Amines in the Earth’s Atmosphere: A Density Functional Theory Study of the Thermochemistry of Pre-Nucleation Clusters
Abstract
:1. Introduction
2. Methods
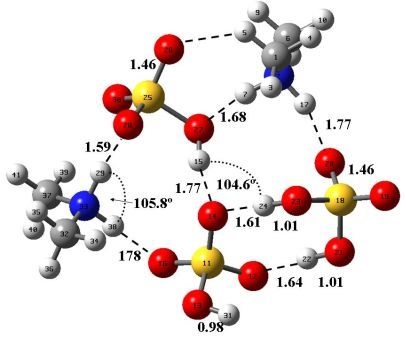
3. Results and Discussion





| ∆H | ∆S | ∆G | |
| CH3NH2 + H2O ⇔ (CH3NH)1(H2O)1 | −7.73 | −22.25 | −1.10 |
| (CH3)2NH + H2O ⇔ [(CH3)2NH]1(H2O)1 | −6.58 | −23.75 | 0.50 |
| H2SO4 + H2O ⇔ (H2SO4)1(H2O)1 | −11.76a | −31.80a | −2.28a |
| (CH3NH)1(H2O)1 + H2O ⇔ (CH3NH)1(H2O)2 | −8.70 | −34.42 | 1.56 |
| ((CH3)2NH)1(H2O)1 + H2O ⇔ [(CH3)2NH]1(H2O)2 | −8.77 | −34.74 | 1.59 |
| (H2SO4)1(H2O)1 + H2O ⇔ (H2SO4)1(H2O)2 | −12.57a | −32.08a | −3.00a |
| (CH3NH)1(H2SO4)1 + H2O ⇔ (CH3NH)1(H2SO4)1(H2O)1 | −13.02 | −32.50 | −3.33 |
| ((CH3)2NH)1(H2SO4)1 + H2O ⇔ [(CH3)2NH]1(H2SO4)1(H2O)1 | −12.65 | −30.11 | −3.67 |
| (NH3)1(H2SO4)1 + H2O ⇔ (NH3)1(H2SO4)1(H2O)1 | −10.96a | −32.03a | −1.41a |
| (CH3NH)1(H2SO4)1(H2O)1 + H2O ⇔ (CH3NH)1(H2SO4)1(H2O)2 | −13.85 | −34.66 | −3.52 |
| ((CH3)3N)1(H2SO4)1 + H2O ⇔ [(CH3)3N]1(H2SO4)1(H2O)1 | −10.88 | −32.51 | −1.19 |
| ((CH3)3N)1(H2SO4)1(H2O)1 + H2O ⇔ [(CH3)3N]1(H2SO4)1(H2O)2 | −10.74 | −32.76 | −0.97 |
| ((CH3)2NH)1(H2SO4)1(H2O)1 + H2O ⇔ [(CH3)2NH]1(H2SO4)(H2O)2 | −12.66 | −36.13 | −1.89 |
| (NH3)1(H2SO4)1(H2O)1 + H2O ⇔ (NH3)1 (H2SO4)1(H2O)2 | −11.92a | −32.34a | −2.28a |
| (H2SO4)2(CH3NH)1 + H2O ⇔ (H2SO4)2(CH3NH)1(H2O)1 | −10.50 | −31.47 | −1.13 |
| (H2SO4)2((CH3)2NH)1 + H2O ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −9.82 | −30.09 | −0.85 |
| (H2SO4)2(NH3)1 + H2O ⇔ (H2SO4)2(NH3)1(H2O)1 | −11.68 | −31.32 | −2.31 |
| ∆H | ∆S | ∆G | |
| (H2SO4)(H2O) + CH3NH ⇔ (CH3NH)1(H2SO4)1(H2O)1 | −21.66 | −32.11 | −12.08 |
| (H2SO4)1(H2O)1 + (CH3)2NH ⇔ [(CH3)2NH]1(H2SO4)1(H2O)1 | −22.24 | −31.78 | −12.76 |
| (H2SO4)1(H2O)1 + (CH3)3N ⇔ [(CH3)3N]1(H2SO4)1(H2O)1 | −19.37 | −35.69 | −8.73 |
| (H2SO4)1(H2O)1 + NH3 ⇔ (NH3)1(H2SO4)1(H2O)1 | −15.91a | −30.23a | −6.90a |
| (H2SO4)1(H2O)2 + CH3NH ⇔ (CH3NH)1(H2SO4)1(H2O)2 | −22.94 | −34.69 | −12.59 |
| (H2SO4)1(H2O)2 + (CH3)2NH ⇔ [(CH3)2NH]1(H2SO4)1(H2O)2 | −22.33 | −35.84 | −11.65 |
| (H2SO4)1(H2O)2 + (CH3)3N ⇔ [(CH3)3N]1(H2SO4)1(H2O)2 | −17.51 | −36.37 | −6.66 |
| (H2SO4)1(H2O)2 + NH3 ⇔ (NH3)1(H2SO4)1(H2O)2 | −15.27a | −30.49a | −6.18a |
| (H2SO4)1(CH3NH)1 + CH3NH ⇔ (H2SO4)1(CH3NH)2 | −15.93 | −39.96 | −4.02 |
| (H2SO4)1[(CH3)2NH]1 + (CH3)2NH ⇔ (H2SO4)1[(CH3)2NH]2 | −14.53 | −35.61 | −3.92 |
| (H2SO4)1[(CH3)3N] + (CH3)3N ⇔ (H2SO4)1[(CH3)3N]2 | −14.18 | −35.64 | −3.56 |
| (H2SO4)1(NH3)1 + NH3 ⇔ (H2SO4)1(NH3)2 | −13.68 | −29.96 | −4.74 |
| (H2SO4)2 + CH3NH ⇔ (H2SO4)2(CH3NH)1 | −31.65 | −40.13 | −19.69 |
| (H2SO4)2 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH] | −32.56 | −41.79 | −20.10 |
| (H2SO4)2 + NH3 ⇔ (H2SO4)2(NH3) | −25.67a | −39.68a | −13.83a |
| (H2SO4)2(H2O)1 + CH3NH ⇔ (H2SO4)2(CH3NH)1(H2O)1 | −27.72 | −34.60 | −17.40 |
| (H2SO4)2(H2O)1 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −27.94 | −34.89 | −17.55 |
| (H2SO4)2(H2O)1 + NH3 ⇔ (H2SO4)2(NH3)1 (H2O)1 | −15.9b | ||
| (H2SO4)2(CH3NH)1 + CH3NH ⇔ (H2SO4)2(CH3NH)2 | −20.13 | −28.86 | −11.52 |
| (H2SO4)2[(CH3)2NH]1 + (CH3)2NH ⇔ (H2SO4)2[(CH3)2NH]2 | −24.45 | −36.25 | −13.64 |
| (H2SO4)2(NH3)1 + NH3 ⇔ (H2SO4)2(NH3)2 | −18.16 | −19.87 | −8.74 |
| (H2SO4)3 + CH3NH ⇔ (H2SO4)3(CH3NH)1 | −32.44 | −32.08 | −22.88 |
| (H2SO4)3 + (CH3)2NH ⇔ (H2SO4)3[(CH3)2NH]1 | −31.63 | −30.68 | −22.48 |
| (H2SO4)3 + NH3 ⇔ (H2SO4)3(NH3)1 | −25.58 | −32.17 | −16.01 |
| CH3NH + CH3NH ⇔ (CH3NH)2 | −4.93 | −21.07 | 1.35 |
| (CH3NH)2 + CH3NH ⇔ (CH3NH)3 | −3.17 | −24.50 | 4.13 |
| (CH3)2NH + (CH3)2NH ⇔ [(CH3)2NH]2 | −2.83 | −23.58 | 4.20 |
| [(CH3)2NH]2 + CH3NH ⇔ [(CH3)2NH]3 | −3.83 | −32.22 | 5.77 |
| ∆H | ∆S | ∆G | |
| CH3NH2 + H2SO4 ⇔ (CH3NH)1 (H2SO4)1 | −20.40 (−20.87) | −31.42 (−36.62) | −11.03(−9.95) |
| (CH3)2NH + H2SO4 ⇔ [(CH3)2NH]1 (H2SO4)1 | −21.36 (−24.73) | −33.48 (−37.14) | −11.38 (−13.66) −7.28* |
| (CH3)3N + H2SO4 ⇔ [(CH3)3N]1(H2SO4)1 | −20.58 (−26.01) | −33.61 (−36.08) | −10.56 (−15.26) |
| NH3 + H2SO4 ⇔ (NH3)1 (H2SO4)1 | −16.72a | −30.01a | −7.77a |
| (CH3NH)(H2O) + (H2SO4) ⇔ (CH3NH)1 (H2SO4)1 (H2O)1 | −25.69 | −13.91 | −13.26 |
| [(CH3)2NH]1(H2O)1 + (H2SO4) ⇔ [(CH3)2NH]1 (H2SO4)1 (H2O)1 | −27.43 | −39.84 | −15.55 |
| (CH3NH)1 (H2O)2 + (H2SO4) ⇔ (CH3NH)1 (H2SO4)1 (H2O)2 | −30.83 | −41.91 | −18.34 |
| [(CH3)2NH]1 (H2O)2 + (H2SO4) ⇔ [(CH3)2NH]1 (H2SO4)1 (H2O)2 | −31.32 | −41.23 | −19.02 |
| (H2SO4)1(CH3NH)1 + H2SO4 ⇔ (H2SO4)2(CH3NH)1 | −27.42 | −44.17 | −14.25 |
| (H2SO4)1[(CH3)2NH]1 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]1 | −27.36 (−32.70) | −43.77 (−44.97) | −14.30 (−19.29) |
| (H2SO4)1[(CH3)3N]1 + H2SO4 ⇔ (H2SO4)2[(CH3)3N]1 | −22.88 | −41.79 | −10.41 |
| (H2SO4)1(NH3)1 + H2SO4 ⇔ (H2SO4)2(NH3)1 | −25.11a | −45.14a | −11.65a |
| (H2SO4)1(CH3NH)1(H2O)1 + H2SO4 ⇔ (H2SO4)2(CH3NH)1 (H2O)1 | −24.91 | −43.14 | −12.05 |
| (H2SO4)1 [(CH3)2NH)]1(H2O)1 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]1(H2O)1 | −24.54 | −43.76 | −11.49 |
| (H2SO4)1(NH3)1(H2O)1 + H2SO4 ⇔ (H2SO4)2 (NH3)1(H2O)1 | −25.83 | −44.42 | −12.59 |
| (H2SO4)1 (CH3NH)2 + H2SO4 ⇔ (H2SO4)2(CH3NH)2 | −31.62 | −33.06 | −21.76 |
| (H2SO4)1[(CH3)2NH]2 + H2SO4 ⇔ (H2SO4)2[(CH3)2NH]2 | −37.28 | −44.41 | −24.04 |
| (H2SO4)1 (NH3)2 + H2SO4 ⇔(H2SO4)2(NH3)2 | −29.59 | −46.77 | −15.66 |
| (H2SO4)2(CH3NH)1 + H2SO4 ⇔ (H2SO4)3(CH3NH)1 | −17.05 | −35.39 | −6.50 |
| (H2SO4)2[(CH3)2NH]1 + H2SO4 ⇔ (H2SO4)3[(CH3)2NH]1 | −15.33 | −32.34 | −5.69 |
| (H2SO4)2(NH3)1 + H2SO4 ⇔ (H2SO4)3(NH3)1 | −16.20 | −35.93 | −5.49 |
4. Conclusions
Acknowledgements
References
- Wilson, C.T.R. Condensation of water vapour in the presence of dust-free air and other gases. Phil. Trans. R. Soc. Lond. A 1897, 189, 265–307. [Google Scholar] [CrossRef]
- Becker, R.; Doring, W. Kinetishe Behandlung der Keimbildung in ubersatuignen Dampfen. Ann. Physik 1935, 24, 719–752. [Google Scholar] [CrossRef]
- Charlson, R.J.; Seinfeld, J.H.; Nenes, A.; Kulmala, M.; Laaksonen, A.; Facchini, M.C. Atmospheric science: Reshaping the theory of cloud formation. Science 2001, 292, 2025–2026. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, M. How particles nucleate and grow. Science 2003, 302, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Saxon, D.; Diaz-Sanchez, D. Air pollution and allergy: You are what you breathe. Nature Immunol. 2005, 6, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, P.; Timonen, K.L.; Tiittanen, P.; Mirme, A.; Ruuskanen, J.; Pekkanen, J. Ultrafine particles in urban air and respiratory health among adult asthmatics. Euro. Respir. J. 2001, 17, 428–435. [Google Scholar] [CrossRef]
- Oberdorster, G.; Utell, M. Ultrafine particles in the urban air: To the respiratory tract—Ang beyond? Environ. Health. Persp. 2002, 110, A440–A441. [Google Scholar] [CrossRef]
- Hamill, P.; Turco, R.P.; Kiang, C.S.; Toon, O.B.; Whitten, R.C. An analysis of various nucleation mechanisms for sulfate particles in the stratosphere. J. Aerosol Sci 1982, 13, 561–585. [Google Scholar] [CrossRef]
- Berndt, T.; Böge, O.; Stratmann, F.; Heintzenberg, J.; Kulmala, M. Rapid formation of sulfuric acid particles at near-atmospheric conditions. Science 2005, 307, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Turco, R.P. Ultrafine aerosol formation via ion-mediated nucleation. Geophys. Res. Lett. 2000, 27, 883–886. [Google Scholar] [CrossRef]
- Napari, I.; Noppel, M.; Vehkamaki, H.; Kulmala, M. Parametrization of ternary nucleation rates for H2SO4-NH3-H2O vapors. J. Geophys. Res. 2002, 107, 4381–4386. [Google Scholar] [CrossRef]
- Yu, F. Effect of ammonia on new particle formation: A kinetic H2SO4-H2O-NH3 nucleation model constrained by laboratory measurements. J. Geophys. Res. 2006, 111, D01204. [Google Scholar] [CrossRef]
- Merikanto, J.; Napari, I.; Vehkamäki, H.; Anttila, T.; Kulmala, M. New parameterization of sulfuric acid-ammonia-water ternary nucleation rates at tropospheric conditions. J. Geophys. Res. 2007, 112, D1520. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; Jimenez, J.L.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H.; Hämerl, K.; Pirjola, L.; Kulmala, M.; Jennings, S.G.; Hoffmann, T. Marine aerosol formation from biogenic iodine emissions. Nature 2002, 417, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Suh, I.; Zhao, J.; Zhang, D.; Fortner, E.C.; Tie, X.; Molina, L.T.; Molina, M.J. Atmospheric new particle formation enhanced by organic acids. Science 2004, 304, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ariya, P.A. Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): A review. Atmos. Environ. 2006, 40, 795–807. [Google Scholar] [CrossRef]
- Adachi, K.; Buseck, P.R. Internally mixed soot, sulfates, and organic matter in aerosol particles from Mexico City. Atmos. Chem. Phys. 2008, 8, 6469–6487. [Google Scholar] [CrossRef]
- Smith, J.N.; Dunn, M.J.; VanReken, T.M.; Iida, K.; Stolzenburg, M.R.; McMurry, P.H.; Huey, L.G. Chemical composition of atmospheric nanoparticles formed from nucleation in Tecamac, Mexico: Evidence for an important role for organic species in nanoparticle growth. Geophys. Res. Lett. 2008, 35, L04808. [Google Scholar] [CrossRef]
- Sellegri, K.; Hanke, M.; Umann, B.; Arnold, F.; Kulmala, M. Measurements of organic gases during aerosol formation events in the boreal forest atmosphere during QUEST. Atmos. Chem. Phys. 2005, 5, 373–384. [Google Scholar] [CrossRef]
- Boy, M.; Karl, T.; Turnipseed, A.; Mauldin, R.L.; Kosciuch, E.; Greenberg, J.; Rathbone, J.; Smith, J.; Held, A.; Barsanti, K.; et al. New particle formation in the front range of the Colorado Rocky Mountains. Atmos. Chem. Phys. 2007, 7, 15581–15617. [Google Scholar] [CrossRef]
- Bonn, B.; Hirsikko, A.; Hakola, H.; Kurtén, T.; Laakso, L.; Boy, M.; Dal Maso, M.; Mäkelä, J.M.; Kulmala, M. Ambient sesquiterpene concentration and its link to air ion measurements. Atmos. Chem. Phys. 2007, 7, 2893–2916. [Google Scholar] [CrossRef]
- Parshintsev, J.; Nurmi, J.; Kilpeläinen, I.; Hartonen, K.; Kulmala, M.; Riekkola, M.-L. Preparation of ß-caryophyllene oxidation products and their determination in ambient aerosol samples. Analytic. Bioanalytic. Chem 2008, 390(3), 913–927. [Google Scholar]
- Bonn, B.; Kulmala, M.; Riipinen, I.; Sihto, S.-L.; Ruuskanen, T.M. How biogenic terpenes govern the correlation between sulfuric acid concentrations and new particles formation. J. Geophys. Res. D Atmos. 2008, 113, D12209. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Al Natsheh, A.; Yu, F.; Mikkelsen, K.V.; Ruuskanen, J. Quantum nature of the sign preference in ion-induced nucleation. Phys. Rev. Lett. 2006, 98, 125701. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Al Natsheh, A.; Yu, F.; Mikkelsen, K.V.; Ruuskanen, J. Sulfuric acid and sulfuric acid hydrates in the gas phase: A DFT investigation. J. Phys. Chem. A 2004, 108, 8914–8923. [Google Scholar]
- Nadykto, A.B.; .Du, H.; Yu, F. Quantum DFT and DF-DFT study of vibrational spectra of sulfuric acid, sulfuric acid monohydrate, formic acid and its cyclic dimer. Vibr. Spectr. 2007, 44, 2, 286–296. [Google Scholar] [CrossRef]
- Lewandowski, H.; Koglin, E.; Meier, R.J. Computational study of the infrared spectrum of acetic acid, its cyclic dimer, and its methyl ester. Vibr. Spec. 2005, 39, 15–22. [Google Scholar] [CrossRef]
- Ding, C.-G.; Laasonen, K.; Laaksonen, A. Two sulfuric acids in small water clusters. J. Phys. Chem. A. 2003, 107, 8648–8658. [Google Scholar] [CrossRef]
- Kurtén, T.; Sundberg, M.R.; Vehkamäki, H.; Noppel, M.; Blomqvist, J.; Kulmala, M. Ab initio and density functional theory reinvestigation of gas-phase sulfuric acid monohydrate and ammonium hydrogen sulfate. J. Phys. Chem. A 2006, 110, 7178–7188. [Google Scholar] [CrossRef] [PubMed]
- Kurtén, T.; Torpo, L.; Ding, C.-G.; Vehkamäki, H.; Sundberg, M.R.; Laasonen, K.; Kulmala, M. A density functional study on water-sulfuric acid-ammonia clusters and implications for atmospheric cluster formation. J. Geophys. Res. 2007, 112, D04210. [Google Scholar] [CrossRef]
- Ortega, I.K.; Kurtén, T.; Vehkamäki, H.; Kulmala, M. The role of ammonia in sulfuric acid ion induced nucleation. Atmos. Chem. Phys. 2008, 8, 2859–2867. [Google Scholar] [CrossRef]
- Kurtén, T.; Loukonen, V.; Vehkaméki, H.; Kulmala, M. Amines are likely to enhance neutral and ion-induced sulfuric acid-water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 2008, 8, 4095–4103. [Google Scholar] [CrossRef]
- Kurtén, T.; Ortega, I.K.; Vehkamäki, H. The sign preference in sulfuric acid nucleation. J. Mol. Structure: Theochem. 2009, 15901, 169–176. [Google Scholar] [CrossRef]
- Nadykto, A.B.; Yu, F. Strong hydrogen bonding between atmospheric nucleation precursors and common organics. Chem. Phys. Lett. 2007, 435, 14–18. [Google Scholar] [CrossRef]
- Zhao, J.; Khalizov, A.; Zhang, R.; McGraw, R. Hydrogen-bonding interaction in molecular complexes and clusters of aerosol nucleation precursors. J. Phys. Chem. A 2009, 113, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nadykto, A.B.; Yu, F.; Herb, J. Towards understanding the sign preference in binary atmospheric nucleation. Phys. Chem. Chem. Phys. 2008, 10, 7073–7078. [Google Scholar] [CrossRef] [PubMed]
- Erupe, M.E.; Viggiano, A.A.; Lee, S.-H. The effect of trimethylamine on atmospheric nucleation involving H2SO4. Atmos. Chem. Phys. Disc. 2006, 10, 27673–27693. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Petäjä, T.; Manninen, H.E.; Paasonen, P.; Nieminen, T.; Sipilä, M.; Junninen, H.; Ehn, M.; Gagné, S.; Laakso, L.; Riipinen, I.; et al. Atmospheric nucleation: Highlights of the EUCAARI project and future directions. Atmos. Chem. Phys. 2010, 10, 10829–10848. [Google Scholar]
- Berndt, T.; Stratmann, F.; Sipilä, M.; Vanhanen, J.; Petäjä, T.; Mikkilä, J.; Grüner, A.; Spindler, G.; Lee Mauldin, R., III; Curtius, J.; et al. Laboratory study on new particle formation from the reaction OH + SO2: Influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. Disc. 2010, 10, 6447–6484. [Google Scholar] [CrossRef]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric amines—Part I. A review. Atmos. Env. 2011, 45, 524–546. [Google Scholar] [CrossRef]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric amines—Part II. Thermodynamic properties and gas/particle partitioning. Atmos. Env. 2011, 45, 561–577. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Ridge, D.P.; Johnston, M.V. Amine exchange into ammonium bisulfate and ammonium nitrate nuclei. Atmos. Chem. Phys. 2010, 10, 3495–3508. [Google Scholar] [CrossRef]
- Angelino, A.; Suess, D.T.; Prather, K. Formation of aerosol particles from reactions of secondary and tertiary alkylamines:Characterization by aerosol rime-of-flight mass spectrometry. Environ. Sci. Technol. 2001, 35, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Sorooshian, A.; Kroll, J.H.; Ng, N.L.; Chhabra, P.; Tong, C.; Surratt, J.D.; Knipping, E.; Flagan, R.C.; Seinfeld, J.H. Secondary aerosol formation from atmospheric reactions ofaliphatic amines. Atmos. Chem. Phys. 2007, 7, 2313–2337. [Google Scholar] [CrossRef]
- Makela, J.M.; Yli-Koivisto, S.; Hiltunen, V.; Seidl, W.; Swietlicki, E.; Teinila, K.; Sillanpaa, M.; Koponen, I.K.; Paatero, J.; Rosman, K.; Hameri, K. Chemical composition of aerosol duringparticle formation events in boreal forest. Tellus 2001, 53B, 380–393. [Google Scholar] [CrossRef]
- Cook, D.B. Handbook of Computational Quantum Chemistry; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Kurtén, T.; Vehkamäki, H. Investigating atmospheric sulfuric acid-water-ammonia particle formation using quantum chemistry. Adv. Quant. Chem. 2008, 55, 407–427. [Google Scholar]
- Cabaleiro-Lago, E.M.; Rios, M.A. Ab initio study of interactions in methylamine clusters. The significance of cooperative effects. J. Chem. Phys. 2000, 112, 2155–2163. [Google Scholar]
- Cabaleiro-Lago, E.M.; Rios, M.A. An ab initio study of the interaction in dimethylamine dimer and trimer. J. Chem. Phys. 2000, 113, 9523–9531. [Google Scholar]
- Sverdlov, L.M.; Kovner, M.A.; Krainov, E.P. Vibrational Spectra of Polyatomic Molecules; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Benson, D.; Markovich, A.; Lee, S. Atmospheric homogeneous nucleation of H2SO4 and H2O. Atmos. Chem. Phys. Discuss. 2010, 10, 22395–22414. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadykto, A.B.; Yu, F.; Jakovleva, M.V.; Herb, J.; Xu, Y. Amines in the Earth’s Atmosphere: A Density Functional Theory Study of the Thermochemistry of Pre-Nucleation Clusters. Entropy 2011, 13, 554-569. https://doi.org/10.3390/e13020554
Nadykto AB, Yu F, Jakovleva MV, Herb J, Xu Y. Amines in the Earth’s Atmosphere: A Density Functional Theory Study of the Thermochemistry of Pre-Nucleation Clusters. Entropy. 2011; 13(2):554-569. https://doi.org/10.3390/e13020554
Chicago/Turabian StyleNadykto, Alexey B., Fangqun Yu, Marina V. Jakovleva, Jason Herb, and Yisheng Xu. 2011. "Amines in the Earth’s Atmosphere: A Density Functional Theory Study of the Thermochemistry of Pre-Nucleation Clusters" Entropy 13, no. 2: 554-569. https://doi.org/10.3390/e13020554
APA StyleNadykto, A. B., Yu, F., Jakovleva, M. V., Herb, J., & Xu, Y. (2011). Amines in the Earth’s Atmosphere: A Density Functional Theory Study of the Thermochemistry of Pre-Nucleation Clusters. Entropy, 13(2), 554-569. https://doi.org/10.3390/e13020554