Approximate Entropy of Brain Network in the Study of Hemispheric Differences
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Data Recordings and Preprocessing
2.3. Entropy Analysis
- The first sequence of length m is compared to all the other sequences of the same length point by point. Those sequences for which all points are within r of their corresponding point in the original sequence are counted. r is also known as similarity criterion, and more clearly is a tuning parameter used to identify a meaningful range in which fluctuations in data are similar. So, a point of a sequence is similar to its corresponding point in the original sequence, when its value is not above its original value plus r.
- The same process is applied to sequences of length m + 1, starting with the first sequence of m + 1 points.
- The amount of similar sequences for m + 1 long one is divided by the one resulting from m long sequences comparison. The natural logarithm of the ratio is taken.
- The process is repeated for all possible sequences.
- All logarithms results are summed and normalized for N, the total number of data samples, and m.
2.4. Statistical Evaluation
3. Results
Control Analyses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
References
- Ma, Y.; Shi, W.; Peng, C.-K.; Yang, A.C.C. Nonlinear dynamical analysis of sleep electroencephalography using fractal and entropy approaches. Sleep Med. Rev. 2018, 37, 85–93. [Google Scholar] [CrossRef]
- Ecoben, R.; Emohammad-Rezazadeh, I. Neural Connectivity in Epilepsy as Measured by Granger Causality. Front. Human Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Ferri, R.; Binetti, G.; Vecchio, F.; Frisoni, G.B.; Lanuzza, B.; Miniussi, C.; Nobili, F.; Rodriguez, G.; Rundo, F.; et al. Directionality of EEG synchronization in Alzheimer’s disease subjects. Neurobiol. Aging 2009, 30, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Vecchio, F.; Cappa, S.; Pasqualetti, P.; Rossi, S.; Miniussi, C.; Rossini, P.M. Functional frontoparietal connectivity during encoding and retrieval processes follows HERA model. Brain Res. Bull. 2006, 68, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Vecchio, F.; Babiloni, C.; Buffo, P.; Rossini, P.M.; Bertini, M. Inter-hemispherical functional coupling of EEG rhythms during the perception of facial emotional expressions. Clin. Neurophysiol. 2013, 124, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Eichele, T.; Calhoun, V.D.; Debener, S. Mining EEG–fMRI using independent component analysis. Int. J. Psychophysiol. 2009, 73, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhan, Q.; Yan, T.; Imtiaz, S.; Xiang, J.; Niu, Y.; Liu, M.; Wang, G.; Cao, R.; Li, D. Hemisphere and Gender Differences in the Rich-Club Organization of Structural Networks. Cereb. Cortex 2019, 29, 4889–4901. [Google Scholar] [CrossRef]
- Basar, E. Eeg-Brain Dynamics: Relation between EEG and Brain Evoked Potentials; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands; New York, NY, USA, 1980. [Google Scholar]
- Başar, E. Memory and Brain Dynamics: Oscillations Integrating Attention, Perception, Learning, and Memory; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Rosso, O.A. Entropy changes in brain function. Int. J. Psychophysiol. 2007, 64, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Durner, G.; Pala, A.; Federle, L.; Grolik, B.; Wirtz, C.R.; Coburger, J. Comparison of hemispheric dominance and correlation of evoked speech responses between functional magnetic resonance imaging and navigated transcranial magnetic stimulation in language mapping. J. Neurosurg. Sci. 2019, 63, 106–113. [Google Scholar] [CrossRef]
- Morillon, B.; Lehongre, K.; Frackowiak, R.S.J.; Ducorps, A.; Kleinschmidt, A.; Poeppel, D.; Giraud, A.-L. Neurophysiological origin of human brain asymmetry for speech and language. Proc. Natl. Acad. Sci. USA 2010, 107, 18688–18693. [Google Scholar] [CrossRef] [Green Version]
- Medina, Y.E.; Fernández, A.P.; Morris, D.M.; Canales-Rodríguez, E.J.; Haroon, H.A.; García-Pentón, L.; Augath, M.; García, L.G.; Logothetis, N.; Parker, G.J.; et al. Brain Hemispheric Structural Efficiency and Interconnectivity Rightward Asymmetry in Human and Nonhuman Primates. Cereb. Cortex 2010, 21, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; He, Y.; Shu, H.; Gong, G. Developmental Changes in Topological Asymmetry between Hemispheric Brain White Matter Networks from Adolescence to Young Adulthood. Cereb. Cortex 2016, 27, 2560–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuvel, M.P.V.D.; Sporns, O. Rich-Club Organization of the Human Connectome. J. Neurosci. 2011, 31, 15775–15786. [Google Scholar] [CrossRef]
- Heuvel, M.P.V.D.; Kahn, R.S.; Goñi, J.; Sporns, O. High-cost, high-capacity backbone for global brain communication. Proc. Natl. Acad. Sci. USA 2012, 109, 11372–11377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Verma, R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA 2013, 111, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Hagmann, P.; Cammoun, L.; Gigandet, X.; Meuli, R.; Honey, C.J.; Wedeen, V.J.; Sporns, O. Mapping the Structural Core of Human Cerebral Cortex. PLoS Biol. 2008, 6, e159. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Rossini, P.M. Connectome: Graph theory application in functional brain network architecture. Clin. Neurophysiol. Pr. 2017, 2, 206–213. [Google Scholar] [CrossRef]
- Clausius, R. Ueber eine veränderte Form des zweiten Hauptsatzes der mechanischen Wärmetheorie. Ann. Phys. 1854, 169, 481–506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.D.; Shen, B. Entropy for the Complexity of Physiological Signal Dynamics. Adv. Exp. Med. Biol. 2017, 1028, 39–53. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1963. [Google Scholar]
- Kolmogorov, A.N. A new metric invariant of transient dynamical systems and automorphisms in Lebesgue spaces. Dokl. Akad. Nauk SSSR 1958, 119, 861–864. [Google Scholar]
- Renyi, A. In Proceedings of the Fourth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Contributions to the Theory of Statistics; University of California Press: Berkeley, CA, USA, 1961; pp. 547–561. [Google Scholar]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale Entropy Analysis of Complex Physiologic Time Series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-C. Complexity and 1/f noise. A phase space approach. J. Phys. I 1991, 1, 971–977. [Google Scholar] [CrossRef]
- Bruhn, J.; Röpcke, H.; Rehberg, B.; Bouillon, T.; Hoeft, A. Electroencephalogram approximate entropy correctly classifies the occurrence of burst suppression pattern as increasing anesthetic drug effect. Anesthesiology 2000, 93, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Posener, J.A.; Debattista, C.; Veldhuis, J.D.; Province, M.A.; Williams, G.H.; Schatzberg, A.F. Process irregularity of cortisol and adrenocorticotropin secretion in men with major depressive disorder. Psychoneuroendocrinology 2004, 29, 1129–1137. [Google Scholar] [CrossRef]
- Pincus, S.M. Assessing Serial Irregularity and Its Implications for Health. Ann. N. Y. Acad. Sci. 2006, 954, 245–267. [Google Scholar] [CrossRef]
- Pincus, S.M.; Viscarello, R.R. Approximate entropy: A regularity measure for fetal heart rate analysis. Obstet. Gynecol. 1992, 79, 249–255. [Google Scholar]
- Bruhn, J.; Lehmann, L.E.; Röpcke, H.; Bouillon, T.W.; Hoeft, A. Shannon Entropy Applied to the Measurement of the Electroencephalographic Effects of Desflurane. Anesthesiology 2001, 95, 30–35. [Google Scholar] [CrossRef]
- Lee, G.M.H.; Efattinger, S.; Emouthon, A.-L.; Noirhomme, Q.; Huber, R. Electroencephalogram approximate entropy influenced by both age and sleep. Front. Aging Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneddon, R. The Tsallis entropy of natural information. Phys. A Stat. Mech. Its Appl. 2007, 386, 101–118. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 2002, 17, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, M.J.; Kilmartin, L.; Keane, M.; Collins, P.; Staff, R.; Kaiser, J.; Lai, R.; Upton, N. Electrophysiological entropy in younger adults, older controls and older cognitively declined adults. Brain Res. 2012, 1445, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmaso, D.; Longoni, A.M. Problems in the Assessment of Hand Preference. Cortex 1985, 21, 533–549. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Gorgoni, M.; Ferrara, M.; Iberite, F.; Bramanti, P.; De Gennaro, L.; Rossini, P.M. Cortical connectivity modulation during sleep onset: A study via graph theory on EEG data. Human Brain Mapp. 2017, 38, 5456–5464. [Google Scholar] [CrossRef] [Green Version]
- Miraglia, F.; Vecchio, F.; Marra, C.; Quaranta, D.; Alù, F.; Peroni, B.; Granata, G.; Judica, E.; Cotelli, M.; Rossini, P.M. Small World Index in Default Mode Network Predicts Progression from Mild Cognitive Impairment to Dementia. Int. J. Neural Syst. 2020, 30, 2050004. [Google Scholar] [CrossRef]
- Vecchio, F.; Tomino, C.; Miraglia, F.; Iodice, F.; Erra, C.; Di Iorio, R.; Judica, E.; Alù, F.; Fini, M.; Rossini, P.M. Cortical connectivity from EEG data in acute stroke: A study via graph theory as a potential biomarker for functional recovery. Int. J. Psychophysiol. 2019, 146, 133–138. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Quaranta, D.; Lacidogna, G.; Marra, C.; Rossini, P.M. Learning Processes and Brain Connectivity in A Cognitive-Motor Task in Neurodegeneration: Evidence from EEG Network Analysis. J. Alzheimer’s Dis. 2018, 66, 471–481. [Google Scholar] [CrossRef]
- Hoffmann, S.; Falkenstein, M. The Correction of Eye Blink Artefacts in the EEG: A Comparison of Two Prominent Methods. PLoS ONE 2008, 3, e3004. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, J.; Urrestarazu, E.; Valencia, M.; Alegre, M.; Malanda, A.; Viteri, C.; Artieda, J. Independent Component Analysis as a Tool to Eliminate Artifacts in EEG: A Quantitative Study. J. Clin. Neurophysiol. 2003, 20, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, L.; Castaldo, R.; Pecchia, L. On the use of approximate entropy and sample entropy with centre of pressure time-series. J. Neuroeng. Rehabil. 2018, 15, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abásolo, D.; Hornero, R.; Espino, P.; Poza, J.; Sánchez, C.I.; de la Rosa, R. Analysis of regularity in the EEG background activity of Alzheimer’s disease patients with Approximate Entropy. Clin. Neurophysiol. 2005, 116, 1826–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abásolo, D.; Escudero, J.; Hornero, R.; Gómez, C.; Espino, P. Approximate entropy and auto mutual information analysis of the electroencephalogram in Alzheimer’s disease patients. Med. Biol. Eng. Comput. 2008, 46, 1019–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burioka, N.; Miyata, M.; Cornélissen, G.; Halberg, F.; Takeshima, T.; Kaplan, D.T.; Suyama, H.; Endo, M.; Maegaki, Y.; Nomura, T.; et al. Approximate Entropy in the Electroencephalogram during Wake and Sleep. Clin. EEG Neurosci. 2005, 36, 21–24. [Google Scholar] [CrossRef]
- Sun, R.; Wong, W.-W.; Wang, J.; Tong, R.K.Y. Changes in Electroencephalography Complexity using a Brain Computer Interface-Motor Observation Training in Chronic Stroke Patients: A Fuzzy Approximate Entropy Analysis. Front. Human Neurosci. 2017, 11, 444. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Pachori, R.B.; Acharya, U.R. Application of Entropy Measures on Intrinsic Mode Functions for the Automated Identification of Focal Electroencephalogram Signals. Entropy 2015, 17, 669–691. [Google Scholar] [CrossRef]
- Eliang, Z.; Ewang, Y.; Esun, X.; Eli, D.; Voss, L.J.; Sleigh, J.W.; Ehagihira, S.; Li, X. EEG entropy measures in anesthesia. Front. Comput. Neurosci. 2015, 9, 16. [Google Scholar] [CrossRef]
- Aydin, S.; Saraoğlu, H.M.; Kara, S. Log Energy Entropy-Based EEG Classification with Multilayer Neural Networks in Seizure. Ann. Biomed. Eng. 2009, 37, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, M. ECG Classification Using Wavelet Packet Entropy and Random Forests. Entropy 2016, 18, 285. [Google Scholar] [CrossRef]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Entropy analysis of heart rate variability and its application to recognize major depressive disorder: A pilot study. Technol. Heal. Care 2019, 27, 407–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarà, M.; Pistoia, F.; Pasqualetti, P.; Sebastiano, F.; Onorati, P.; Rossini, P.M. Functional isolation within the cerebral cortex in the vegetative state: A nonlinear method to predict clinical outcomes. Neurorehabil Neural Repair 2011, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.L.; Snyder, A.Z.; Hacker, C.D.; Mitra, A.; Shimony, J.S.; Limbrick, D.D.; Raichle, M.E.; Smyth, M.D.; Leuthardt, E.C. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 13278–13283. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, J.R. Posterior parietal cortex. Curr. Biol. 2017, 27, R691–R695. [Google Scholar] [CrossRef] [Green Version]
- Jawabri, K.; Sharma, S. Physiology, Cerebral Cortex Functions; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Huff, T.; Mahabadi, N.; Tadi, P. Neuroanatomy, Visual Cortex; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Caillaud, M.; Bejanin, A.; Laisney, M.; Gagnepain, P.; Gaubert, M.; Viard, A.; Clochon, P.; De La Sayette, V.; Allain, P.; Eustache, F.; et al. Influence of emotional complexity on the neural substrates of affective theory of mind. Human Brain Mapp. 2019, 41, 139–149. [Google Scholar] [CrossRef]
- Chaudhry, F.B.; Raza, S.; Ahmad, U. Anton’s syndrome: A rare and unusual form of blindness. BMJ Case Rep. 2019, 12, e228103. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Zhang, H.; Li, X.; Zou, F.; Wang, Y.; Wu, X.; Zhang, H.; Meng, Z. The spontaneous activity and functional network of the occipital cortex is correlated with state anxiety in healthy adults. Neurosci. Lett. 2019, 715, 134596. [Google Scholar] [CrossRef]
- Luchins, D.J.; Meltzer, H.Y. A blind, controlled study of occipital cerebral asymmetry in schizophrenia. Psychiatry Res. 1983, 10, 87–95. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Heier, L.A.; Vannucci, S.J. Cerebral asymmetry during development using linear measures from MRI. Early Human Dev. 2019, 139, 104853. [Google Scholar] [CrossRef] [PubMed]
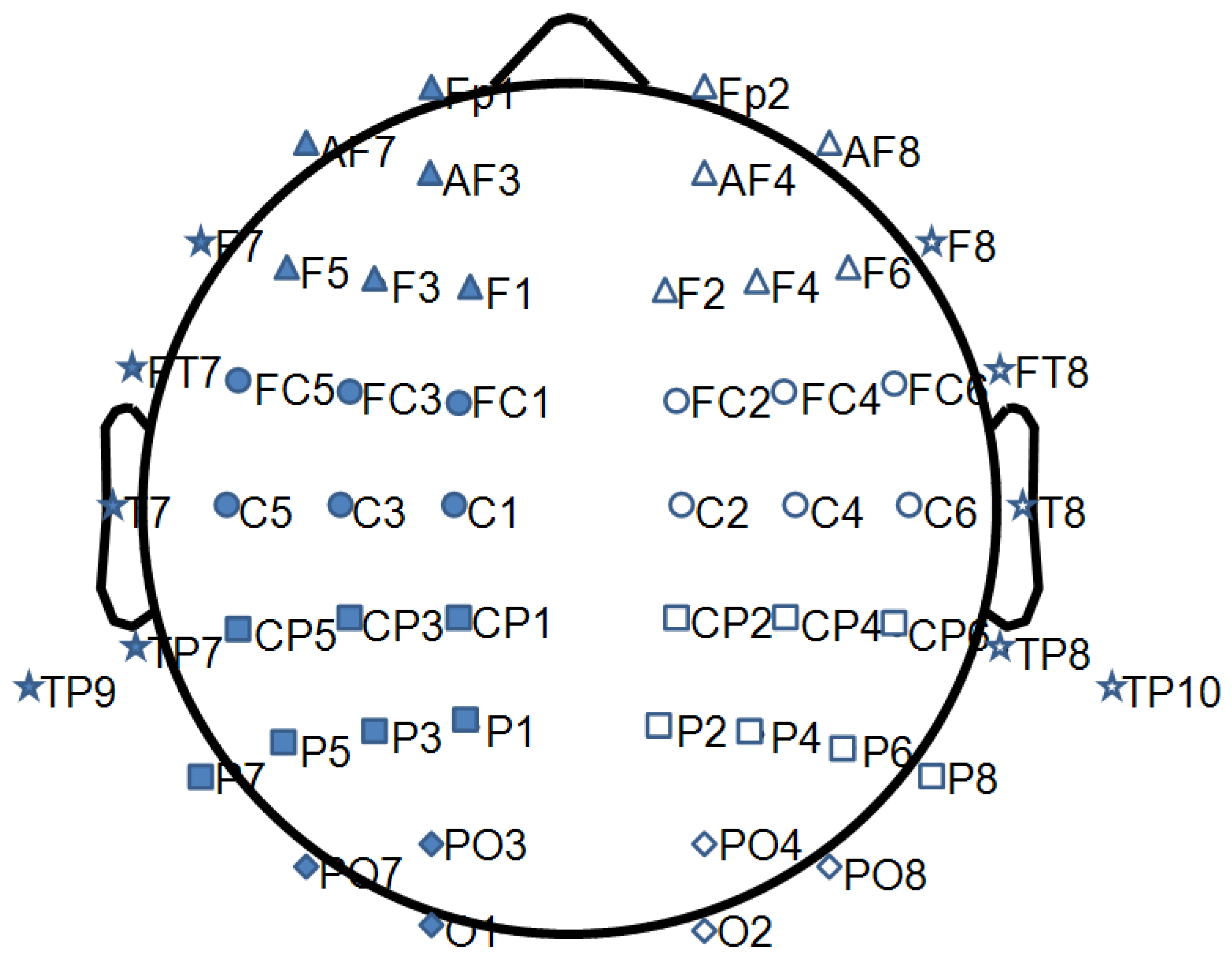


| Brain Region | Electrodes | ||||||
|---|---|---|---|---|---|---|---|
| Frontal Left | FP1 | AF3 | AF7 | F1 | F3 | F5 | |
| Frontal Right | FP2 | AF4 | AF8 | F2 | F4 | F6 | |
| Central Left | FC1 | FC3 | FC5 | C1 | C3 | C5 | |
| Central Right | FC2 | FC4 | FC6 | C2 | C4 | C6 | |
| Parietal Left | CP1 | CP3 | CP5 | P1 | P3 | P5 | P7 |
| Parietal Right | CP2 | CP4 | CP6 | P2 | P4 | P6 | P8 |
| Occipital Left | PO3 | PO7 | O1 | ||||
| Occipital Right | PO4 | PO8 | O2 | ||||
| Temporal Left | F7 | FT7 | T7 | TP7 | TP9 | ||
| Temporal Right | F8 | FT8 | T8 | TP8 | TP10 | ||
| Medial | FPz | Fz | Cz | CPz | Pz | POz | Oz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alù, F.; Miraglia, F.; Orticoni, A.; Judica, E.; Cotelli, M.; Rossini, P.M.; Vecchio, F. Approximate Entropy of Brain Network in the Study of Hemispheric Differences. Entropy 2020, 22, 1220. https://doi.org/10.3390/e22111220
Alù F, Miraglia F, Orticoni A, Judica E, Cotelli M, Rossini PM, Vecchio F. Approximate Entropy of Brain Network in the Study of Hemispheric Differences. Entropy. 2020; 22(11):1220. https://doi.org/10.3390/e22111220
Chicago/Turabian StyleAlù, Francesca, Francesca Miraglia, Alessandro Orticoni, Elda Judica, Maria Cotelli, Paolo Maria Rossini, and Fabrizio Vecchio. 2020. "Approximate Entropy of Brain Network in the Study of Hemispheric Differences" Entropy 22, no. 11: 1220. https://doi.org/10.3390/e22111220
APA StyleAlù, F., Miraglia, F., Orticoni, A., Judica, E., Cotelli, M., Rossini, P. M., & Vecchio, F. (2020). Approximate Entropy of Brain Network in the Study of Hemispheric Differences. Entropy, 22(11), 1220. https://doi.org/10.3390/e22111220







