Investigating Aging-Related Changes in the Coordination of Agonist and Antagonist Muscles Using Fuzzy Entropy and Mutual Information
Abstract
:1. Introduction
2. Methods
2.1. Subjects
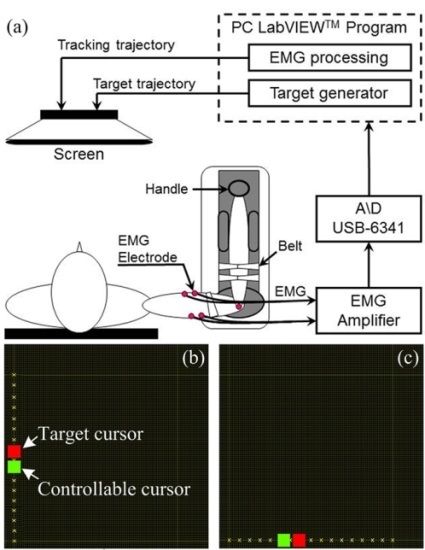
2.2. Apparatus and Procedure
2.3. Data and Statistical Analysis
3. Results
3.1. Normalized Agonist and Antagonist Activation
3.2. FuzzyEn Values of Agonist and Antagonist EMG
3.3. Mutual Information
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EMG | Electromyogram |
| ECG | Electrocardiography |
| EEG | Electroencephalograms |
| MVC | Maximal voluntary contraction |
| MCI | Myoelectric-controlled interface |
| SamEn | Sample entropy |
| ApEn | Approximate entropy |
| FuzzyEn | Fuzzy entropy |
| MI | Mutual information |
| CNS | Central nervous system |
References
- Macaluso, A.; Nimmo, M.A.; Foster, J.E.; Cockburn, M.; McMillan, N.C.; de Vito, G. Contractile muscle volume and agonist–antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 2002, 25, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Klass, M.; Baudry, S.; Duchateau, J. Voluntary activation during maximal contraction with advancing age: A brief review. Eur. J. Appl. Physiol. 2007, 100, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Billot, M.; Duclay, J.; Simoneau-Buessinger, E.M.; Ballay, Y.; Martin, A. Is co-contraction responsible for the decline in maximal knee joint torque in older males? Age 2014, 36, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Devita, P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc. Sport. Sci. Rev. 2006, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Kwon, M.; Fox, E.J.; Christou, E.A. Altered activation of the antagonist muscle during practice compromises motor learning in older adults. J. Neurophysiol. 2014, 112, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; Martin, P.E. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 2010, 31, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Laughton, C.A.; Slavin, M.; Katdare, K.; Nolan, L.; Bean, J.F.; Kerrigan, D.C.; Phillips, E.; Lipsitz, L.A.; Collins, J.J. Aging, muscle activity, and balance control: Physiologic changes associated with balance impairment. Gait Posture 2003, 18, 101–108. [Google Scholar] [CrossRef]
- Hortobágyi, T.; DeVita, P. Muscle pre-and coactivity during downward stepping are associated with leg stiffness in aging. J. Electromyogr. Kinesiol. 2000, 10, 117–126. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Dynamics and stability of muscle activations during walking in healthy young and older adults. J. Biomech. 2009, 42, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, P. Sample entropy analysis of surface EMG for improved muscle activity onset detection against spurious background spikes. J. Electromyogr. Kinesiol. 2012, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.; Torres, A.; Sarlabous, L.; Jane, R. Improvement in neural respiratory drive estimation from diaphragm electromyographic signals using fixed sample entropy. IEEE J. Biomed. Health Inform. 2016, 20, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, X.; Gao, X.; Chen, X.; Zhou, P. Complexity analysis of surface EMG for overcoming ECG interference toward proportional myoelectric control. Entropy 2016, 18. [Google Scholar] [CrossRef]
- Giannasi, L.C.; Matsui, M.Y.; Politti, F.; Batista, S.R.F.; Caldas, B.F.; Amorim, J.B.; de Oliveira, L.V.; Oliveira, C.S.; Gomes, M.F. Test–retest reliability of electromyographic variables of masseter and temporal muscles in patients with cerebral palsy. Arch. Oral Biol. 2014, 59, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Zhao, X.; Han, J.; Liu, G. Application of the chaos theory in the analysis of EMG on patients with facial paralysis. Adv. Intell. Syst. Comput. 2014, 274, 805–819. [Google Scholar]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of surface EMG signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Sun, R.; Tong, K.Y.; Song, R. Characterization of stroke- and aging-related changes in the complexity of EMG signals during tracking tasks. Ann. Biomed. Eng. 2014, 43, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Song, R.; Tong, K.Y. Complexity analysis of EMG signals for patients after stroke during robot-aided rehabilitation training using fuzzy approximate entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Power, H.A.; Norton, J.A.; Porter, C.L.; Doyle, Z.; Hui, I.; Chan, K.M. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J. Physiol. 2006, 577, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Asaka, T.; Wang, Y. Effects of aging on feedforward postural synergies. J. Hum. Kinetics 2009, 20, 63–70. [Google Scholar] [CrossRef]
- Keenan, K.G.; Massey, W.V.; Walters, T.J.; Collins, J.D. Sensitivity of EMG-EMG coherence to detect the common oscillatory drive to hand muscles in young and older adults. J. Neurophysiol. 2012, 107, 2866–2875. [Google Scholar] [PubMed]
- Jin, S.H.; Lin, P.; Hallett, M. Linear and nonlinear information flow based on time-delayed mutual information method and its application to corticomuscular interaction. Clin. Neurophysiol. 2010, 121, 392–401. [Google Scholar] [PubMed]
- Jung, T.I.; Vogiatzian, F.; Har-Shemesh, O.; Fitzsimons, C.P.; Quax, R. Applying information theory to neuronal networks: From theory to experiments. Entropy 2014, 16, 5721–5737. [Google Scholar]
- Svendsen, J.H.; Samani, A.; Mayntzhusen, K.; Madeleine, P. Muscle coordination and force variability during static and dynamic tracking tasks. Hum. Mov. Sci. 2011, 30, 1039–1051. [Google Scholar] [PubMed]
- Madeleine, P.; Samani, A.; Binderup, A.T.; Stensdotter, A.K. Changes in the spatio-temporal organization of the trapezius muscle activity in response to eccentric contractions. Scand. J. Med. Sci. Sports 2011, 21, 277–286. [Google Scholar] [PubMed]
- Gordon, K.E.; Ferris, D.P. Proportional myoelectric control of a virtual object to investigate human efferent control. Exp. Brain Res. 2004, 159, 478–486. [Google Scholar] [PubMed]
- Missenard, O.; Mottet, D.; Perrey, S. The role of cocontraction in the impairment of movement accuracy with fatigue. Exp. Brain Res. 2008, 185, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Burden, A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. 2010, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Ada, L.; O’Dwyer, N.J. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J. Neurol. Sci. 2000, 176, 45–56. [Google Scholar] [CrossRef]
- Moon, Y.I.; Rajagopalan, B.; Lall, U. Estimation of mutual information using kernel density estimators. Phys. Rev. E. 1995, 52, 2318–2321. [Google Scholar] [CrossRef]
- Klein, C.S.; Rice, C.L.; Marsh, G.D. Normalized force, activation, and coactivation in the arm muscles of young and old men. J. Appl. Physiol. 2001, 91, 1341–1349. [Google Scholar] [PubMed]
- Gordon, M.T. Effect of Age, Elbow Muscle Co-Contraction Level, and Elbow Moment Loading Characteristics on Elbow Angle Positional Variability in Postural Tasks by Positional Variability in Postural Tasks. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2012. [Google Scholar]
- Bautmans, I.; Vantieghem, S.; Gorus, E.; Grazzini, Y.R.; Fierens, Y.; Pool-Goudzwaard, A.; Mets, T. Age-related differences in pre-movement antagonist muscle co-activation and reaction-time performance. Exp. Gerontol. 2011, 46, 637–642. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Mambrito, B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J. Neurophysiol. 1987, 58, 525–542. [Google Scholar] [PubMed]
- Nielsen, J.; Kagamihara, Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J. Physiol. 1993, 464, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Pousson, M.; Lepers, R.; Van Hoecke, J. Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp. Gerontol. 2001, 36, 1687–1698. [Google Scholar] [CrossRef]
- Valour, D.; Ochala, J.; Ballay, Y.; Pousson, M. The influence of ageing on the force-velocity-power characteristics of human elbow flexor muscles. Exp. Gerontol. 2003, 38, 387–395. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Riccio, M.E.; Felici, F. Tennis players show a lower coactivation of the elbow antagonist muscles during isokinetic exercises. J. Electromyogr. Kinesiol. 2008, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Amarantini, D.; Bru, B. Training-related changes in the EMG-moment relationship during isometric contractions: Further evidence of improved control of muscle activation in strength-trained men? J. Electromyogr. Kinesiol. 2015, 25, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Tanaka, N.; Stein, R.B. Spinal excitation and inhibition decrease as humans’ age. Can. J. Physiol. Pharmacol. 2004, 82, 238–248. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Liang, J.; Yang, Y.; Wu, Y.; Yan, T.; Song, R. Investigating Aging-Related Changes in the Coordination of Agonist and Antagonist Muscles Using Fuzzy Entropy and Mutual Information. Entropy 2016, 18, 229. https://doi.org/10.3390/e18060229
Sun W, Liang J, Yang Y, Wu Y, Yan T, Song R. Investigating Aging-Related Changes in the Coordination of Agonist and Antagonist Muscles Using Fuzzy Entropy and Mutual Information. Entropy. 2016; 18(6):229. https://doi.org/10.3390/e18060229
Chicago/Turabian StyleSun, Wenbo, Jingtao Liang, Yuan Yang, Yuanyu Wu, Tiebin Yan, and Rong Song. 2016. "Investigating Aging-Related Changes in the Coordination of Agonist and Antagonist Muscles Using Fuzzy Entropy and Mutual Information" Entropy 18, no. 6: 229. https://doi.org/10.3390/e18060229
APA StyleSun, W., Liang, J., Yang, Y., Wu, Y., Yan, T., & Song, R. (2016). Investigating Aging-Related Changes in the Coordination of Agonist and Antagonist Muscles Using Fuzzy Entropy and Mutual Information. Entropy, 18(6), 229. https://doi.org/10.3390/e18060229