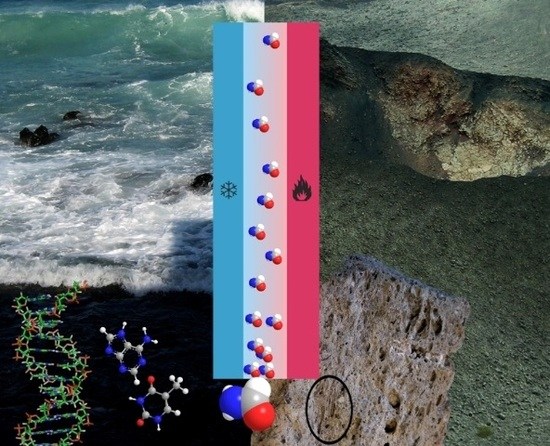
Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores
Abstract
Share and Cite
Niether, D.; Wiegand, S. Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores. Entropy 2017, 19, 33. https://doi.org/10.3390/e19010033
Niether D, Wiegand S. Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores. Entropy. 2017; 19(1):33. https://doi.org/10.3390/e19010033
Chicago/Turabian StyleNiether, Doreen, and Simone Wiegand. 2017. "Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores" Entropy 19, no. 1: 33. https://doi.org/10.3390/e19010033
APA StyleNiether, D., & Wiegand, S. (2017). Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores. Entropy, 19(1), 33. https://doi.org/10.3390/e19010033