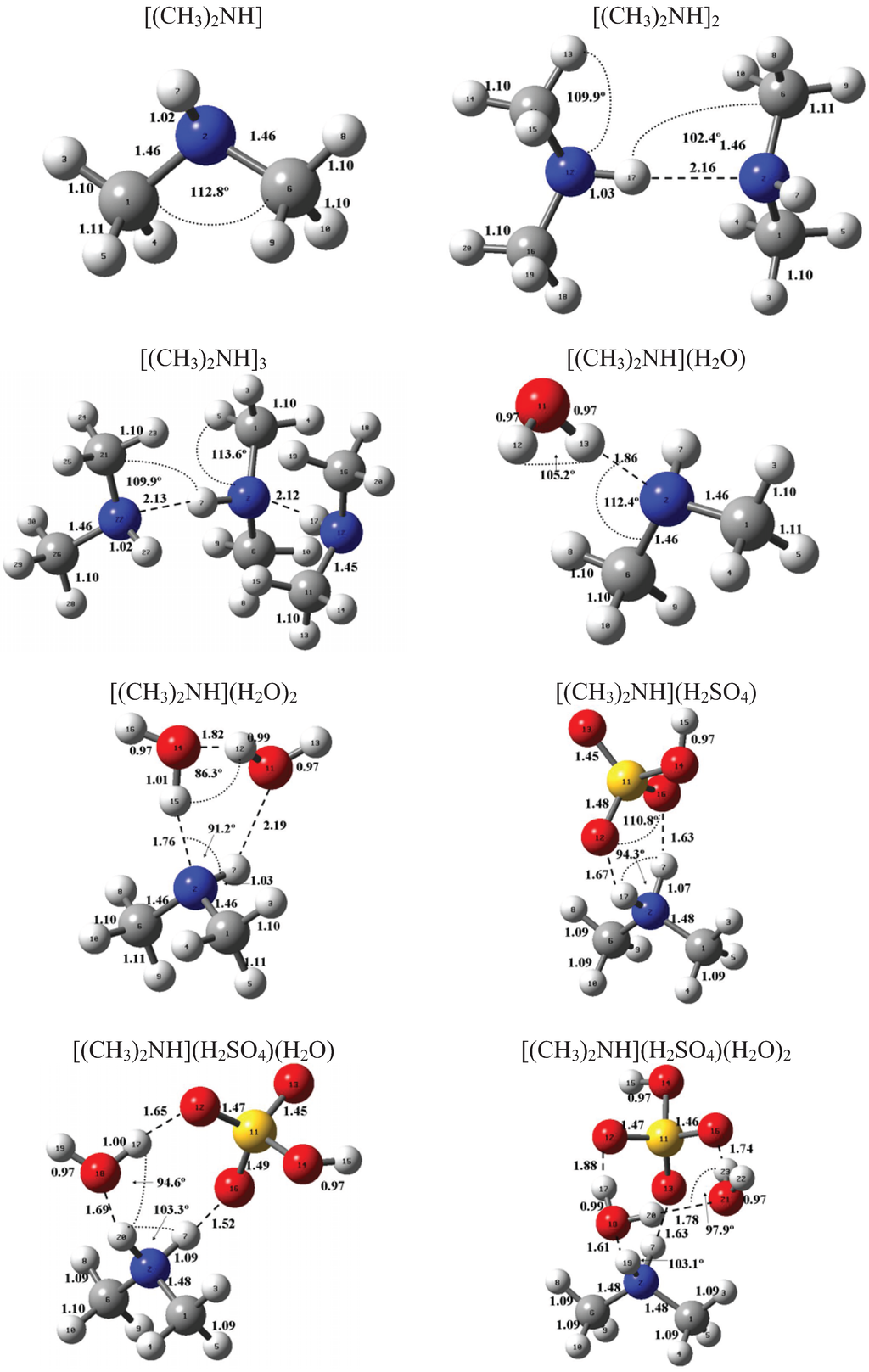
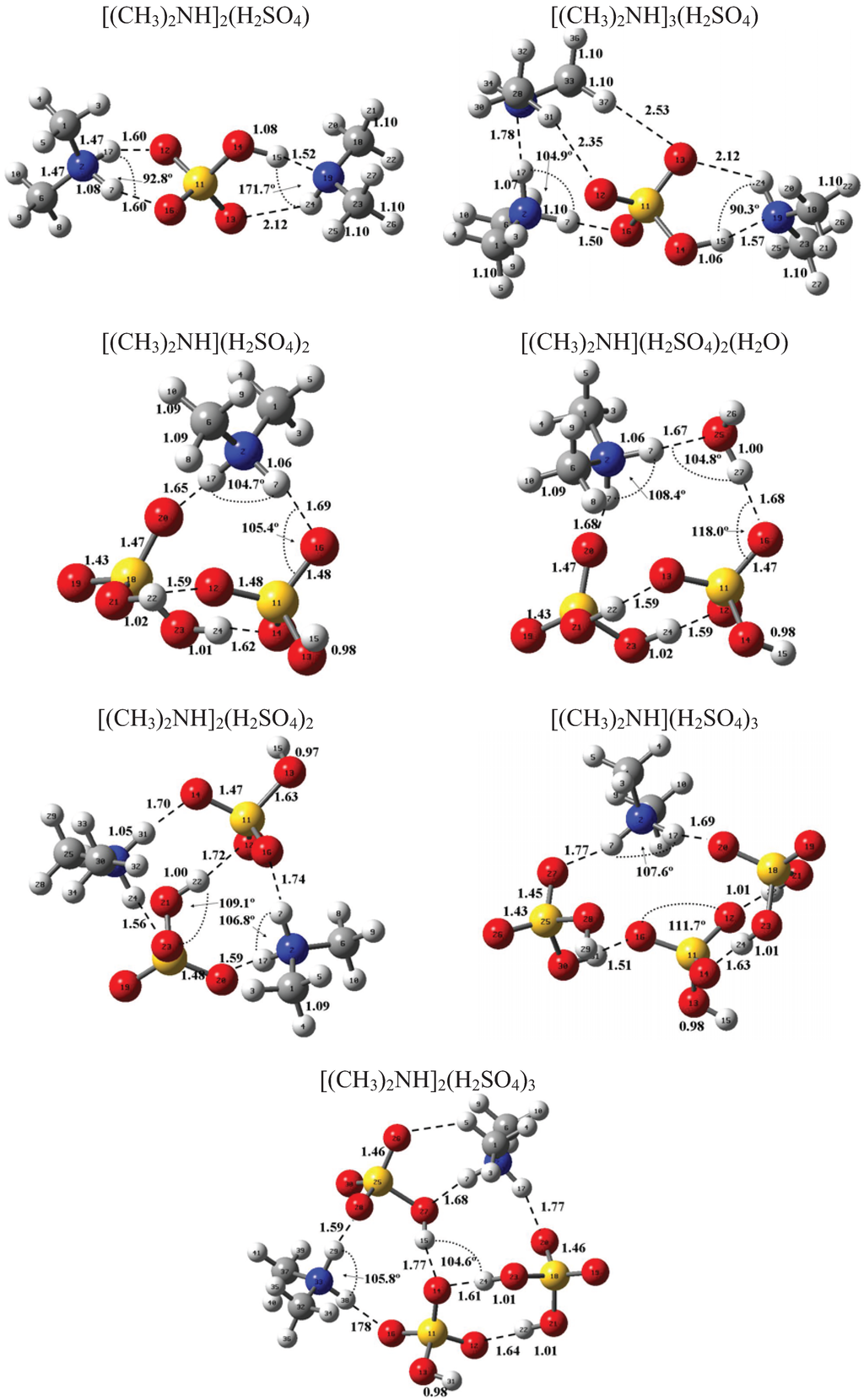
Figure 2.
Equilibrium geometries of the most stable isomers of [(CH3)2NH]m(H2SO4)n(H2O)k complexes. Distances and angles are given in angstroms and degrees, respectively.
Figure 2.
Equilibrium geometries of the most stable isomers of [(CH3)2NH]m(H2SO4)n(H2O)k complexes. Distances and angles are given in angstroms and degrees, respectively.
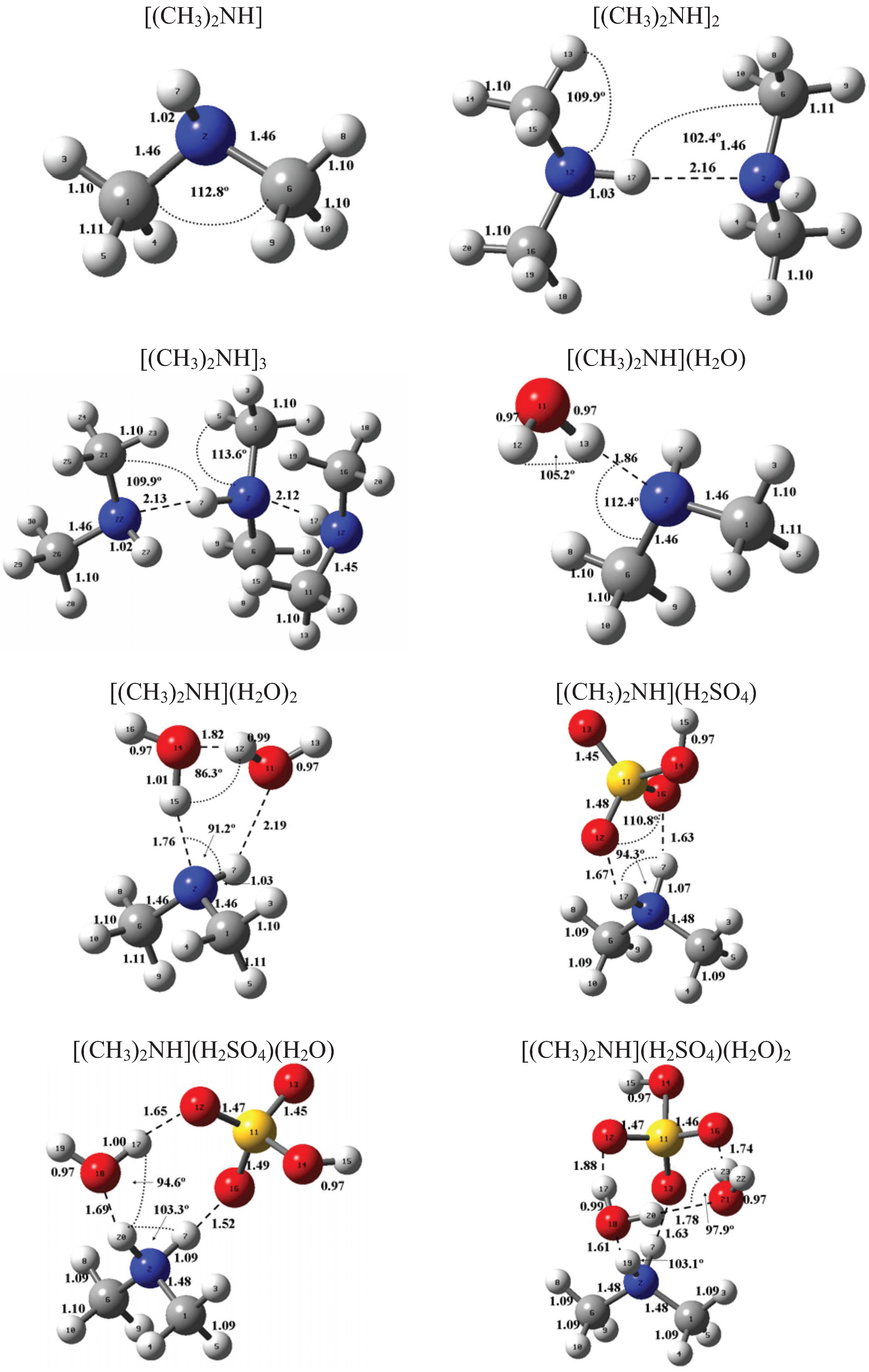
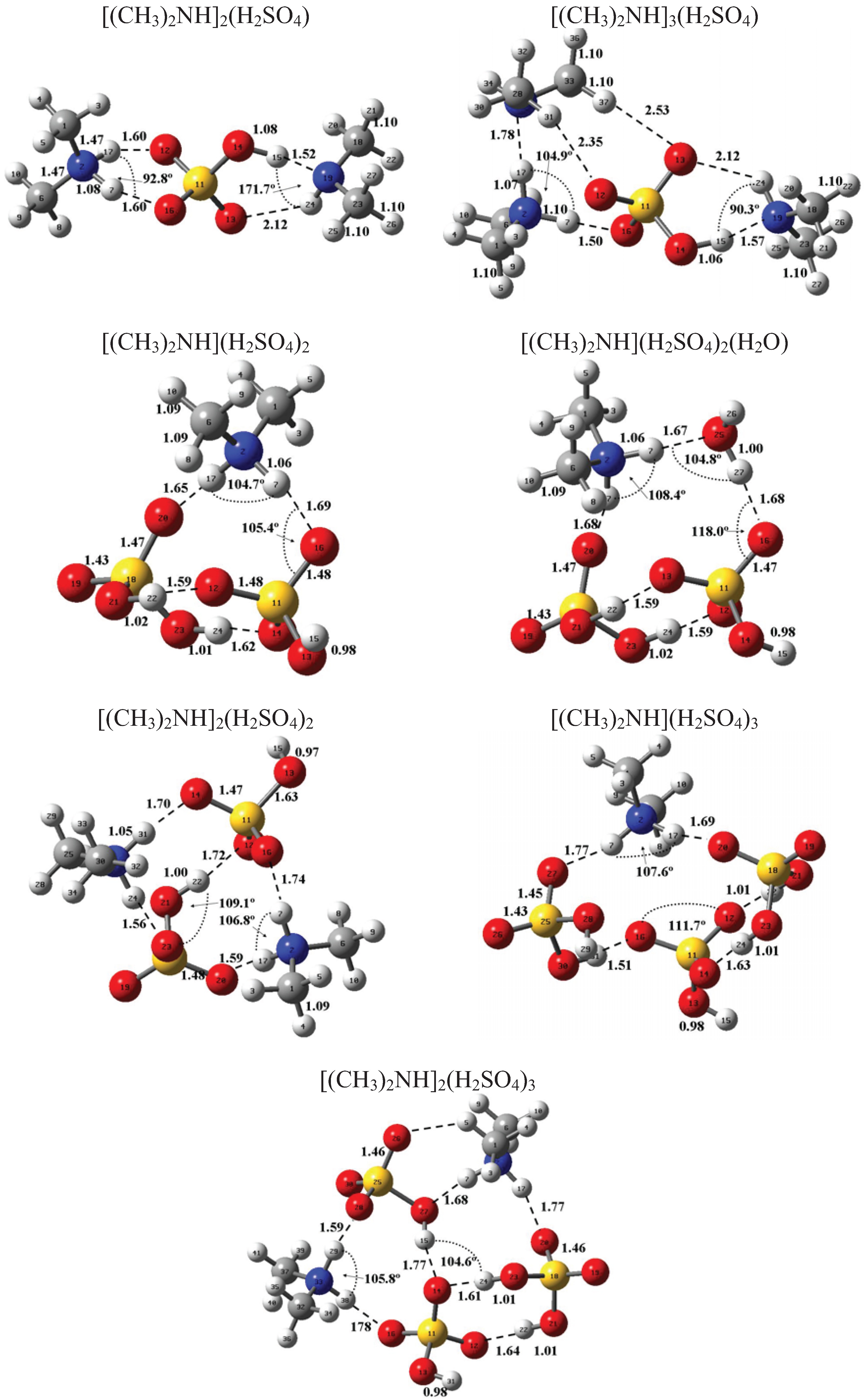
Table 1, Table 2, and Table 3 present the key thermochemical properties controlling the thermodynamic stability: attachment of H2SO4, affinity of amines to clusters being formed, and hydration. As may be seen from Table 1, the qualitative conclusion about the hydration of amines made based on the structural data agrees well with the obtained thermochemical data. The hydration of amines is weak, and, thus, hydrated sulfuric acid-amine clusters are thermodynamically unstable under typical atmospheric conditions. The above-mentioned conclusion is also applicable to the weakly hydrated (H2SO4)2(CH3NH2)(H2O) and (H2SO4)2[(CH3)2NH](H2O) complexes. In contrast, the hydration of both the (H2SO4)(CH3NH) and (H2SO4)2[(CH3)2NH], and (H2SO4)2(CH3NH)(H2O) and (H2SO4)2[(CH3)2NH](H2O) is strong enough to expect the existence of such clusters in the Earth’s atmosphere. On average, the hydration of amine-sulfuric acid-water complexes is moderately weak and is close to that of ammonia-sulfuric acid-water complexes, for which the hydration is considered to be much less important than the attachment of the sulfuric acid and ammonia. As seen from Table 2 and Table 3, the bonding energies associated with attachment of CH3NH2 and (CH3)2NH to clusters of identical chemical composition are very close. The difference between them is less than 0.7 kcal mole−1 on average. The hydration affects the attachment of both sulfuric acid and amines; however, its effect is weak. The comparison of affinities of amines and ammonia to the pre-nucleation clusters is favorable for amines because the free energies of [(CH3)2NH]m−1(H2SO4)n + (CH3)2NH ⇔ [(CH3)2NH]m(H2SO4)n reactions are in most cases higher than those of the (NH3)m−1 (H2SO4)n + NH3 ⇔ (NH3)m(H2SO4)n reactions. In some cases, the replacement of ammonia with amines leads to a considerable (up to 2–3.5 kcal mole−1) enhancement in the affinity of the sulfuric acid, the key atmospheric nucleation precursor, to the pre-nucleation clusters.
It is important to note that the enhanced thermodynamic stability does not necessarily imply stronger stabilizing effects under the real atmospheric conditions. The stabilizing effect is controlled by two different factors: stepwise Gibbs free energy changes and concentration ratio of amines with respect to ammonia, which is ~ 10−2–10−3 under typical atmospheric conditions [32,37,38,39,40,41,42]. As it may be seen from Table 2 and Table 3, the difference in formation free energies of (amine)(H2SO4) and (ammonia-H2SO4) clusters is ~ 3–4 kcal mole−1, which may imply the possibility of the domination of clusters containing amines over ammonia bisulfate clusters under favorable condition (high concentration of amines and low temperatures). However, the difference in the free energies of (amine)(H2SO4) + H2SO4 ⇔ (amine)(H2SO4)2 and (NH3)(H2SO4) + H2SO4 ⇔ (NH3)(H2SO4)2 reaction underlining the further cluster growth does not exceed ~2.5 kcal mole−1 and tends to decrease with hydration. Another important details are that the free energies of (amine)(H2SO4) + amine ⇔ (amine)2(H2SO4) are very small (3.7–4.7 kcal mole−1) and that the (amine)2(H2SO4) clusters, to which the sulfuric acid could easily attach, are unstable thermodynamically. Other possible pathways of the formation of large clusters such as (H2SO4)2 + amine ⇔ (H2SO4)2(amine) or (amine)2 + H2SO4 ⇔ (H2SO4)(amine)2 are impossible under the atmospheric conditions due to the insufficient stability of (H2SO4)2 and amine dimers. The obtained results lead us to conclude that under typical atmospheric conditions (H2SO4)2(amine) formation is the limiting stage of the pre-nucleation cluster formation.

Table 1.
Comparison of changes in enthalpies ∆H (kcal mole−1), entropies ∆S (cal mole−1 K−1), and Gibbs free energies ∆G (kcal mole−1) associated with formation of (CH3NH2)m(H2SO4)n(H2O)k, ((CH3)2NH)m(H2SO4)n(H2O)k and ((CH3)3N)m(H2SO4)n(H2O)k complexes via hydration with those associated with the formation of (NH3)m(H2SO4)n(H2O)k at temperature of 298.15K and pressure of 101.3 KPa.
| ∆H | ∆S | ∆G | |
| CH3NH2 + H2O ⇔ (CH3NH)1(H2O)1 | −7.73 | −22.25 | −1.10 |
| (CH3)2NH + H2O ⇔ [(CH3)2NH]1(H2O)1 | −6.58 | −23.75 | 0.50 |
| H2SO4 + H2O ⇔ (H2SO4)1(H2O)1 | −11.76a | −31.80a | −2.28a |
| (CH3NH)1(H2O)1 + H2O ⇔ (CH3NH)1(H2O)2 | −8.70 | −34.42 | 1.56 |
| ((CH3)2NH)1(H2O)1 + H2O ⇔ [(CH3)2NH]1(H2O)2 | −8.77 | −34.74 | 1.59 |
| (H2SO4)1(H2O)1 + H2O ⇔ (H2SO4)1(H2O)2 | −12.57a | −32.08a | −3.00a |
| (CH3NH)1(H2SO4)1 + H2O ⇔ (CH3NH)1(H2SO4)1(H2O)1 | −13.02 | −32.50 | −3.33 |
| ((CH3)2NH)1(H2SO4)1 + H2O ⇔ [(CH3)2NH]1(H2SO4)1(H2O)1 | −12.65 | −30.11 | −3.67 |
| (NH3)1(H2SO4)1 + H2O ⇔ (NH3)1(H2SO4)1(H2O)1 | −10.96a | −32.03a | −1.41a |
| (CH3NH)1(H2SO4)1(H2O)1 + H2O ⇔ (CH3NH)1(H2SO4)1(H2O)2 | −13.85 | −34.66 | −3.52 |
| ((CH3)3N)1(H2SO4)1 + H2O ⇔ [(CH3)3N]1(H2SO4)1(H2O)1 | −10.88 | −32.51 | −1.19 |
| ((CH3)3N)1(H2SO4)1(H2O)1 + H2O ⇔ [(CH3)3N]1(H2SO4)1(H2O)2 | −10.74 | −32.76 | −0.97 |
| ((CH3)2NH)1(H2SO4)1(H2O)1 + H2O ⇔ [(CH3)2NH]1(H2SO4)(H2O)2 | −12.66 | −36.13 | −1.89 |
| (NH3)1(H2SO4)1(H2O)1 + H2O ⇔ (NH3)1 (H2SO4)1(H2O)2 | −11.92a | −32.34a | −2.28a |
| (H2SO4)2(CH3NH)1 + H2O ⇔ (H2SO4)2(CH3NH)1(H2O)1 | −10.50 | −31.47 | −1.13 |
| (H2SO4)2((CH3)2NH)1 + H2O ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −9.82 | −30.09 | −0.85 |
| (H2SO4)2(NH3)1 + H2O ⇔ (H2SO4)2(NH3)1(H2O)1 | −11.68 | −31.32 | −2.31 |
a [34].
It is also important to note that the difference in the absolute values of the dimerization free energies between PW91PW91 and RI-MP2/CC2 [8] is quite large. The absolute free energies of the formation (H2SO4)(amine) dimers obtained using these two methods deviate by 1.5–5 kcal mole−1. This finding is quite surprising, because in the case of clusters composed of H2SO4, NH3 and H2O the agreement between PW91PW91 and RI-MP2/CC2 is very good. RI-MP2/CC2 energies [32] are higher than those produced by PW91PW91; however, they were obtained neglecting the Basis Set Superposition Error (BSSE), which is significant and may reach several Kcal mole−1. In contrast, the BSSE at the DFT level with large basis sets is very small, and seldom exceed 0.5 kcal mole−1. This means that absolute RI-MP2/CC2 reaction free energies [32] would be significantly lower, and thus, in better agreement with the DFT results in the case, when the BSSE in the previous RI-MP2/CC2 work [32] is corrected. It is important to note that while BSSE affect mainly the absolute values, the basis set dependency of the MP2 calculations exceeding 6 kcal mole−1 in the case of (CH3)3N + H2SO4 ⇔ (CH3)3N(H2SO4) reaction [32,33] and anharmonic correction are essential sources of uncertainties in both absolute and relative energies. These uncertainties may significantly affect the conclusions about the role of amines in the atmospheric nucleation, and, therefore, further research is needed in order to reach a final conclusion about the importance of amines for atmospheric nucleation.

Table 2.
Comparison of changes in enthalpies ∆H (kcal mole−1), entropies ∆S (cal mole−1 K−1), and Gibbs free energies ∆G (kcal mole−1) associated with formation of (CH3NH2)m(H2SO4)n(H2O)k, ((CH3)2NH)m(H2SO4)n(H2O)k and ((CH3)3N)m(H2SO4)n(H2O)k complexes via the attachment of amines with those associated with the formation of (NH3)m(H2SO4)n(H2O)k at temperature of 298.15K and pressure of 101.3 KPa.
| ∆H | ∆S | ∆G | |
| (H2SO4)(H2O) + CH3NH ⇔ (CH3NH)1(H2SO4)1(H2O)1 | −21.66 | −32.11 | −12.08 |
| (H2SO4)1(H2O)1 + (CH3)2NH ⇔ [(CH3)2NH]1(H2SO4)1(H2O)1 | −22.24 | −31.78 | −12.76 |
| (H2SO4)1(H2O)1 + (CH3)3N ⇔ [(CH3)3N]1(H2SO4)1(H2O)1 | −19.37 | −35.69 | −8.73 |
| (H2SO4)1(H2O)1 + NH3 ⇔ (NH3)1(H2SO4)1(H2O)1 | −15.91a | −30.23a | −6.90a |
| (H2SO4)1(H2O)2 + CH3NH ⇔ (CH3NH)1(H2SO4)1(H2O)2 | −22.94 | −34.69 | −12.59 |
| (H2SO4)1(H2O)2 + (CH3)2NH ⇔ [(CH3)2NH]1(H2SO4)1(H2O)2 | −22.33 | −35.84 | −11.65 |
| (H2SO4)1(H2O)2 + (CH3)3N ⇔ [(CH3)3N]1(H2SO4)1(H2O)2 | −17.51 | −36.37 | −6.66 |
| (H2SO4)1(H2O)2 + NH3 ⇔ (NH3)1(H2SO4)1(H2O)2 | −15.27a | −30.49a | −6.18a |
| (H2SO4)1(CH3NH)1 + CH3NH ⇔ (H2SO4)1(CH3NH)2 | −15.93 | −39.96 | −4.02 |
| (H2SO4)1[(CH3)2NH]1 + (CH3)2NH ⇔ (H2SO4)1[(CH3)2NH]2 | −14.53 | −35.61 | −3.92 |
| (H2SO4)1[(CH3)3N] + (CH3)3N ⇔ (H2SO4)1[(CH3)3N]2 | −14.18 | −35.64 | −3.56 |
| (H2SO4)1(NH3)1 + NH3 ⇔ (H2SO4)1(NH3)2 | −13.68 | −29.96 | −4.74 |
| (H2SO4)2 + CH3NH ⇔ (H2SO4)2(CH3NH)1 | −31.65 | −40.13 | −19.69 |
| (H2SO4)2 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH] | −32.56 | −41.79 | −20.10 |
| (H2SO4)2 + NH3 ⇔ (H2SO4)2(NH3) | −25.67a | −39.68a | −13.83a |
| (H2SO4)2(H2O)1 + CH3NH ⇔ (H2SO4)2(CH3NH)1(H2O)1 | −27.72 | −34.60 | −17.40 |
| (H2SO4)2(H2O)1 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −27.94 | −34.89 | −17.55 |
| (H2SO4)2(H2O)1 + NH3 ⇔ (H2SO4)2(NH3)1 (H2O)1 | −15.9b | ||
| (H2SO4)2(CH3NH)1 + CH3NH ⇔ (H2SO4)2(CH3NH)2 | −20.13 | −28.86 | −11.52 |
| (H2SO4)2[(CH3)2NH]1 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH]2 | −24.45 | −36.25 | −13.64 |
| (H2SO4)2(NH3)1 + NH3 ⇔ (H2SO4)2(NH3)2 | −18.16 | −19.87 | −8.74 |
| (H2SO4)3 + CH3NH ⇔ (H2SO4)3(CH3NH)1 | −32.44 | −32.08 | −22.88 |
| (H2SO4)3 + (CH3)2NH ⇔ (H2SO4)3[(CH3)2NH]1 | −31.63 | −30.68 | −22.48 |
| (H2SO4)3 + NH3 ⇔ (H2SO4)3(NH3)1 | −25.58 | −32.17 | −16.01 |
| CH3NH + CH3NH ⇔ (CH3NH)2 | −4.93 | −21.07 | 1.35 |
| (CH3NH)2 + CH3NH ⇔ (CH3NH)3 | −3.17 | −24.50 | 4.13 |
| (CH3)2NH + (CH3)2NH ⇔ [(CH3)2NH]2 | −2.83 | −23.58 | 4.20 |
| [(CH3)2NH]2 + CH3NH ⇔ [(CH3)2NH]3 | −3.83 | −32.22 | 5.77 |
a [34]; b [30].

Table 3.
Comparison of changes in enthalpies ∆H (kcal mole−1), entropies ∆S (cal mole−1 K−1), and Gibbs free energies ∆G (kcal mole−1) associated with formation of (CH3NH2)m(H2SO4)n(H2O)k, ((CH3)2NH)m(H2SO4)n(H2O)k and ((CH3)3N)m(H2SO4)n(H2O)k complexes via the attachment of the sulfuric acid with those associated with the formation of (NH3)m(H2SO4)n(H2O)k at temperature of 298.15K and pressure of 101.3 KPa.
| ∆H | ∆S | ∆G | |
| CH3NH2 + H2SO4 ⇔ (CH3NH)1 (H2SO4)1 | −20.40 (−20.87) | −31.42 (−36.62) | −11.03(−9.95) |
| (CH3)2NH + H2SO4 ⇔ [(CH3)2NH]1 (H2SO4)1 | −21.36 (−24.73) | −33.48 (−37.14) | −11.38 (−13.66) −7.28* |
| (CH3)3N + H2SO4 ⇔ [(CH3)3N]1(H2SO4)1 | −20.58 (−26.01) | −33.61 (−36.08) | −10.56 (−15.26) |
| NH3 + H2SO4 ⇔ (NH3)1 (H2SO4)1 | −16.72a | −30.01a | −7.77a |
| (CH3NH)(H2O) + (H2SO4) ⇔ (CH3NH)1 (H2SO4)1 (H2O)1 | −25.69 | −13.91 | −13.26 |
| [(CH3)2NH]1(H2O)1 + (H2SO4) ⇔ [(CH3)2NH]1 (H2SO4)1 (H2O)1 | −27.43 | −39.84 | −15.55 |
| (CH3NH)1 (H2O)2 + (H2SO4) ⇔ (CH3NH)1 (H2SO4)1 (H2O)2 | −30.83 | −41.91 | −18.34 |
| [(CH3)2NH]1 (H2O)2 + (H2SO4) ⇔ [(CH3)2NH]1 (H2SO4)1 (H2O)2 | −31.32 | −41.23 | −19.02 |
| (H2SO4)1(CH3NH)1 + H2SO4 ⇔ (H2SO4)2(CH3NH)1 | −27.42 | −44.17 | −14.25 |
| (H2SO4)1[(CH3)2NH]1 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]1 | −27.36 (−32.70) | −43.77 (−44.97) | −14.30 (−19.29) |
| (H2SO4)1[(CH3)3N]1 + H2SO4 ⇔ (H2SO4)2[(CH3)3N]1 | −22.88 | −41.79 | −10.41 |
| (H2SO4)1(NH3)1 + H2SO4 ⇔ (H2SO4)2(NH3)1 | −25.11a | −45.14a | −11.65a |
| (H2SO4)1(CH3NH)1(H2O)1 + H2SO4 ⇔ (H2SO4)2(CH3NH)1 (H2O)1 | −24.91 | −43.14 | −12.05 |
| (H2SO4)1 [(CH3)2NH)]1(H2O)1 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −24.54 | −43.76 | −11.49 |
| (H2SO4)1(NH3)1(H2O)1 + H2SO4 ⇔ (H2SO4)2 (NH3)1(H2O)1 | −25.83 | −44.42 | −12.59 |
| (H2SO4)1 (CH3NH)2 + H2SO4 ⇔ (H2SO4)2(CH3NH)2 | −31.62 | −33.06 | −21.76 |
| (H2SO4)1[(CH3)2NH]2 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]2 | −37.28 | −44.41 | −24.04 |
| (H2SO4)1 (NH3)2 + H2SO4 ⇔(H2SO4)2(NH3)2 | −29.59 | −46.77 | −15.66 |
| (H2SO4)2(CH3NH)1 + H2SO4 ⇔ (H2SO4)3(CH3NH)1 | −17.05 | −35.39 | −6.50 |
| (H2SO4)2[(CH3)2NH]1 + H2SO4 ⇔ (H2SO4)3[(CH3)2NH]1 | −15.33 | −32.34 | −5.69 |
| (H2SO4)2(NH3)1 + H2SO4 ⇔ (H2SO4)3(NH3)1 | −16.20 | −35.93 | −5.49 |
a [34];* [33]; ( ) [32].
4. Conclusions
In the present paper, the hydrogen-bonded complexes of binary sulfuric acid-water clusters with methylamine, dimethylamine and trimethylamine—common atmospheric organic species, vegetation products and laboratory impurities, have studied using the computational quantum methods. The present study shows that amines form strongly hydrogen-bonded complexes with the pre‑nucleation sulfuric acid-water clusters. The replacement of ammonia with amines leads to a moderately large enhancement in the stabilization of pre-nucleation clusters and sulfuric acid- pre‑nucleation clusters bonding energies. However, the difference in the formation free energies between the critically important (H2SO4)(amine) + H2SO4 ⇔ (H2SO4)2(amine) and (H2SO4)(NH3) + H2SO4 ⇔ (H2SO4)2(NH3) reactions is not large enough to account for the large (a factor of 102–103 [37,38,39,40,41,42]) difference in atmospheric typical concentrations of amines and ammonia. This leads us to a logical conclusion that under atmospheric conditions the formation of (H2SO4)2(amine) is a limiting stage. This indicates that under typical atmospheric conditions the stabilizing effect of amines is unlikely to exceed that of ammonia.
We also found that the difference in the dimerization free energies between the present study and earlier work [32] is considerable. There exist several sources of uncertainties, the basis set dependency of the MP2 calculations, which exceeds 6 kcal mole−1 in the case of (CH3)3N + H2SO4 ⇔ (CH3)3N(H2SO4) reaction [32,33], the vibrational anharmonicty, moderately large BSSE in the MP2/CC2 study [32] and possible problems of the PW91PW91 method in describing the hydrogen bonding. The aforementioned uncertainties directly affect the conclusions about the importance of amines for the atmospheric nucleation. It is important to note the qualitative conclusions appear to be quite sensitive to moderately large differences between calculated thermodynamic data of the present study and Kurten et al. [32]. While the present more comprehensive study concludes that the presence of amines does not enhance the cluster stability enough to overcome a concentration difference (in favour of ammonia) of around 2–3 orders of magnitude, the predictions of Kurten et al. [32], which are based on the dimerization free energies only, leads to the opposite conclusion. In contrast to [32], the recent experimental study [37] shows that in the case of trimethylamine the threshold [H2SO4] needed to produce the unity nucleation rate ([H2SO4] of 106–107 cm−3) and the number of precursor molecules in the critical cluster (nH2SO4 = 4−6; nTMA = 1) are surprisingly similar to those found in the ammonia (NH3) ternary nucleation study [51]. At lower RH some enhancement in nucleation rates due to trimethylamine was observed; hovewer, its value was up to an order of magnitude only. The recent experimental study [37] agrees with our theoretical results and supports our conclusion that under typical atmospheric conditions the stabilizing effect of amines is unlikely to exceed that of ammonia. Further computations using the high level ab initio or compound methods taking both the BSSE and anharmonic correction into accounts are needed to address these issues and to clarify the role of amines in the atmospheric nucleation.
Acknowledgements
Support of this work by the U.S. National Science Foundation under grant 0942106 is gratefully acknowledged. Authors thank the CRAES Supercomputing Facilities (SGI 4700 super computers) for providing computational resources.
References
- Wilson, C.T.R. Condensation of water vapour in the presence of dust-free air and other gases. Phil. Trans. R. Soc. Lond. A 1897, 189, 265–307. [Google Scholar] [CrossRef]
- Becker, R.; Doring, W. Kinetishe Behandlung der Keimbildung in ubersatuignen Dampfen. Ann. Physik 1935, 24, 719–752. [Google Scholar] [CrossRef]
- Charlson, R.J.; Seinfeld, J.H.; Nenes, A.; Kulmala, M.; Laaksonen, A.; Facchini, M.C. Atmospheric science: Reshaping the theory of cloud formation. Science 2001, 292, 2025–2026. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, M. How particles nucleate and grow. Science 2003, 302, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Saxon, D.; Diaz-Sanchez, D. Air pollution and allergy: You are what you breathe. Nature Immunol. 2005, 6, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, P.; Timonen, K.L.; Tiittanen, P.; Mirme, A.; Ruuskanen, J.; Pekkanen, J. Ultrafine particles in urban air and respiratory health among adult asthmatics. Euro. Respir. J. 2001, 17, 428–435. [Google Scholar] [CrossRef]
- Oberdorster, G.; Utell, M. Ultrafine particles in the urban air: To the respiratory tract—Ang beyond? Environ. Health. Persp. 2002, 110, A440–A441. [Google Scholar] [CrossRef]
- Hamill, P.; Turco, R.P.; Kiang, C.S.; Toon, O.B.; Whitten, R.C. An analysis of various nucleation mechanisms for sulfate particles in the stratosphere. J. Aerosol Sci 1982, 13, 561–585. [Google Scholar] [CrossRef]
- Berndt, T.; Böge, O.; Stratmann, F.; Heintzenberg, J.; Kulmala, M. Rapid formation of sulfuric acid particles at near-atmospheric conditions. Science 2005, 307, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Turco, R.P. Ultrafine aerosol formation via ion-mediated nucleation. Geophys. Res. Lett. 2000, 27, 883–886. [Google Scholar] [CrossRef]
- Napari, I.; Noppel, M.; Vehkamaki, H.; Kulmala, M. Parametrization of ternary nucleation rates for H2SO4-NH3-H2O vapors. J. Geophys. Res. 2002, 107, 4381–4386. [Google Scholar] [CrossRef]
- Yu, F. Effect of ammonia on new particle formation: A kinetic H2SO4-H2O-NH3 nucleation model constrained by laboratory measurements. J. Geophys. Res. 2006, 111, D01204. [Google Scholar] [CrossRef]
- Merikanto, J.; Napari, I.; Vehkamäki, H.; Anttila, T.; Kulmala, M. New parameterization of sulfuric acid-ammonia-water ternary nucleation rates at tropospheric conditions. J. Geophys. Res. 2007, 112, D1520. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; Jimenez, J.L.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H.; Hämerl, K.; Pirjola, L.; Kulmala, M.; Jennings, S.G.; Hoffmann, T. Marine aerosol formation from biogenic iodine emissions. Nature 2002, 417, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Suh, I.; Zhao, J.; Zhang, D.; Fortner, E.C.; Tie, X.; Molina, L.T.; Molina, M.J. Atmospheric new particle formation enhanced by organic acids. Science 2004, 304, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ariya, P.A. Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): A review. Atmos. Environ. 2006, 40, 795–807. [Google Scholar] [CrossRef]
- Adachi, K.; Buseck, P.R. Internally mixed soot, sulfates, and organic matter in aerosol particles from Mexico City. Atmos. Chem. Phys. 2008, 8, 6469–6487. [Google Scholar] [CrossRef]
- Smith, J.N.; Dunn, M.J.; VanReken, T.M.; Iida, K.; Stolzenburg, M.R.; McMurry, P.H.; Huey, L.G. Chemical composition of atmospheric nanoparticles formed from nucleation in Tecamac, Mexico: Evidence for an important role for organic species in nanoparticle growth. Geophys. Res. Lett. 2008, 35, L04808. [Google Scholar] [CrossRef]
- Sellegri, K.; Hanke, M.; Umann, B.; Arnold, F.; Kulmala, M. Measurements of organic gases during aerosol formation events in the boreal forest atmosphere during QUEST. Atmos. Chem. Phys. 2005, 5, 373–384. [Google Scholar] [CrossRef]
- Boy, M.; Karl, T.; Turnipseed, A.; Mauldin, R.L.; Kosciuch, E.; Greenberg, J.; Rathbone, J.; Smith, J.; Held, A.; Barsanti, K.; et al. New particle formation in the front range of the Colorado Rocky Mountains. Atmos. Chem. Phys. 2007, 7, 15581–15617. [Google Scholar] [CrossRef]
- Bonn, B.; Hirsikko, A.; Hakola, H.; Kurtén, T.; Laakso, L.; Boy, M.; Dal Maso, M.; Mäkelä, J.M.; Kulmala, M. Ambient sesquiterpene concentration and its link to air ion measurements. Atmos. Chem. Phys. 2007, 7, 2893–2916. [Google Scholar] [CrossRef]
- Parshintsev, J.; Nurmi, J.; Kilpeläinen, I.; Hartonen, K.; Kulmala, M.; Riekkola, M.-L. Preparation of ß-caryophyllene oxidation products and their determination in ambient aerosol samples. Analytic. Bioanalytic. Chem 2008, 390(3), 913–927. [Google Scholar]
- Bonn, B.; Kulmala, M.; Riipinen, I.; Sihto, S.-L.; Ruuskanen, T.M. How biogenic terpenes govern the correlation between sulfuric acid concentrations and new particles formation. J. Geophys. Res. D Atmos. 2008, 113, D12209. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Al Natsheh, A.; Yu, F.; Mikkelsen, K.V.; Ruuskanen, J. Quantum nature of the sign preference in ion-induced nucleation. Phys. Rev. Lett. 2006, 98, 125701. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Al Natsheh, A.; Yu, F.; Mikkelsen, K.V.; Ruuskanen, J. Sulfuric acid and sulfuric acid hydrates in the gas phase: A DFT investigation. J. Phys. Chem. A 2004, 108, 8914–8923. [Google Scholar]
- Nadykto, A.B.; .Du, H.; Yu, F. Quantum DFT and DF-DFT study of vibrational spectra of sulfuric acid, sulfuric acid monohydrate, formic acid and its cyclic dimer. Vibr. Spectr. 2007, 44, 2, 286–296. [Google Scholar] [CrossRef]
- Lewandowski, H.; Koglin, E.; Meier, R.J. Computational study of the infrared spectrum of acetic acid, its cyclic dimer, and its methyl ester. Vibr. Spec. 2005, 39, 15–22. [Google Scholar] [CrossRef]
- Ding, C.-G.; Laasonen, K.; Laaksonen, A. Two sulfuric acids in small water clusters. J. Phys. Chem. A. 2003, 107, 8648–8658. [Google Scholar] [CrossRef]
- Kurtén, T.; Sundberg, M.R.; Vehkamäki, H.; Noppel, M.; Blomqvist, J.; Kulmala, M. Ab initio and density functional theory reinvestigation of gas-phase sulfuric acid monohydrate and ammonium hydrogen sulfate. J. Phys. Chem. A 2006, 110, 7178–7188. [Google Scholar] [CrossRef] [PubMed]
- Kurtén, T.; Torpo, L.; Ding, C.-G.; Vehkamäki, H.; Sundberg, M.R.; Laasonen, K.; Kulmala, M. A density functional study on water-sulfuric acid-ammonia clusters and implications for atmospheric cluster formation. J. Geophys. Res. 2007, 112, D04210. [Google Scholar] [CrossRef]
- Ortega, I.K.; Kurtén, T.; Vehkamäki, H.; Kulmala, M. The role of ammonia in sulfuric acid ion induced nucleation. Atmos. Chem. Phys. 2008, 8, 2859–2867. [Google Scholar] [CrossRef]
- Kurtén, T.; Loukonen, V.; Vehkaméki, H.; Kulmala, M. Amines are likely to enhance neutral and ion-induced sulfuric acid-water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 2008, 8, 4095–4103. [Google Scholar] [CrossRef]
- Kurtén, T.; Ortega, I.K.; Vehkamäki, H. The sign preference in sulfuric acid nucleation. J. Mol. Structure: Theochem. 2009, 15901, 169–176. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Yu, F. Strong hydrogen bonding between atmospheric nucleation precursors and common organics. Chem. Phys. Lett. 2007, 435, 14–18. [Google Scholar] [CrossRef]
- Zhao, J.; Khalizov, A.; Zhang, R.; McGraw, R. Hydrogen-bonding interaction in molecular complexes and clusters of aerosol nucleation precursors. J. Phys. Chem. A 2009, 113, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nadykto, A.B.; Yu, F.; Herb, J. Towards understanding the sign preference in binary atmospheric nucleation. Phys. Chem. Chem. Phys. 2008, 10, 7073–7078. [Google Scholar] [CrossRef] [PubMed]
- Erupe, M.E.; Viggiano, A.A.; Lee, S.-H. The effect of trimethylamine on atmospheric nucleation involving H2SO4. Atmos. Chem. Phys. Disc. 2006, 10, 27673–27693. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Petäjä, T.; Manninen, H.E.; Paasonen, P.; Nieminen, T.; Sipilä, M.; Junninen, H.; Ehn, M.; Gagné, S.; Laakso, L.; Riipinen, I.; et al. Atmospheric nucleation: Highlights of the EUCAARI project and future directions. Atmos. Chem. Phys. 2010, 10, 10829–10848. [Google Scholar]
- Berndt, T.; Stratmann, F.; Sipilä, M.; Vanhanen, J.; Petäjä, T.; Mikkilä, J.; Grüner, A.; Spindler, G.; Lee Mauldin, R., III; Curtius, J.; et al. Laboratory study on new particle formation from the reaction OH + SO2: Influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. Disc. 2010, 10, 6447–6484. [Google Scholar] [CrossRef]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric amines—Part I. A review. Atmos. Env. 2011, 45, 524–546. [Google Scholar] [CrossRef]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric amines—Part II. Thermodynamic properties and gas/particle partitioning. Atmos. Env. 2011, 45, 561–577. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Ridge, D.P.; Johnston, M.V. Amine exchange into ammonium bisulfate and ammonium nitrate nuclei. Atmos. Chem. Phys. 2010, 10, 3495–3508. [Google Scholar] [CrossRef]
- Angelino, A.; Suess, D.T.; Prather, K. Formation of aerosol particles from reactions of secondary and tertiary alkylamines:Characterization by aerosol rime-of-flight mass spectrometry. Environ. Sci. Technol. 2001, 35, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Sorooshian, A.; Kroll, J.H.; Ng, N.L.; Chhabra, P.; Tong, C.; Surratt, J.D.; Knipping, E.; Flagan, R.C.; Seinfeld, J.H. Secondary aerosol formation from atmospheric reactions ofaliphatic amines. Atmos. Chem. Phys. 2007, 7, 2313–2337. [Google Scholar] [CrossRef]
- Makela, J.M.; Yli-Koivisto, S.; Hiltunen, V.; Seidl, W.; Swietlicki, E.; Teinila, K.; Sillanpaa, M.; Koponen, I.K.; Paatero, J.; Rosman, K.; Hameri, K. Chemical composition of aerosol duringparticle formation events in boreal forest. Tellus 2001, 53B, 380–393. [Google Scholar] [CrossRef]
- Cook, D.B. Handbook of Computational Quantum Chemistry; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Kurtén, T.; Vehkamäki, H. Investigating atmospheric sulfuric acid-water-ammonia particle formation using quantum chemistry. Adv. Quant. Chem. 2008, 55, 407–427. [Google Scholar]
- Cabaleiro-Lago, E.M.; Rios, M.A. Ab initio study of interactions in methylamine clusters. The significance of cooperative effects. J. Chem. Phys. 2000, 112, 2155–2163. [Google Scholar]
- Cabaleiro-Lago, E.M.; Rios, M.A. An ab initio study of the interaction in dimethylamine dimer and trimer. J. Chem. Phys. 2000, 113, 9523–9531. [Google Scholar]
- Sverdlov, L.M.; Kovner, M.A.; Krainov, E.P. Vibrational Spectra of Polyatomic Molecules; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Benson, D.; Markovich, A.; Lee, S. Atmospheric homogeneous nucleation of H2SO4 and H2O. Atmos. Chem. Phys. Discuss. 2010, 10, 22395–22414. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
