Altered Causal Coupling Pathways within the Central-Autonomic-Network in Patients Suffering from Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
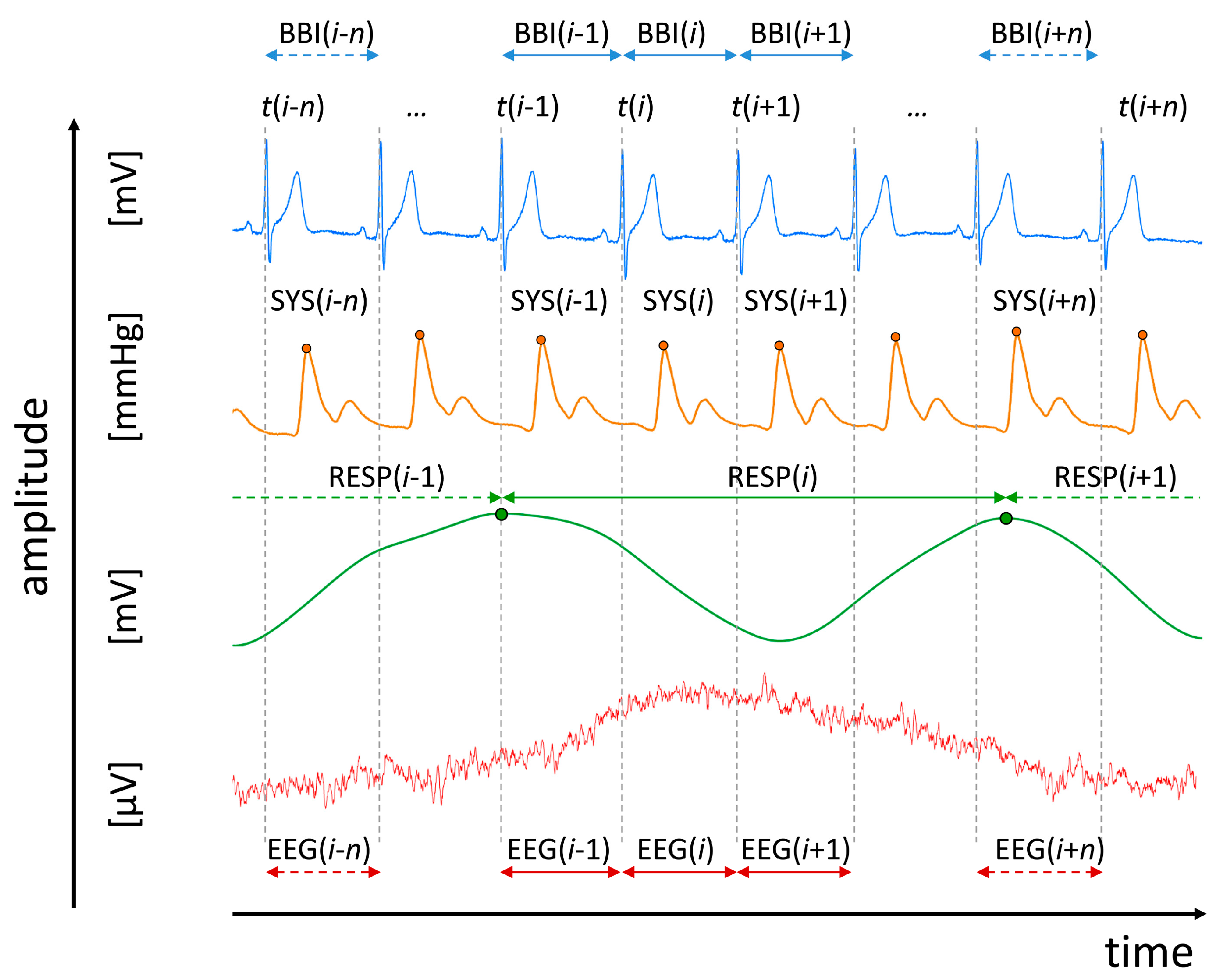
2.2. Data Recordings and Pre-Processing
- Heart rate (lead I) consisting of successive beat-to-beat intervals (BBI, tachogram, (msec));
- Maximum successive end-systolic blood pressure amplitude values over time in relation to the previous R-peak (SYS, systogram, (mmHg));
- Respiratory frequency (RESP, (sec)) as time intervals between consecutive breathing cycles.
2.3. Standard Indices from Electroencephalogram, Heart Rate-, Blood Pressure- and Respiratory Variability in the Frequency-and Time Domains
- meanNN: the mean value of the NN intervals of BBI (msec), of systolic (SYS) blood pressure (mmHg) values, and RESP (sec) as respiratory cycle length;
- sdNN: the standard deviation of the NN intervals of BBI (msec), of systolic (SYS) blood pressure (mmHg) values, and RESP (sec);
- BF: the breathing frequency characterizing the number of breaths per minute (1/min).
2.4. Central-Cardiovascular and Central-Cardiorespiratory Coupling Analyses
2.4.1. Normalized Short-Time Partial Directed Coherence
- NF = {−2 | 2} (where −2 denotes yPEEG as the driver): Strong unidirectional coupling;
- NF = {−1.5 to −2} or NF = {1.5 to 2}: Weak unidirectional coupling;
- NF = {−1 | 1} (−1 denotes yPEEG as the driver): Strong bidirectional coupling;
- NF = {−0.5 to −1} or NF = {0.5 to 1}: Weak bidirectional coupling;
- NF = 0: Equal influence in both directions and/or no coupling in respect to the coupling strengths (If both area indices reveal equal values larger than zero an equal influence in both directions is present, if both area indices reveal equal values but are zero no coupling is present).
2.4.2. Multivariate Transfer Entropy
2.4.3. Surrogate Data
2.5. Statistics
3. Results
3.1. Standard Indices from Electroencephalogram, Heart Rate Variability, Blood Pressure Variability and Respiratory Variability in the Frequency-and Time Domains
3.2. Central-Cardiovascular Coupling-Linear
3.2.1. Cardiovascular Coupling
3.2.2. Central-Cardiac Coupling
3.2.3. Central-Vascular Coupling
3.3. Central-Cardiovascular Coupling-Nonlinear
3.3.1. Cardiovascular Coupling
3.3.2. Central-Cardiac Coupling
3.3.3. Central-Vascular Coupling
3.4. Central-Cardiorespiratory Coupling-Linear
3.4.1. Cardiorespiratory Coupling
3.4.2. Central-Cardiac Coupling
3.4.3. Central-Respiratory Coupling
3.5. Central-Cardiorespiratory Coupling-Nonlinear
3.5.1. Cardiorespiratory Coupling
3.5.2. Central-Cardiac Coupling
3.5.3. Central-Respiratory Coupling
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bartsch, R.P.; Liu, K.K.; Bashan, A.; Ivanov, P.C. Network Physiology: How Organ Systems Dynamically Interact. PLoS ONE 2015, 10, e0142143. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.C.; Liu, K.K.; Bartsch, R.P. Focus on the emerging new fields of network physiology and network medicine. New J. Phys. 2016, 18, 100201. [Google Scholar] [CrossRef] [PubMed]
- Bashan, A.; Bartsch, R.P.; Kantelhardt, J.W.; Havlin, S.; Ivanov, P.C. Network physiology reveals relations between network topology and physiological function. Nat. Commun. 2012, 3, 702. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D.; Casey, D.E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Mortensen, Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.-J.; Boettger, M.K.; Koschke, M.; Schulz, S.; Chokka, P.; Yeragani, V.K.; Voss, A. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin. Neurophysiol. 2007, 118, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.; Letzsch, A.; Jochum, T.; Wagner, G.; Greiner, W.; Sauer, H. Loss of efferent vagal activity in acute schizophrenia. J. Psychiatr. Res. 2005, 39, 519–527. [Google Scholar] [CrossRef]
- Chang, J.S.; Yoo, C.S.; Yi, S.H.; Hong, K.H.; Oh, H.S.; Hwang, J.Y.; Kim, S.-G.; Ahn, Y.M.; Kim, Y.S. Differential pattern of heart rate variability in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 991–995. [Google Scholar] [CrossRef]
- Valkonen-Korhonen, M.; Tarvainen, M.P.; Ranta-Aho, P.; Karjalainen, P.A.; Partanen, J.; Karhu, J.; Lehtonen, J. Heart rate variability in acute psychosis. Psychophysiology 2003, 40, 716–726. [Google Scholar] [CrossRef]
- Schulz, S.; Tupaika, N.; Berger, S.; Haueisen, J.; Bär, K.-J.; Voss, A. Cardiovascular coupling analysis with high-resolution joint symbolic dynamics in patients suffering from acute schizophrenia. Physiol. Meas. 2013, 34, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.-J.; Rachow, T.; Schulz, S.; Bassarab, K.; Haufe, S.; Berger, S.; Koch, K.; Voss, A. The phrenic component of acute schizophrenia—A name and its physiological reality. PLoS ONE 2012, 7, e33459. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Bär, K.J.; Voss, A. Cardiovascular and cardiorespiratory coupling in unmedicated schizophrenic patients in comparison to healthy subjects. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3664–3667. [Google Scholar]
- Steffen, S.; Karl-Jürgen, B.; Haueisen, J.; Andreas, V. Quantification of cardiorespiratory coupling in acute schizophrenia applying high resolution joint symbolic dynamics. In Proceedings of the Computing in Cardiology Conference (CinC), Zaragoza, Spain, 22–25 September 2013; pp. 101–104. [Google Scholar]
- Schulz, S.; Bär, K.J.; Voss, A. Respiratory variability and cardiorespiratory coupling analyses in patients suffering from schizophrenia and their healthy first-degree relatives. Biomed. Tech. 2012, 57, 1044. [Google Scholar] [CrossRef]
- Peupelmann, J.; Boettger, M.K.; Ruhland, C.; Berger, S.; Ramachandraiah, C.T.; Yeragani, V.K.; Bär, K.-J. Cardio-respiratory coupling indicates suppression of vagal activity in acute schizophrenia. Schizophr. Res. 2009, 112, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Haueisen, J.; Bär, K.; Voss, A. Changed cardiorespiratory phase-coupling pattern in patients suffering from schizophrenia. Biomed. Tech. 2014, 59, S178. [Google Scholar]
- Aguirre, R.R.; Mustafa, M.Z.; Dumenigo, A.; Schulz, S.; Voss, A.; Goubran, B.; Dumenigo, R.; Sanchez-Gonzalez, M.A. Influence of Acute Antipsychotic Treatment on Cardiorespiratory Coupling and Heart Rate Variability. Cureus 2018, 10, e2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, S.; Bär, K.J.; Voss, A. Analyses of Heart Rate, Respiration and Cardiorespiratory Coupling in Patients with Schizophrenia. Entropy 2015, 17, 483–501. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Bolz, M.; Bär, K.-J.; Voss, A. Central- and autonomic nervous system coupling in schizophrenia. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150178. [Google Scholar] [CrossRef]
- Schulz, S.; Castro, M.R.; Giraldo, B.; Haueisen, J.; Bär, K.-J.; Voss, A. Multivariate high resolution joint symbolic dynamics (mHRJSD): A new tool to analyze Altered central-cardiovascular network pattern in neuropathological disease—application of the three dimensional high resolution joint symbolic dynamics. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; volume 44, pp. 260–475. [Google Scholar]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. A Math. Phys. Eng. Sci. 2008, 367, 277–296. [Google Scholar] [CrossRef]
- Cohen, M.A.; Taylor, J.A. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J. Physiol. 2002, 542, 669–683. [Google Scholar] [CrossRef]
- Eckberg, D.L. The human respiratory gate. J. Physiol. 2003, 548, 339–352. [Google Scholar] [CrossRef]
- Eckberg, D.L. Point:counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J. Appl. Physiol. 2009, 106, 1740–1742. [Google Scholar] [CrossRef]
- Gilbey, M.; Jordan, D.; Richter, D.; Spyer, K. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J. Physiol. 1984, 356, 65–78. [Google Scholar] [CrossRef]
- Triedman, J.K.; Saul, J.P. Blood pressure modulation by central venous pressure and respiration. Buffering effects of the heart rate reflexes. Circulation 1994, 89, 169–179. [Google Scholar] [CrossRef]
- Sin, P.Y.; Galletly, D.C.; Tzeng, Y.C. Influence of breathing frequency on the pattern of respiratory sinus arrhythmia and blood pressure: old questions revisited. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1588–H1599. [Google Scholar] [CrossRef]
- Angelone, A.; Coulter, J.R.N. Respiratory sinus arrhythmia: a frequency dependent phenomenon. J. Appl. Physiol. 1964, 19, 479–482. [Google Scholar] [CrossRef]
- Grossman, P.; Taylor, E.W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef]
- Bartsch, R.P.; Schumann, A.Y.; Kantelhardt, J.W.; Penzel, T.; Ivanov, P.C. Phase transitions in physiologic coupling. Proc. Natl. Acad. Sci. USA 2012, 109, 10181–10186. [Google Scholar] [CrossRef] [Green Version]
- Tonhajzerova, I.; Mestanik, M.; Mestanikova, A.; Jurko, A. Respiratory sinus arrhythmia as a non-invasive index of ‘brain-heart’ interaction in stress. Indian J. Med. Res. 2016, 144, 815–822. [Google Scholar]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef]
- McAllen, R.M. Proceedings: Inhibition of the baroreceptor input to the medulla by stimulation of the hypothalamic defence area. J. Physiol. 1976, 257, 45P–46P. [Google Scholar]
- Taylor, E.; Al-Ghamdi, M.; Ihmied, I.; Wang, T.; Abe, A.S. The neuranatomical basis of central control of cardiorespiratory interactions in vertebrates. Exp. Physiol. 2001, 86, 771–776. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Rea, P. Overview of the nervous system. In Essential Clinically Applied Anatomy of the Peripheral Nervous System in the Head and Neck; Chapter, 1, Rea, P., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–20. [Google Scholar]
- Shoemaker, J.K.; Norton, K.N.; Baker, J.; Luchyshyn, T. Forebrain organization for autonomic cardiovascular control. Auton. Neurosci. 2015, 188, 5–9. [Google Scholar] [CrossRef]
- Ziegler, G.; Dahnke, R.; Yeragani, V.K.; Bär, K.J. The relation of ventromedial prefrontal cortex activity and heart rate fluctuations at rest. Eur. J. Neurosci. 2009, 30, 2205–2210. [Google Scholar] [CrossRef]
- Beissner, F.; Meissner, K.; Bär, K.-J.; Napadow, V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013, 33, 10503–10511. [Google Scholar] [CrossRef]
- Anderson, N.B.; Bulatao, R.A.; Cohen, B.; on Race, P.; National Research Council. A neurovisceral integration model of health disparities in aging. In Critical Perspectives on Racial and Ethnic Differences in Health in Late Life; The National Academies Press: Washington, DC, USA, 2014; p. 752. [Google Scholar]
- Faes, L.; Marinazzo, D.; Jurysta, F.; Nollo, G. Linear and non-linear brain-heart and brain-brain interactions during sleep. Physiol. Meas. 2015, 36, 683–698. [Google Scholar] [CrossRef]
- Schulz, S.; Adochiei, F.-C.; Edu, I.-R.; Schroeder, R.; Costin, H.; Bär, K.-J.; Voss, A. Cardiovascular and cardiorespiratory coupling analyses: a review. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120191. [Google Scholar] [CrossRef] [Green Version]
- Marwan, N.; Zou, Y.; Wessel, N.; Riedl, M.; Kurths, J. Estimating coupling directions in the cardiorespiratory system using recurrence properties. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20110624. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, A.; Guaragnella, C.; Amoroso, N.; Monaco, A.; Fazio, L.; Taurisano, P.; Pergola, G.; Blasi, G.; Bertolino, A.; Bellotti, R. Modelling cognitive loads in schizophrenia by means of new functional dynamic indexes. Neuroimage 2019, 195, 150–164. [Google Scholar] [CrossRef]
- Novak, V.; Novak, P.; de Champlain, J.; Le Blanc, A.; Martin, R.; Nadeau, R. Influence of respiration on heart rate and blood pressure fluctuations. J. Appl. Physiol. 1993, 74, 617–626. [Google Scholar] [CrossRef]
- Bell, C.C. DSM-IV: Diagnostic and statistical manual of mental disorders. JAMA 1994, 272, 828–829. [Google Scholar] [CrossRef]
- LeBlanc, J.; Ducharme, M.B.; Thompson, M. Study on the correlation of the autonomic nervous system responses to a stressor of high discomfort with personality traits. Physiol. Behav. 2004, 82, 647–652. [Google Scholar] [CrossRef]
- Shanbao, T.; Thankor, N.V. Quantitative EEG Analysis Methods and Applications; Artech House: Boston, MA, USA, 2009. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Maiorana, E.; Solé-Casals, J.; Campisi, P. EEG signal preprocessing for biometric recognition. Mach. Vis. Appl. 2016, 27, 1351–1360. [Google Scholar] [CrossRef]
- Wessel, N.; Voss, A.; Malberg, H.; Ziehmann, C.; Voss, H.U.; Schirdewan, A.; Meyerfeldt, U.; Kurths, J. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmachertherapie und Elektrophysiologie 2000, 11, 159–173. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Lombardi, F. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Adochiei, F.; Schulz, S.; Edu, I.; Costin, H.; Voss, A. A New Normalised Short Time PDC for Dynamic Coupling Analyses. Biomed. Tech. 2013. [Google Scholar] [CrossRef]
- Montalto, A.; Faes, L.; Marinazzo, D. MuTE: A MATLAB toolbox to compare established and novel estimators of the multivariate transfer entropy. PLoS ONE 2014, 9, e109462. [Google Scholar] [CrossRef]
- Baccala, L.A.; Sameshima, K. Partial directed coherence: a new concept in neural structure determination. Biol. Cybern. 2001, 84, 463–474. [Google Scholar] [CrossRef]
- Milde, T.; Schwab, K.; Walther, M.; Eiselt, M.; Schelenz, C.; Voss, A.; Witte, H. Time-variant partial directed coherence in analysis of the cardiovascular system. A methodological study. Physiol. Meas. 2011, 32, 1787–1805. [Google Scholar] [CrossRef]
- Neumaier, A.; Schneider, T. Estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans. Math. Softw. 2001, 27, 27–57. [Google Scholar] [CrossRef]
- Schneider, T.; Neumaier, A. Algorithm 808: ARfit—A matlab package for the estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans. Math. Softw. 2001, 27, 58–65. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461–464. [Google Scholar] [CrossRef]
- Schreiber, T.; Schmitz, A. Surrogate time series. Phys. D 2000, 142, 346–382. [Google Scholar] [CrossRef] [Green Version]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Farmer, J.D. Testing for nonlinearity in time series: The method of surrogate data. Phys. D 1992, 58, 77–94. [Google Scholar] [CrossRef]
- Stankovski, T.; Ticcinelli, V.; McClintock, P.V.; Stefanovska, A. Neural Cross-Frequency Coupling Functions. Front. Syst. Neurosci. 2017, 11, 33. [Google Scholar] [CrossRef]
- de Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef]
- Ribolsi, M.; Daskalakis, Z.J.; Siracusano, A.; Koch, G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014, 8, 1010. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Collinson, S.L.; Bezerianos, A.; Sim, K. Reduced Hemispheric Asymmetry of Brain Anatomical Networks Is Linked to Schizophrenia: A Connectome Study. Cereb. Cortex 2017, 27, 602–615. [Google Scholar] [CrossRef]
- Foster, P.S.; Drago, V.; Ferguson, B.J.; Harrison, D.W. Cerebral moderation of cardiovascular functioning: A functional cerebral systems perspective. Clin. Neurophysiol. 2008, 119, 2846–2854. [Google Scholar] [CrossRef]
- Foster, P.S.; Harrison, D.W. Magnitude of cerebral asymmetry at rest: covariation with baseline cardiovascular activity. Brain Cogn. 2006, 61, 286–297. [Google Scholar] [CrossRef]
- Thayer, J.F. What the Heart Says to the Brain (and vice versa) and Why We Should Listen. Psychol. Top. 2007, 16, 241–250. [Google Scholar]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Levy, M.N. Neural control of cardiac function. Baillieres Clin. Neurol. 1997, 6, 227–244. [Google Scholar]
- Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Armour, J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: Functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1740–H1752. [Google Scholar] [CrossRef]
- Guyenet, P.G. Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 2014, 4, 1511–1562. [Google Scholar]
- Gourine, A.V.; Spyer, K. Autonomic Nervous System: Central Respiratory Control. In Encyclopedia of Neuroscience; pp. 883–890. Available online: http://discovery.ucl.ac.uk/148767/ (accessed on 23 July 2019).
- Turner, D.L. Cardiovascular and respiratory control mechanisms during exercise: an integrated view. J. Exp. Biol. 1991, 160, 309–340. [Google Scholar]
- Schulz, S.; Haueisen, J.; Bär, K.-J.; Voss, A. Antipsychotic medication influences cardiovascular coupling in patients suffering from acute schizophrenia. In Computing in Cardiology; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Agelink, M.W.; Majewski, T.; Wurthmann, C.; Lukas, K.; Ullrich, H.; Linka, T.; Klieser, E. Effects of newer atypical antipsychotics on autonomic neurocardiac function: A comparison between amisulpride, olanzapine, sertindole, and clozapine. J. Clin. Psychopharmacol. 2001, 21, 8–13. [Google Scholar] [CrossRef]
- Bär, K.-J.; Koschke, M.; Berger, S.; Schulz, S.; Tancer, M.; Voss, A.; Yeragani, V.K. Influence of olanzapine on QT variability and complexity measures of heart rate in patients with schizophrenia. J. Clin. Psychopharmacol. 2008, 28, 694–698. [Google Scholar] [CrossRef]
- Bär, K.J.; Boettger, M.K.; Voss, A. Differences between heart rate and blood pressure variability in schizophrenia. Biomed. Tech. 2006, 51, 237–239. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef]
- Horacek, J.; Bubenikova-Valesova, V.; Kopecek, M.; Palenicek, T.; Dockery, C.; Mohr, P.; Höschl, C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006, 20, 389–409. [Google Scholar] [CrossRef]
- Pérez-Neri, I.; Ramírez-Bermúdez, J.; Montes, S.; Ríos, C. Possible Mechanisms of Neurodegeneration in Schizophrenia. Neurochem. Res. 2006, 31, 1279–1294. [Google Scholar] [CrossRef]
- Ben-Shachar, D. Mitochondrial dysfunction in schizophrenia: A possible linkage to dopamine. J. Neurochem. 2002, 83, 1241–1251. [Google Scholar] [CrossRef]
- Homma, I.; Masaoka, Y. Breathing rhythms and emotions. Exp. Physiol. 2008, 93, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Masaoka, Y.; Homma, I. Anxiety and respiratory patterns: Their relationship during mental stress and physical load. Int. J. Psychophysiol. 1997, 27, 153–159. [Google Scholar] [CrossRef]
- Masaoka, Y.; Homma, I. Expiratory time determined by individual anxiety levels in humans. J. Appl. Physiol. 1999, 86, 1329–1336. [Google Scholar] [CrossRef]
- Masaoka, Y.; Homma, I. The effect of anticipatory anxiety on breathing and metabolism in humans. Respir. Physiol. 2001, 128, 171–177. [Google Scholar] [CrossRef]
- Tislerova, B.; Brunovsky, M.; Horacek, J.; Novak, T.; Kopecek, M.; Mohr, P.; Krajca, V. LORETA functional imaging in antipsychotic-naive and olanzapine-, clozapine- and risperidone-treated patients with schizophrenia. Neuropsychobiology 2008, 58, 1–10. [Google Scholar] [CrossRef]
- Maccrimmon, D.; Brunet, D.; Criollo, M.; Galin, H.; Lawson, J. Clozapine augments delta, theta, and right frontal EEG alpha power in schizophrenic patients. ISRN Psychiatry 2012, 2012, 596486. [Google Scholar] [CrossRef]
- Small, J.; Milstein, V.; Small, I.; Miller, M.; Kellams, J.; Corsaro, C. Computerized EEG profiles of haloperidol, chlorpromazine, clozapine and placebo in treatment resistant schizophrenia. Clin. Electroencephalogr. 1987, 18, 124–135. [Google Scholar]
- Knott, V.; Labelle, A.; Jones, B.; Mahoney, C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophr. Res. 2001, 50, 41–53. [Google Scholar] [CrossRef]
- Swenne, C.A. Baroreflex sensitivity: Mechanisms and measurement. Neth. Heart J. 2013, 21, 58–60. [Google Scholar] [CrossRef]
- Nosaka, S. Modifications of arterial baroreflexes: Obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn. J. Physiol. 1996, 46, 271–288. [Google Scholar] [CrossRef]
- Steptoe, A.; Sawada, Y. Assessment of baroreceptor reflex function during mental stress and relaxation. Psychophysiology 1989, 26, 140–147. [Google Scholar] [CrossRef]
- Foster, P.S.; Harrison, D.W. The covariation of cortical electrical activity and cardiovascular responding. Int. J. Psychophysiol. 2004, 52, 239–255. [Google Scholar] [CrossRef]
- Wittling, W.; Block, A.; Schweiger, E.; Genzel, S. Hemisphere asymmetry in sympathetic control of the human myocardium. Brain Cogn. 1998, 38, 17–35. [Google Scholar] [CrossRef]
- Tucker, D.M. Lateral brain function, emotion, and conceptualization. Psychol. Bull. 1981, 89, 19–46. [Google Scholar] [CrossRef]
- Dick, T.E.; Hsieh, Y.-H.; Dhingra, R.R.; Baekey, D.M.; Galán, R.F.; Wehrwein, E.; Morris, K.F. Cardiorespiratory coupling: Common rhythms in cardiac, sympathetic, and respiratory activities. Prog. Brain Res. 2014, 209, 191–205. [Google Scholar]
- Porta, A.; Bassani, T.; Bari, V.; Tobaldini, E.; Takahashi, A.C.; Catai, A.M.; Montano, N. Model-based assessment of baroreflex and cardiopulmonary couplings during graded head-up tilt. Comput. Biol. Med. 2012, 42, 298–305. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Information domain approach to the investigation of cardio-vascular, cardio-pulmonary, and vasculo-pulmonary causal couplings. Front. Physiol. 2011, 2, 80. [Google Scholar] [CrossRef]
- Williams, L.M.; Das, P.; Harris, A.W.; Liddell, B.B.; Brammer, M.J.; Olivieri, G.; Skerrett, D.; Phillips, M.L.; David, A.S.; Peduto, A. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am. J. Psychiatry 2004, 161, 480–489. [Google Scholar] [CrossRef]
- Callicott, J.H.; Mattay, V.S.; Verchinski, B.A.; Marenco, S.; Egan, M.F.; Weinberger, D.R. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am. J. Psychiatry 2003, 160, 2209–2215. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Henry, B.L.; Minassian, A.; Paulus, M.P.; Geyer, M.A.; Perry, W. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 2010, 44, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, P.C.; Bartsch, R.P. Network Physiology: Mapping Interactions Between Networks of Physiologic Networks. In Networks of Networks: The Last Frontier of Complexity; D’Agostino, G., Scala, A., Eds.; Springer International Publishing: Cham, Germany, 2014; pp. 203–222. [Google Scholar]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers III, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- He, B.; Liu, Z. Multimodal functional neuroimaging: Integrating functional MRI and EEG/MEG. IEEE Rev. Biomed. Eng. 2008, 1, 23–40. [Google Scholar]
- Porta, A.; Faes, L. Wiener-Granger Causality in Network Physiology with Applications to Cardiovascular Control and Neuroscience. Proc. IEEE 2016, 104, 282–309. [Google Scholar] [CrossRef]



| Index | CON | SZO | |||||
|---|---|---|---|---|---|---|---|
| mean | ± | std | mean | ± | std | ||
| HRV | meanNNBBI | 904.2 | ± | 153.0 | 709.4 | ± | 104.7 *** |
| sdNNBBI | 52.0 | ± | 23.0 | 32.3 | ± | 23.4 * | |
| BPV | meanNNSYS | 134.9 | ± | 19.8 | 121.4 | ± | 15.4 * |
| sdNNSYS | 9.2 | ± | 3.0 | 10.0 | ± | 6.8 | |
| meanNNDIA | 69.8 | ± | 12.8 | 66.7 | ± | 12.2 | |
| sdNNDIA | 0.8 | ± | 0.9 | 2.5 | ± | 3.6 | |
| RESPV | meanNNRESP | 4.0 | ± | 1.1 | 3.7 | ± | 0.8 |
| sdNNRESP | 0.7 | ± | 0.6 | 0.7 | ± | 0.4 | |
| BF | 16.2 | ± | 3.0 | 17.7 | ± | 3.5 | |
| EEG | P | 820.6 | ± | 860.8 | 357.8 | ± | 732.8 *** |
| Index | CON | SZO | |||||
|---|---|---|---|---|---|---|---|
| mean | ± | std | mean | ± | std | ||
| BBISYS | NF | −0.66 | ± | 0.52 | −0.48 | ± | 0.81 *** |
| ABBI→SYS(PEEG) | 0.25 | ± | 0.06 | 0.27 | ± | 0.14 | |
| ASYS→BBI(PEEG) | 0.43 | ± | 0.14 | 0.39 | ± | 0.16 *** | |
| BBIPEEG | NF | −0.64 | ± | 0.86 | −0.81 | ± | 1.03 ** |
| ABBI→PEEG(SYS) | 0.10 | ± | 0.05 | 0.09 | ± | 0.06 * | |
| APEEG→BBI(SYS) | 0.23 | ± | 0.16 | 0.26 | ± | 0.17 * | |
| SYSPEEG | NF | 0.00 | ± | 1.07 | −0.70 | ± | 0.94 *** |
| ASYS→PEEG(BBI) | 0.13 | ± | 0.07 | 0.10 | ± | 0.06 *** | |
| APEEG→SYS(BBI) | 0.14 | ± | 0.10 | 0.20 | ± | 0.13 *** | |
| Index | CON | SZO | |||||
|---|---|---|---|---|---|---|---|
| mean | ± | std | mean | ± | std | ||
| BBISYS | BBI→SYS(PEEG) | 0.098 | ± | 0.035 | 0.064 | ± | 0.042 *** |
| SYS→BBI(PEEG) | 0.053 | ± | 0.034 | 0.093 | ± | 0.037 *** | |
| BBIPEEG | BBI→PEEG(SYS) | 0.012 | ± | 0.011 | 0.007 | ± | 0.009 *** |
| PEEG→BBI(SYS) | 0.012 | ± | 0.009 | 0.007 | ± | 0.008 *** | |
| SYSPEEG | SYS→PEEG(BBI) | 0.012 | ± | 0.011 | 0.006 | ± | 0.008 *** |
| PEEG→SYS(BBI) | 0.008 | ± | 0.008 | 0.006 | ± | 0.008 *** | |
| Index | CON | SZO | |||||
|---|---|---|---|---|---|---|---|
| mean | ± | std | mean | ± | std | ||
| BBIRESP | NF | −1.56 | ± | 0.34 | −1.48 | ± | 0.69 |
| ABBI→RESP(PEEG) | 0.05 | ± | 0.02 | 0.04 | ± | 0.03 *** | |
| ARESP→BBI(PEEG) | 0.25 | ± | 0.08 | 0.27 | ± | 0.17 | |
| BBIPEEG | NF | −0.48 | ± | 0.76 | −0.13 | ± | 0.91 *** |
| ABBI→PEEG(RESP) | 0.10 | ± | 0.05 | 0.12 | ± | 0.06 *** | |
| APEEG→BBI(RESP) | 0.16 | ± | 0.08 | 0.15 | ± | 0.07 * | |
| RESPPEEG | NF | 0.99 | ± | 0.66 | 1.26 | ± | 0.62 *** |
| ARESP→PEEG(BBI) | 0.19 | ± | 0.07 | 0.24 | ± | 0.11 *** | |
| APEEG→RESP(BBI) | 0.06 | ± | 0.03 | 0.05 | ± | 0.03 ***,# | |
| Index | CON | SZO | |||||
|---|---|---|---|---|---|---|---|
| mean | ± | std | mean | ± | std | ||
| BBIRESP | BBI→RESP(PEEG) | 0.020 | ± | 0.013 | 0.015 | ± | 0.012 *** |
| RESP→BBI(PEEG) | 0.033 | ± | 0.009 | 0.026 | ± | 0.012 *** | |
| BBIPEEG | BBI→PEEG(RESP) | 0.014 | ± | 0.011 | 0.012 | ± | 0.011 * |
| PEEG→BBI(RESP) | 0.016 | ± | 0.010 | 0.014 | ± | 0.010 | |
| RESPPEEG | RESP→PEEG(BBI) | 0.017 | ± | 0.010 | 0.014 | ± | 0.009 *** |
| PEEG→RESP(BBI) | 0.015 | ± | 0.008 | 0.012 | ± | 0.009 *** | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, S.; Haueisen, J.; Bär, K.-J.; Voss, A. Altered Causal Coupling Pathways within the Central-Autonomic-Network in Patients Suffering from Schizophrenia. Entropy 2019, 21, 733. https://doi.org/10.3390/e21080733
Schulz S, Haueisen J, Bär K-J, Voss A. Altered Causal Coupling Pathways within the Central-Autonomic-Network in Patients Suffering from Schizophrenia. Entropy. 2019; 21(8):733. https://doi.org/10.3390/e21080733
Chicago/Turabian StyleSchulz, Steffen, Jens Haueisen, Karl-Jürgen Bär, and Andreas Voss. 2019. "Altered Causal Coupling Pathways within the Central-Autonomic-Network in Patients Suffering from Schizophrenia" Entropy 21, no. 8: 733. https://doi.org/10.3390/e21080733
APA StyleSchulz, S., Haueisen, J., Bär, K. -J., & Voss, A. (2019). Altered Causal Coupling Pathways within the Central-Autonomic-Network in Patients Suffering from Schizophrenia. Entropy, 21(8), 733. https://doi.org/10.3390/e21080733




