Structure and Phase Composition of a W-Ta-Mo-Nb-V-Cr-Zr-Ti Alloy Obtained by Ball Milling and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
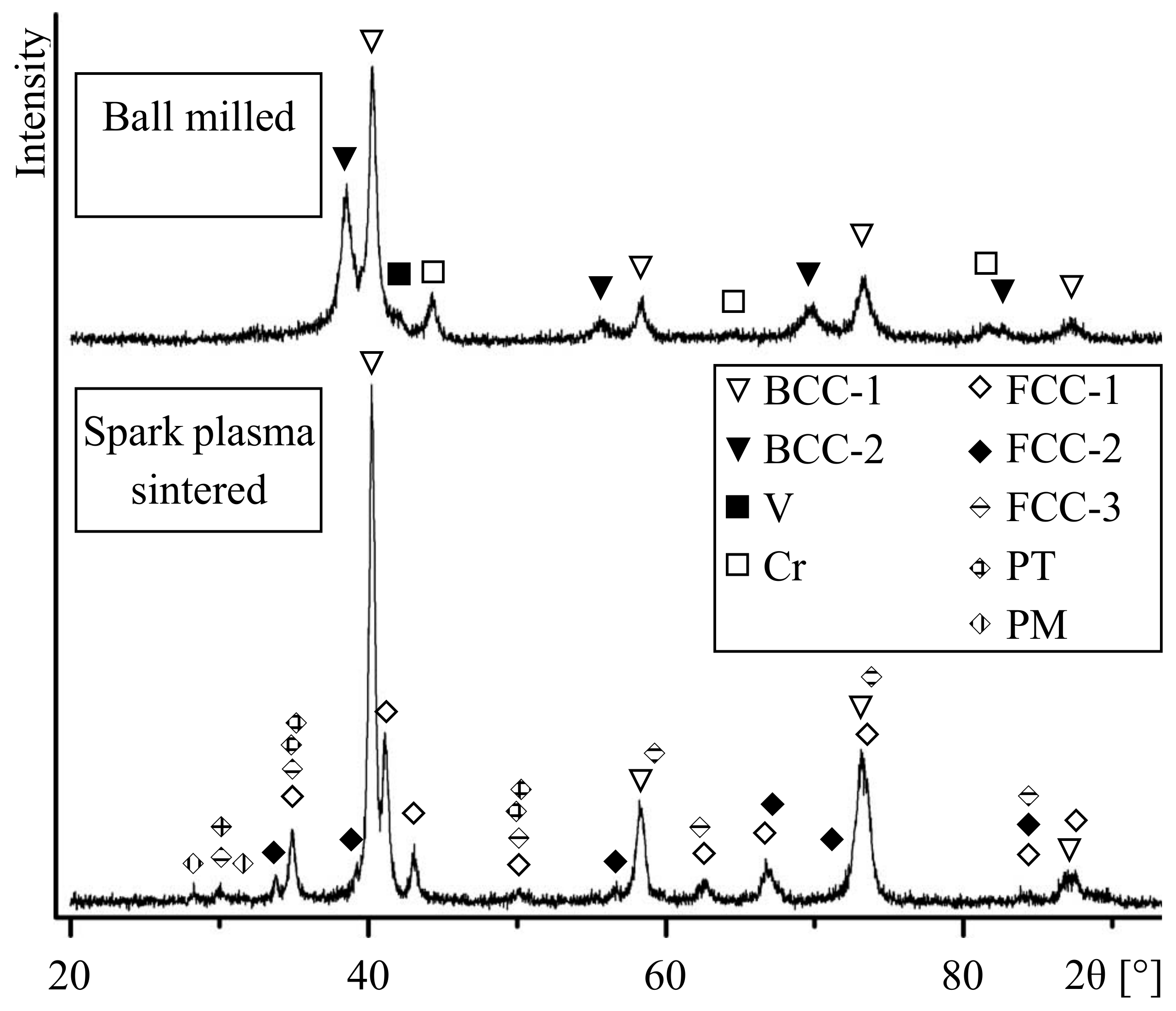
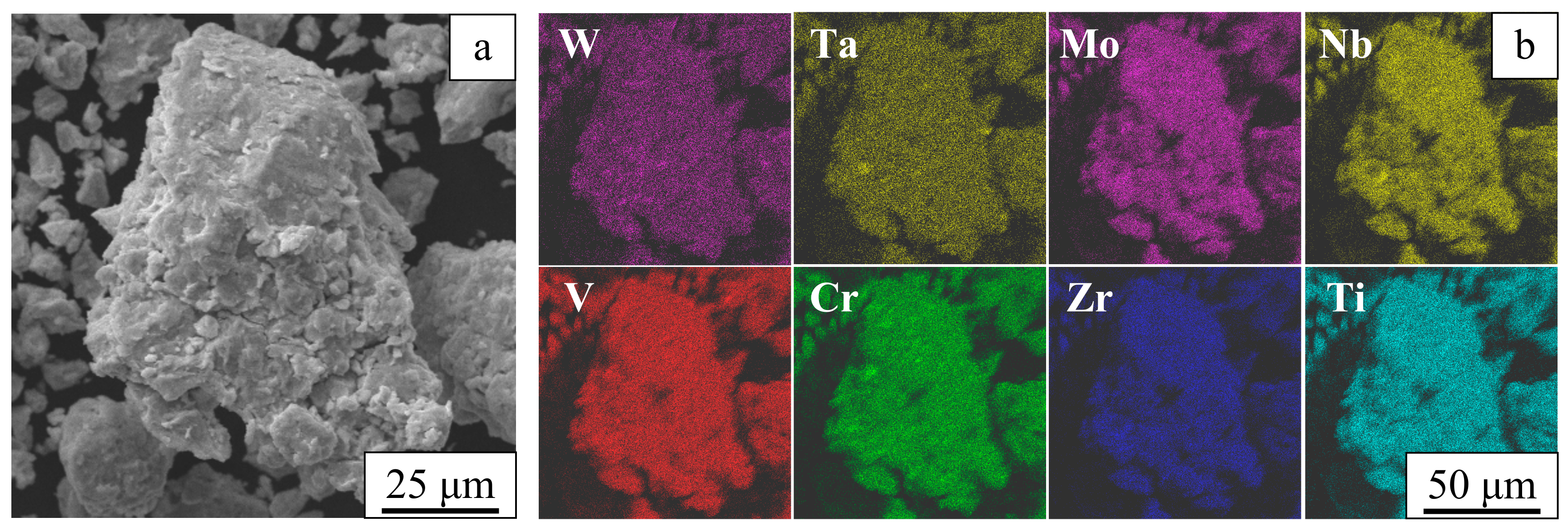
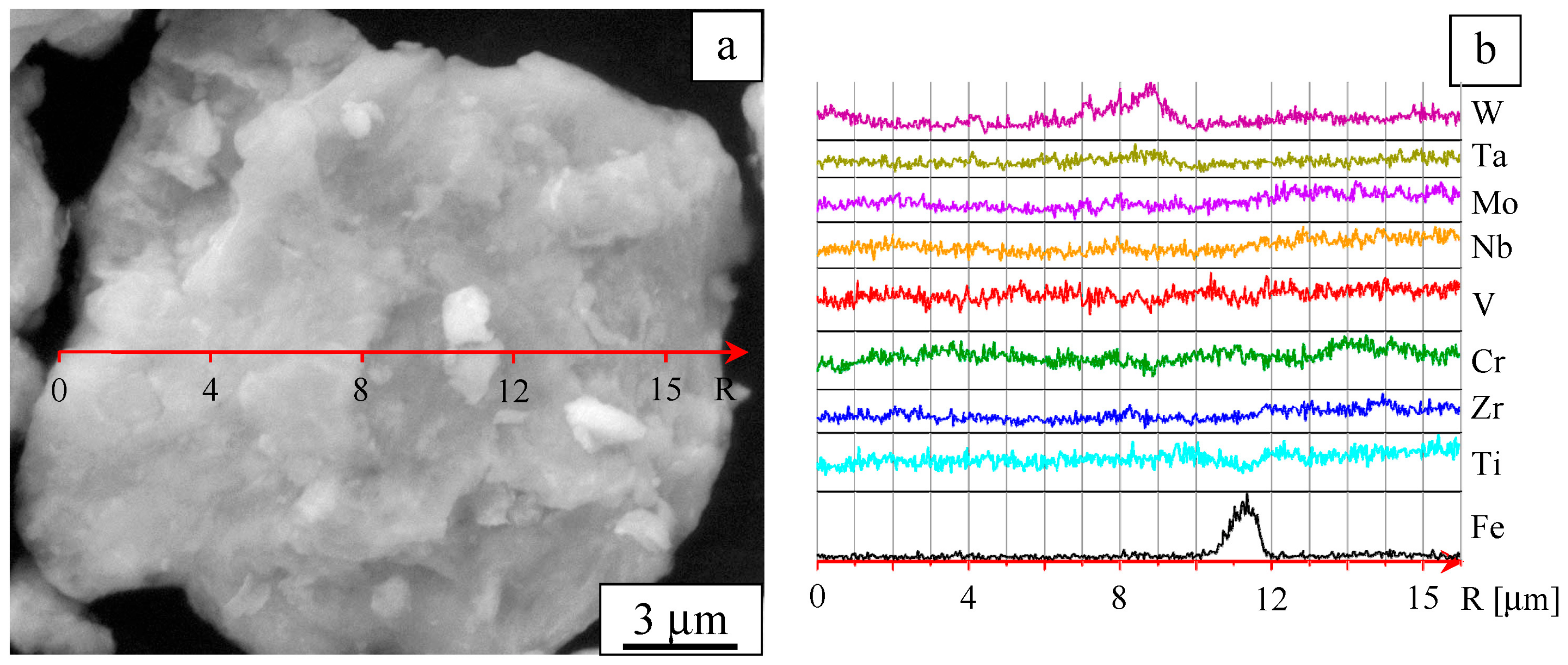
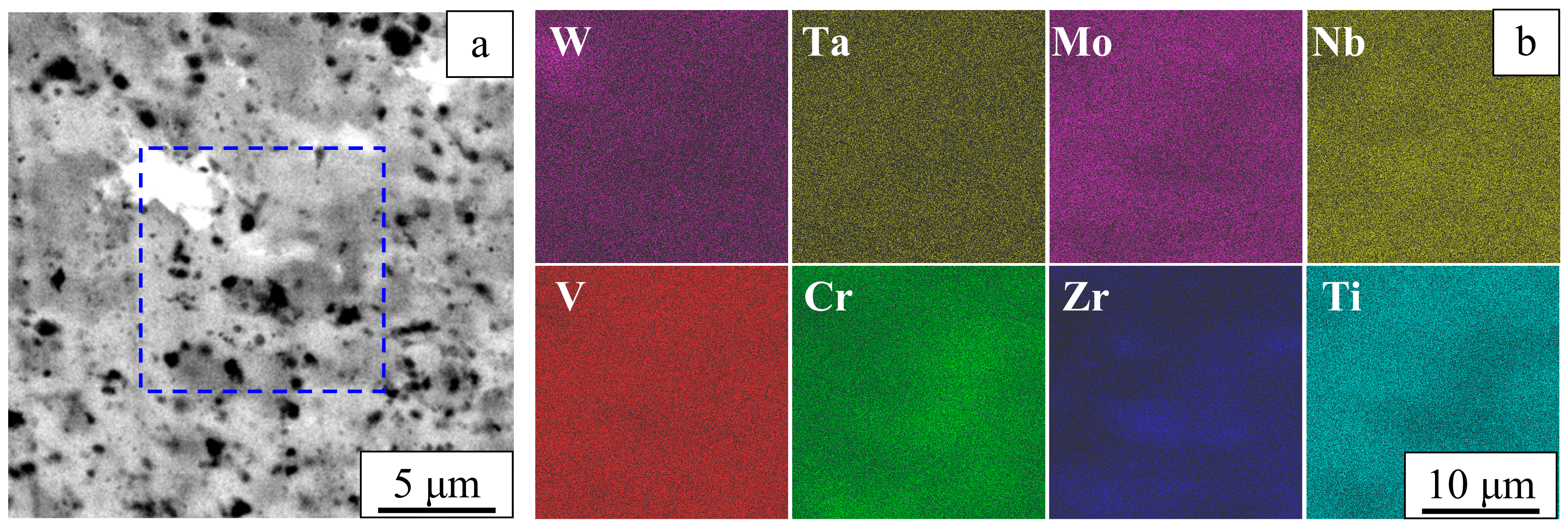
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Miracle, D.; Majumdar, B.; Wertz, K.; Gorsse, S. New strategies and tests to accelerate discovery and development of multi-principal element structural alloys. Scr. Mater. 2017, 127, 195–200. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinie, J.P.; Perriere, L.; Hocini, A.; Keller, C.; Dirras, G.; Guillot, I. Study of a bcc multi-principal element alloy: Tensile and simple shear properties and underlying deformation mechanisms. Acta Mater. 2018, 142, 131–141. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Jiang, H.; Guo, S.; Wang, T.; Cao, Z.; Li, T. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent and High Entropy Alloys. Entropy 2014, 16, 4749–4768. [Google Scholar] [CrossRef] [Green Version]
- Xian, X.; Zhong, Z.; Zhang, B.; Song, K.; Chen, C.; Wang, S.; Cheng, J.; Wu, Y. A high-entropy V35Ti35Fe15Cr10Zr5 alloy with excellent high-temperature strength. Mater. Des. 2017, 121, 229–236. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Hawk, J.A.; Zhou, H.F.; Chen, M.W.; Gao, M.C. Mechanical properties of refractory high-entropy alloys: Experiments and modeling. J. Alloys Compd. 2017, 696, 1139–1150. [Google Scholar] [CrossRef]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline high-entropy alloys: A new paradigm in high-temperature strength and stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Kaigorodova, L.I.; Rasposienko, D.Y.; Pushin, V.G.; Pilyugin, V.P.; Smirnov, S.V. Structure and mechanical properties of aging Al-Li-Cu-Zr-Sc-Ag alloy after severe plastic deformation by high-pressure torsion. Phys. Met. Metallogr. 2015, 116, 346–355. [Google Scholar] [CrossRef]
- Haas, S.; Manzoni, A.M.; Krieg, F.; Glatzel, U. Microstructure and mechanical properties of precipitate strengthened high entropy alloy Al10Co25Cr8Fe15Ni36Ti6 with additions of hafnium and molybdenum. Entropy 2019, 21, 169. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, A.M.; Singh, S.; Daoud, H.M.; Popp, R.; Völkl, R.; Glatzel, U.; Wanderka, N. On the path to optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti high entropy alloy family for high temperature applications. Entropy 2016, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Jang, J.S.C.; Nieh, T.G. Elastic and plastic deformations in a high entropy alloy investigated using a nanoindentation method. Intermetallics 2016, 68, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Sheng, G.U.O.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Liang, X.; Su, K.; Peng, H.; Li, X. A fine-grained NbMoTaWVCr refractory high-entropy alloy with ultra-high strength: Microstructural evolution and mechanical properties. J. Alloys Compd. 2019, 780, 607–617. [Google Scholar] [CrossRef]
- Long, Y.; Su, K.; Zhang, J.; Liang, X.; Peng, H.; Li, X. Enhanced strength of a mechanical alloyed NbMoTaWVTi refractory high entropy alloy. Materials 2018, 11, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Wang, Y.; Qiu, J. Microstructure and mechanical properties of WMoNbCrTi HEAs sintered from the powders milled for different durations. JOM 2019, 71, 2489–2497. [Google Scholar] [CrossRef]
- Pan, J.; Dai, T.; Lu, T.; Ni, X.; Dai, J.; Li, M. Microstructure and mechanical properties of Nb25Mo25Ta25W25 and Ti8Nb23Mo23Ta23W23 high entropy alloys prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2018, 738, 362–366. [Google Scholar] [CrossRef]
- Raman, L.; Guruvidyathri, K.; Kumari, G.; Murty, S.N.; Kottada, R.S.; Murty, B.S. Phase evolution of refractory high-entropy alloy CrMoNbTiW during mechanical alloying and spark plasma sintering. J. Mater. Res. 2019, 34, 756–766. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Powder metallurgy processing of a WxTaTiVCr high-entropy alloy and its derivative alloys for fusion material applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef]
- Couzinié, J.-P.; Dirras, G. Body-centered cubic high-entropy alloys: From processing to underlying deformation mechanisms. Mater. Charact. 2019, 147, 533–544. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Tokita, M. Development of large-size ceramic/metal bulk FGM fabricated by spark plasma sintering. Mater. Sci. Forum 1999, 308, 83–88. [Google Scholar] [CrossRef]
- Spark Plasma Sintering of Materials; Cavaliere, P. (Ed.) Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Kolobov, Y.R.; Valiev, R.Z.; Grabovetskaya, G.P.; Zhilyaev, A.P.; Dudarev, E.F.; Ivanov, K.V.; Ivanov, M.B.; Kashin, O.A.; Naidenkin, E.V. Grain Boundary Diffusion and Properties of Nanostructured Materials; Cambridge International Science Publishing: Cambridge, UK, 2007. [Google Scholar]
- Fultz, B.; Howe, J.M. Transmission Electron Microscopy and Diffractometry of Materials; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Binary Alloy Phase Diagrams; Massalski, T.B. (Ed.) ASM International: Materials Park, OH, USA, 1986. [Google Scholar]
- Vegard, L. Die konstitution der mischkristalle und die raumfüllung der atome. Zeitschrift Physik A Hadrons Nuclei 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Hirsch, P.B.; Howie, A.; Nicholson, R.B.; Pashley, D.W.; Whelan, M.J. Electron Microscopy of Thin Crystals; Butterworths: London, UK, 1965. [Google Scholar]
- Ditenberg, I.A.; Tyumentsev, A.N.; Denisov, K.I.; Korchagin, M.A. Peculiarities of the Formation of High-Defect States in Mechanocomposites and Powders of Niobium and Aluminum under Severe Deformation in Planetary Ball Mills. Phys. Mesomech. 2013, 16, 84–92. [Google Scholar] [CrossRef]
- Tyumentsev, A.N.; Ditenberg, I.A.; Korchagin, M.A. Effect of severe mechanical alloying on the microstructure parameters of 3Ti + Al mechanocomposites. Phys. Met. Metallogr. 2011, 111, 190–196. [Google Scholar] [CrossRef]
- Fromm, E.; Gebhardt, E. Gases and Carbon in Metals; Springer: Berlin, Germany, 1976. [Google Scholar]
- Martin, J.W. Micromechanisms in Particle-Hardened Alloys; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Embury, J.D. Plastic flow in dispersion hardened materials. Metall. Trans. A 1985, 16, 2191–2200. [Google Scholar] [CrossRef]
- Lloyd, D.J. Precipitation hardening. Strength Met. Alloys ICSMA 7 Pergamon 1986, 1745–1778. [Google Scholar] [CrossRef]
- Cutler, R.A.; Reynolds, J.R.; Jones, A. Sintering and characterization of polycrystalline monoclinic, tetragonal, and cubic zirconia. J. Am. Ceram. Soc. 1992, 75, 2173–2183. [Google Scholar] [CrossRef]
- Zhao, X.S.; Shang, S.L.; Liu, Z.K.; Shen, J.Y. Elastic properties of cubic, tetragonal and monoclinic ZrO2 from first-principles calculations. J. Nucl. Mater. 2011, 415, 13–17. [Google Scholar] [CrossRef]
- Blumenthal, W.B. The Chemical Behavior of Zirconium; Van Nostrand: New York, NY, USA, 1958. [Google Scholar]
- Morokhov, I.D.; Petinov, V.I.; Trusov, L.I.; Petrunin, V.F. Structure and properties of fine metallic particles. Soviet Phys. Uspekhi 1981, 24, 295–317. [Google Scholar] [CrossRef]
- Chernov, V.M.; Potapenko, M.M.; Drobyshev, V.A.; Kravtsova, M.V.; Tyumentsev, A.N.; Ovchinnikov, S.V.; Ditenberg, I.A.; Pinzhin, Y.P.; Korotaev, A.D.; Smirnov, I.V.; et al. Microstructure and mechanical properties of V-Me(Cr,W)-Zr alloys as a function of their chemical-thermal treatment. Nucl. Mater. Energy 2015, 3, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Ditenberg, I.A.; Tyumentsev, A.N.; Smirnov, I.V.; Grinyaev, K.V.; Chernov, V.M. Thermal stability of nanostructured internally oxidized vanadium alloy with combined dispersion and substructural hardening. Phys. Mesomech. 2019, 22, 496–503. [Google Scholar] [CrossRef]
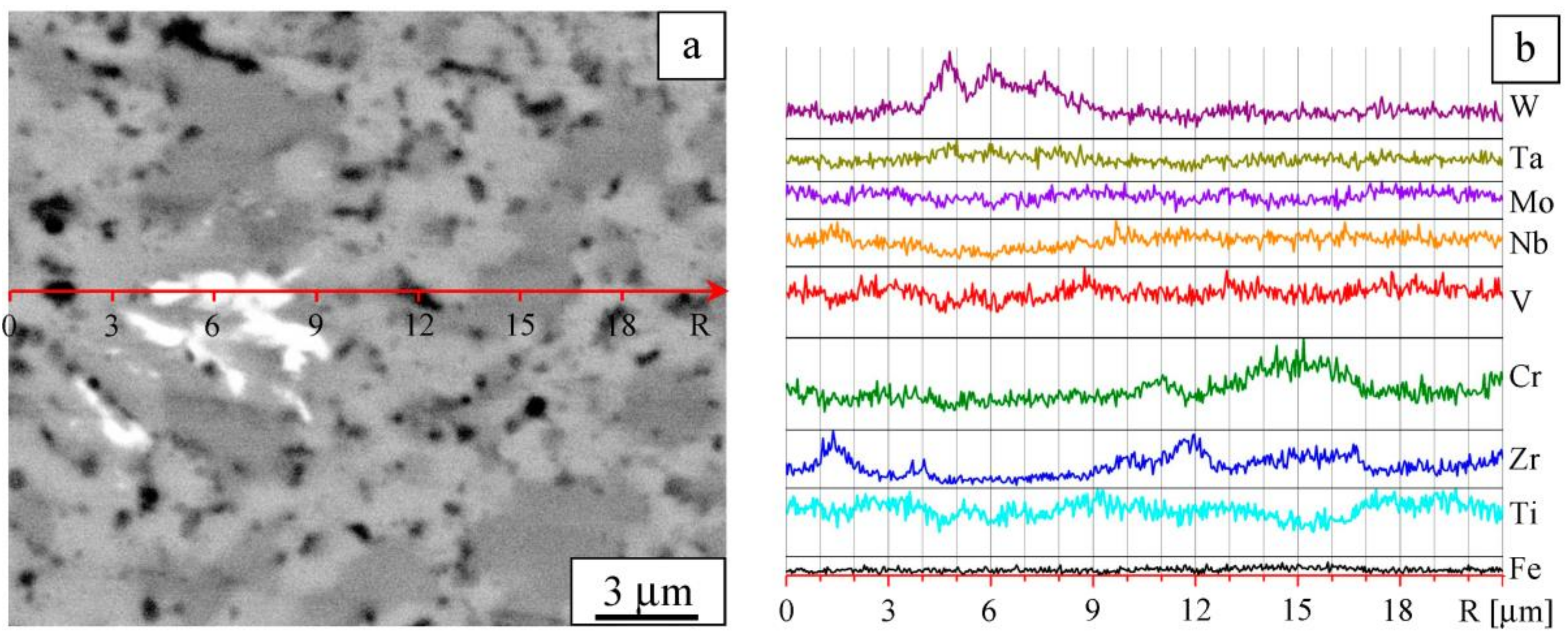
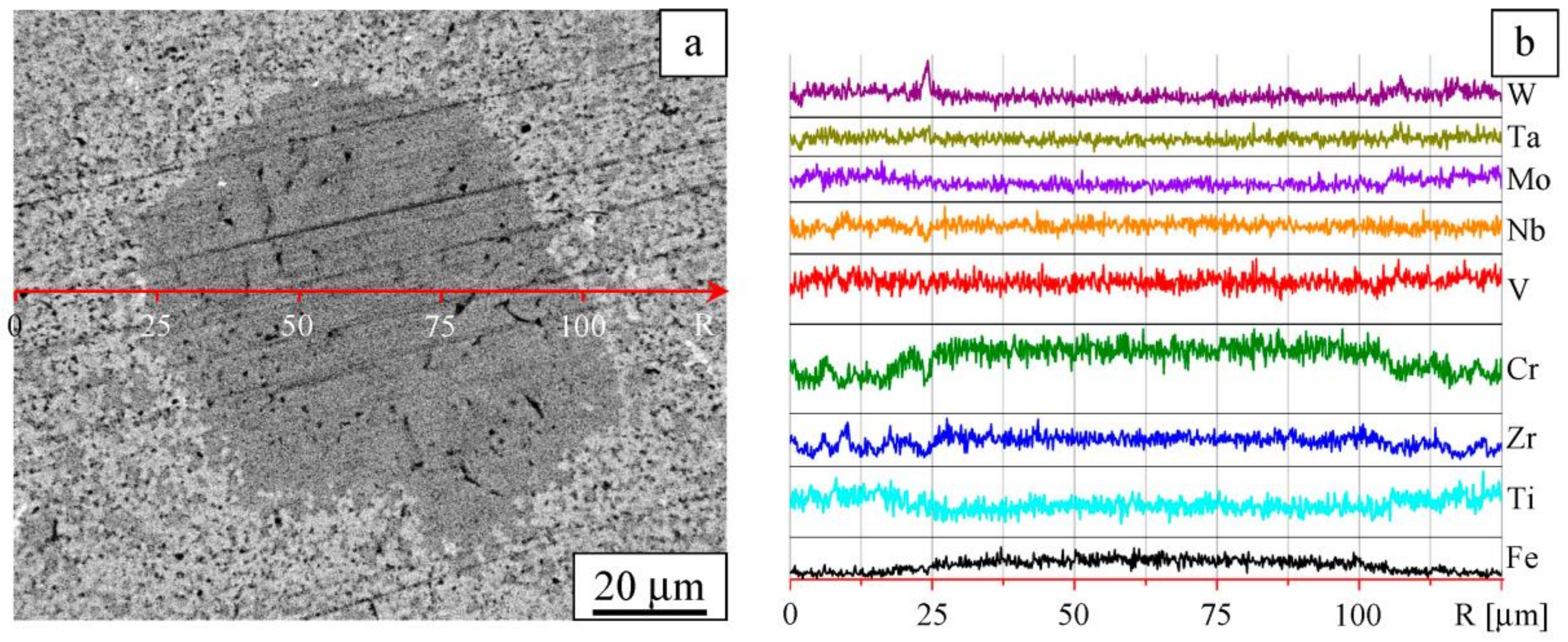
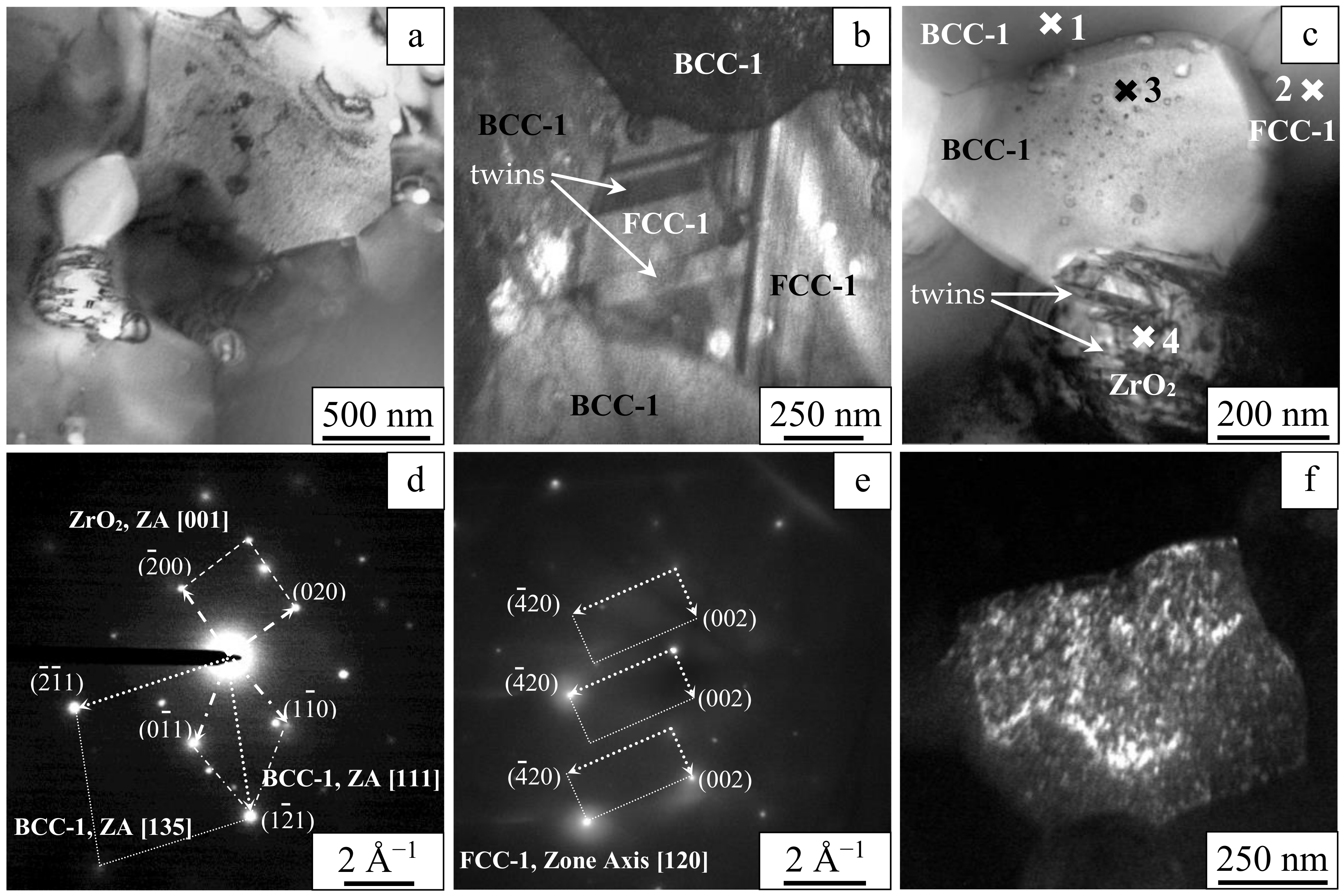
| Composition | W | Ta | Mo | Nb | Zr | Cr | V | Ti |
|---|---|---|---|---|---|---|---|---|
| at% | 5.3 | 5.4 | 10.1 | 10.5 | 10.7 | 18.7 | 19.1 | 20.3 |
| Area | W | Ta | Mo | Nb | Zr | Cr | V | Ti |
|---|---|---|---|---|---|---|---|---|
| 1 (BCC-1) | 9 | 8 | 13 | 14 | 3 | 13 | 17 | 23 |
| 2 (FCC-1) | 6 | 8 | 5 | 9 | 16 | 29 | 16 | 11 |
| 3 (FCC-2, FCC-3) | 2 | 3 | 1 | 3 | 78 | 2 | 1 | 10 |
| 4 (FCC-3) | 3 | 5 | 0 | 4 | 81 | 3 | 2 | 2 |
| Processing | Hμ (GPa) |
|---|---|
| Ball-milled | 9.28 ± 1.31 |
| Spark Plasma Sintered | 8.95 ± 0.42 |
| System | Processing | Hμ (GPa) | Reference |
|---|---|---|---|
| W-Ta-Mo-Nb-V-Cr-Zr-Ti | 10.5 min milling and SPS (1250 °C) | 8.95 | This work |
| W-Ta-Mo-Nb-V-Cr | 40 h milling and SPS (1400–1700 °C) | 10.52 | [20] |
| WMoNbCrTi | 5–40 h milling and SPS (1400 °C) | 10.40 | [23] |
| W-Mo-Nb-Cr-Ti | 7–10 h milling and SPS (1100–1300 °C) | 8.9 | [25] |
| W0.3(TaTiCrV)0.7 | 3 h milling and SPS (1600 °C) | 8.4 (790 Hv) | [28] |
| W-Ta-Mo-Nb | 24 h milling and SPS (1600 °C) | 7.78 | [24] |
| NbMoTaWVTi | 40 h milling and SPS (1400 °C) | – | [21] |
| WNbMoTaV | 6 h milling and SPS (1500–1700 °C) | – | [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditenberg, I.A.; Smirnov, I.V.; Korchagin, M.A.; Grinyaev, K.V.; Melnikov, V.V.; Pinzhin, Y.P.; Gavrilov, A.I.; Esikov, M.A.; Mali, V.I.; Dudina, D.V. Structure and Phase Composition of a W-Ta-Mo-Nb-V-Cr-Zr-Ti Alloy Obtained by Ball Milling and Spark Plasma Sintering. Entropy 2020, 22, 143. https://doi.org/10.3390/e22020143
Ditenberg IA, Smirnov IV, Korchagin MA, Grinyaev KV, Melnikov VV, Pinzhin YP, Gavrilov AI, Esikov MA, Mali VI, Dudina DV. Structure and Phase Composition of a W-Ta-Mo-Nb-V-Cr-Zr-Ti Alloy Obtained by Ball Milling and Spark Plasma Sintering. Entropy. 2020; 22(2):143. https://doi.org/10.3390/e22020143
Chicago/Turabian StyleDitenberg, Ivan A., Ivan V. Smirnov, Michail A. Korchagin, Konstantin V. Grinyaev, Vladlen V. Melnikov, Yuriy P. Pinzhin, Alexander I. Gavrilov, Maksim A. Esikov, Vyacheslav I. Mali, and Dina V. Dudina. 2020. "Structure and Phase Composition of a W-Ta-Mo-Nb-V-Cr-Zr-Ti Alloy Obtained by Ball Milling and Spark Plasma Sintering" Entropy 22, no. 2: 143. https://doi.org/10.3390/e22020143
APA StyleDitenberg, I. A., Smirnov, I. V., Korchagin, M. A., Grinyaev, K. V., Melnikov, V. V., Pinzhin, Y. P., Gavrilov, A. I., Esikov, M. A., Mali, V. I., & Dudina, D. V. (2020). Structure and Phase Composition of a W-Ta-Mo-Nb-V-Cr-Zr-Ti Alloy Obtained by Ball Milling and Spark Plasma Sintering. Entropy, 22(2), 143. https://doi.org/10.3390/e22020143






