Impact of General Anesthesia Guided by State Entropy (SE) and Response Entropy (RE) on Perioperative Stability in Elective Laparoscopic Cholecystectomy Patients—A Prospective Observational Randomized Monocentric Study
Abstract
:1. Introduction
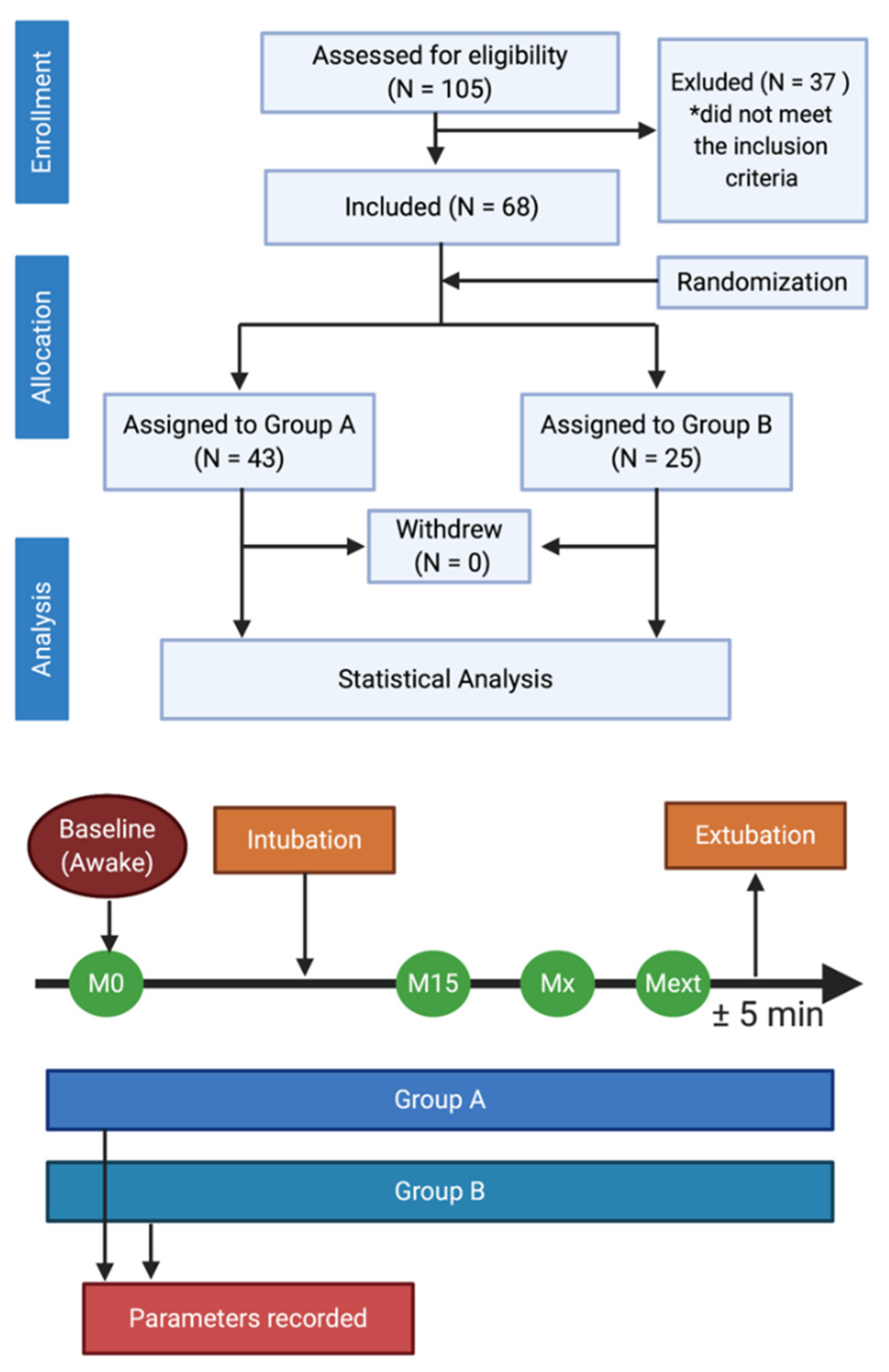
2. Materials and Methods
2.1. Study Population
2.2. Measurements and Data Management
2.3. General Anesthesia and Monitoring
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographical Characteristics
3.2. State Entropy and Response Entropy Expression
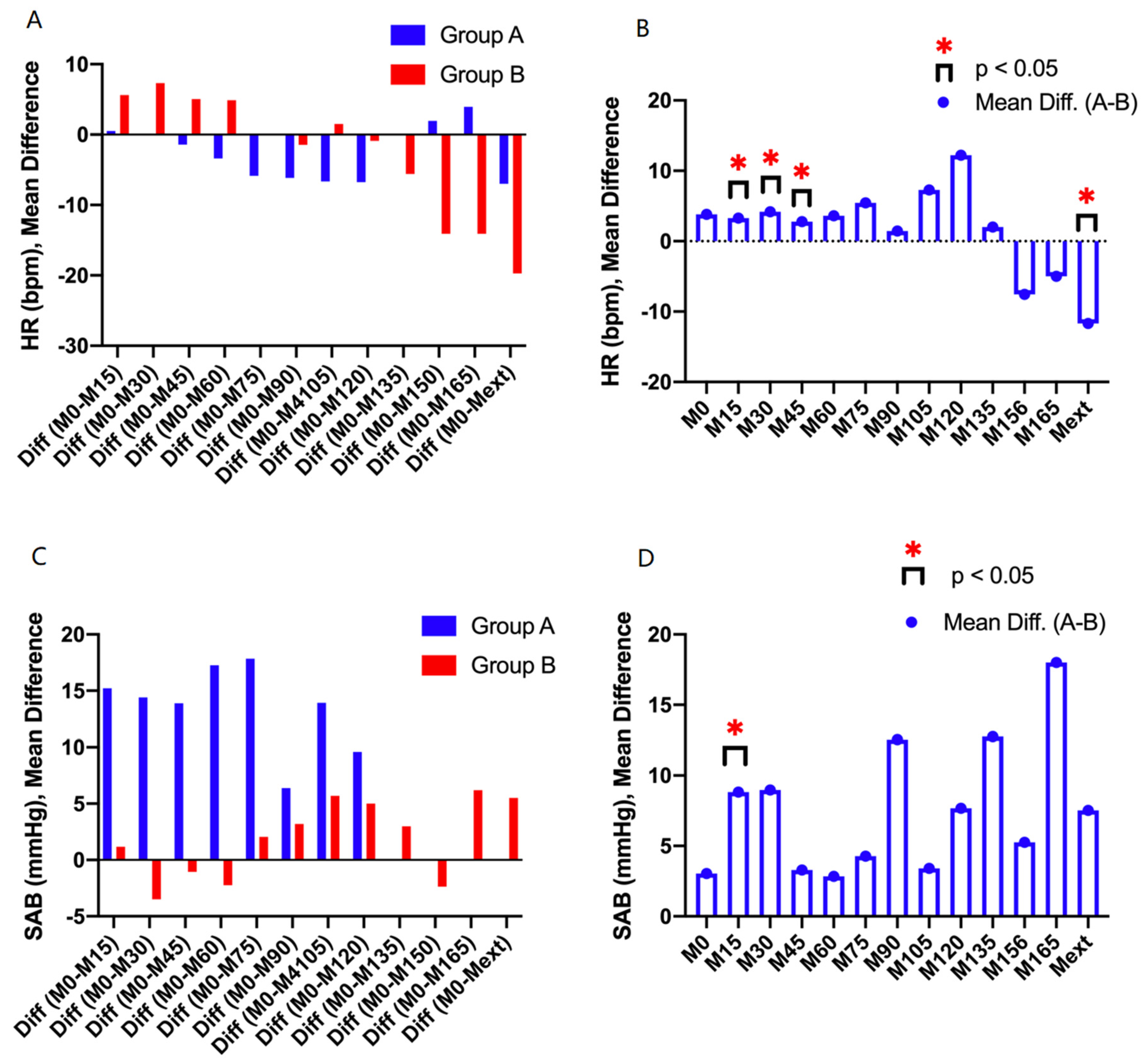
3.3. Hemodynamic Stability During Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanakala, V.; Borowski, D.W.; Pellen, M.G.C.; Dronamraju, S.S.; Woodcock, S.A.A.; Seymour, K.; Attwood, S.E.A.; Horgan, L.F. Risk factors in laparoscopic cholecystectomy: A multivariate analysis. Int. J. Surg. 2011, 9, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Ellakany, M. Comparative study between general and thoracic spinal anesthesia for laparoscopic cholecystectomy. Egypt. J. Anaesth. 2013, 29, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Wycherley, A.S.; Bembridge, J.L. Monitoring techniques; neuromuscular blockade and depth of anaesthesia. Anaesth. Intensive Care Med. 2017, 18, 324–327. [Google Scholar] [CrossRef]
- Ozdogan, H.K.; Cetinkunar, S.; Karateke, F.; Cetinalp, S.; Celik, M.; Ozyazici, S. The effects of sevoflurane and desflurane on the hemodynamics and respiratory functions in laparoscopic sleeve gastrectomy. J. Clin. Anesth. 2016, 35, 441–445. [Google Scholar] [CrossRef]
- Rogobete, A.F.; Bedreag, O.H.; Sandesc, D. Entropy-Guided Depth of Anesthesia in Critically Ill Polytrauma Patients. J. Interdiscip. Med. 2017, 2, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Cotae, A.; Grinţescu, I.M. Entropy—The Need of an Ally for Depth of Anesthesia Monitoring in Emergency Surgery. Cent. Eur. Ann. Clin. Res. 2019, 1, 1. [Google Scholar]
- Shalbaf, R.; Behnam, H.; Sleigh, J.W.; Steyn-Ross, A.; Voss, L.J. Monitoring the depth of anesthesia using entropy features and an artificial neural network. J. Neurosci. Methods 2013, 218, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.; Myles, P.S.; Sneyd, R.; Struys, M.M.R.F. Depth of anaesthesia monitoring: What’s available, what’s validated and what’s next? Br. J. Anaesth. 2006, 97, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortolani, O.; Conti, A.; Di Filippo, A.; Adembri, C.; Moraldi, E.; Evangelisti, A.; Maggini, M.; Roberts, S.J. EEG signal processing in anaesthesia. Use of a neural network technique for monitoring depth of anaesthesia. Br. J. Anaesth. 2002, 88, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Musialowicz, T.; Lahtinen, P.; Pitkänen, O.; Kurola, J.; Parviainen, I. Comparison of Spectral Entropy and BIS VISTATM monitor during general anesthesia for cardiac surgery. J. Clin. Monit. Comput. 2011, 25, 95–103. [Google Scholar] [CrossRef]
- Lehmann, A.; Schmidt, M.; Zeitler, C.; Kiessling, A.-H.; Isgro, F.; Boldt, J. Bispectral index and electroencephalographic entropy in patients undergoing aortocoronary bypass grafting. Eur. J. Anaesthesiol. 2007, 24, 751–760. [Google Scholar] [CrossRef]
- Meybohm, P.; Gruenewald, M.; Höcker, J.; Renner, J.; Graesner, J.-T.; Ilies, C.; Scholz, J.; Bein, B. Correlation and agreement between the bispectral index vs. state entropy during hypothermic cardio-pulmonary bypass. Acta Anaesthesiol. Scand. 2010, 54, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, X.; Xie, Y.; Yu, J.; He, Q.; Li, Z.; Du, J.; Jiang, X. Spectral entropy monitoring reduces anesthetic dosage for patients undergoing off-pump coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2012, 26, 818–821. [Google Scholar]
- Vargas, M.; Brunetti, I.; Pelosi, P. Protective mechanical ventilation during general anaesthesia. Trends Anaesth. Crit. Care 2013, 3, 77–81. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.H.; Jung, H.; Choi, K.Y.; Jeong, J.S. Effects of neuromuscular blockade reversal on bispectral index and frontal electromyogram during steady-state desflurane anesthesia: A randomized trial. Sci. Rep. 2019, 9, 10486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rosa, R.C.; Romanelli, A.; Calabria, M.; Abbate, R.; Montesano, R.; Corcione, A. Continuous Noninvasive Haemoglobin Monitoring in Vascular Surgery within the Goal-Directed Therapy Protocol. CEACR 2019, 1, 1–15. [Google Scholar]
- Cotrau, P.; Hodosan, V.; Vladu, A.; Timar, C.; Daina, L.; Pantis, C.; Negrau, M.; Daina, C.; Vernic, C. Occupational Stress and Burnout Syndrome among ICU Nurses. A Prospective Observational Study. CEACR 2019, 1, 1–10. [Google Scholar]
- Moise, A.; Balescu-arion, C. The Vitamin D and the Immune System. When? Why? How? Cent. Eur. Ann. Clin. Res. 2020, 2, 1–9. [Google Scholar]
- Georgescu, D.; Reisz, D.; Petre, I.; Ionita, I. Ischemic Stroke Secondary to Cerebral Venous Thrombosis: A Case Report. Cent. Eur. Ann. Clin. Res. 2019, 1, 1–7. [Google Scholar]
- Colombo, R.; Raimondi, F.; Rech, R.; Castelli, A.; Fossali, T.; Marchi, A.; Borghi, B.; Corona, A.; Guzzetti, S. Surgical Pleth Index guided analgesia blunts the intraoperative sympathetic response to laparoscopic cholecystectomy. Minerva Anestesiol. 2015, 81, 837–845. [Google Scholar]
- Xie, P.; Li, Z.; Tian, Z. The optimal combination of mechanical ventilator parameters under general anesthesia in obese patients undergoing laparoscopic surgery. J. Clin. Anesth. 2016, 34, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.S.; Kaneva, P.; Demyttenaere, S.; Carli, F.; Fried, G.M.; Mayo, N.E. Validation of a physical activity questionnaire (CHAMPS) as an indicator of postoperative recovery after laparoscopic cholecystectomy. Surgery 2009, 146, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Papurica, M.; Sandesc, D.; Rogobete, A.F.; Nartita, R.; Vernic, C.; Popovici, S.E.; Bedreag, O.H. Cardioprotective Effects Induced by Preconditioning with Halogenated Anesthetics. J. Interdiscip. Med. 2016, 1, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Weihrauch, D.; Ludwig, L.M.; Kersten, J.R.; Pagel, P.S.; Warltier, D.C. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology 2003, 98, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Metry, A.A.; Hussain, N.S.; Nakhla, G.M.; Ragaei, M.Z.; Wahba, R.M. The effect of continuous propofol versus dexmedetomidine infusion on regional cerebral tissue oxygen saturation during cardiopulmonary bypass. Rom. J. Anaesth. Intensive Care 2019, 26, 17–23. [Google Scholar] [PubMed]
- Riazanova, O.V.; Alexandrovich, Y.S.; Guseva, Y.V.; Ioscovich, A.M. A randomized comparison of low dose ropivacaine programmed intermittent epidural bolus with continuous epidural infusion for labour analgesia. Rom. J. Anaesth. Intensive Care 2019, 26, 25–30. [Google Scholar]
- Balan, S.A.; Bubenek-turconi, Ş.I.; Droc, G.; Marinescu, E.; Nita, E.; Popa, C.; Popescu-spineni, D.; Tomescu, D. Burnout syndrome in the Anaesthesia and Intensive Care Unit. Rom. J. Anaesth. Intensive Care 2019, 26, 31–36. [Google Scholar]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. C-Myc represses tumor-suppressive microRNAs, let-7α, miR-16 and miR-29b, and induces cyclin D2-mediated cell proliferation in Ewing’s sarcoma cell line. PLoS ONE 2015, 10, e0138560. [Google Scholar] [CrossRef]
- Maestroni, U.; Sortini, D.; Devito, C.; Morad, F.P.; Brunaldi, K.; Anania, G.; Pavanelli, L.; Pasqualucci, A.; Donini, A. A new method of preemptive analgesia in laparoscopic cholecystectomy. Surg. Endosc. Other Interv. Tech. 2002, 16, 1336–1340. [Google Scholar] [CrossRef]
- Bedreag, O.H.; Rogobete, A.F.; Sandesc, D.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Dumache, R.; Horhat, F.G.; Vernic, C.; Sima, L.V.; et al. Modulation of the Redox Expression and Inflammation Response in the Crtically Ill Polytrauma Patient with Thoracic Injury. Statistical Correlations between Antioxidant Therapy and Clinical Aspects. Clin. Lab. 2016, 62, 1747–1759. [Google Scholar] [CrossRef]
- David, V.L.; Ercisli, F.; Florin, A.; Boia, E.S.; Nitu, R. Early Prediction of Sepsis Incidence in Critically Ill Patients Using Specific Genetic Polymorphisms. Biochem. Genet. 2017, 55, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yang, S.Y.; Choi, G.J.; Kim, B.G.; Kang, H. Comparison of pressure- and volume-controlled ventilation during laparoscopic colectomy in patients with colorectal cancer. Sci. Rep. 2019, 9, 17007. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.; Cowman, S. Anaesthesia for laparoscopic surgery. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.M.; Que, Y.A. A protocol guided by transpulmonary thermodilution and lactate levels for resuscitation of patients with severe burns. Crit. Care 2013, 17, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küntscher, M.V.; Germann, G.; Hartmann, B. Correlations between cardiac output, stroke volume, central venous pressure, intra-abdominal pressure and total circulating blood volume in resuscitation of major burns. Resuscitation 2006, 70, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, T.N.; Li, Y. Depth of anaesthesia monitors and the latest algorithms. Asian Pac. J. Trop. Med. 2014, 7, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Chowdhury, I.; Bhargava, A.; Sabbharwal, B. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 180–185. [Google Scholar] [CrossRef]
- Riad, W.; Schreiber, M.; Saeed, A.B. Monitoring with EEG entropy decreases propofol requirement and maintains cardiovascular stability during induction of anaesthesia in elderly patients. Eur. J. Anaesthesiol. 2007, 24, 684–688. [Google Scholar] [CrossRef]
- Tewari, S.; Bhadoria, P.; Wadhawan, S.; Prasad, S.; Kohli, A. Entropy vs. standard clinical monitoring using total intravenous anesthesia during transvaginal oocyte retrieval in patients for in vitro fertilization. J. Clin. Anesth. 2016, 34, 105–112. [Google Scholar] [CrossRef]
- Wu, S.C.; Wang, P.C.; Liao, W.T.; Shih, T.H.; Chang, K.A.; Lin, K.C.; Chou, A.K. Use of spectral entropy monitoring in reducing the quantity of sevoflurane as sole inhalational anesthetic and in decreasing the need for antihypertensive drugs in total knee replacement surgery. Acta Anaesthesiol. Taiwanica 2008, 46, 106–111. [Google Scholar] [CrossRef]
- Vakkuri, A.; Yli-hankala, A.; Sandin, R.; Mustola, S. Spectral Entropy Monitoring Is Associated with Reduced Propofol Use and Faster Emergence in Propofol–Nitrous Oxide–Alfentanil Anesthesia. Anesthesiol. J. Am. Soc. Anesthesiol. 2005, 130, 274–279. [Google Scholar] [CrossRef] [PubMed]
- El Hor, T.; Van Der Linden, P.; De Hert, S.; Melot, C.; Bidgoli, J. Impact of entropy monitoring on volatile anesthetic uptake. Anesthesiology 2013, 118, 868–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, L.; Sleigh, J. Monitoring consciousness: The current status of EEG-based depth of anaesthesia monitors. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shan, G.J.; Zhang, Y.X.; Cao, S.J.; Zhu, S.N.; Li, H.J.; Ma, D.; Wang, D.X. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br. J. Anaesth. 2018, 121, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Kadoi, Y.; Kawauchi, C.; Saito, S.; Takahashi, K. The comparative effects of equipotent Bispectral Index dosages of propofol and sevoflurane on cerebrovascular carbon dioxide reactivity in elderly patients. J. Clin. Anesth. 2009, 21, 173–177. [Google Scholar] [CrossRef]
- Radtke, F.M.; Franck, M.; Lendner, J.; Krüger, S.; Wernecke, K.D.; Spies, C.D. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 2013, 110, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Llanes-garza, H.A.; López-cabrera, N.G.; Vega, R.C.; Palacios-rios, D. Efficacy of antiemetic therapy in patients undergoing laparoscopic cholecystectomy. Med. Univ. 2015, 17, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Bedreag, O.H.; Papurica, M.; Rogobete, A.F.; Sarandan, M.; Cradigati, C.A.; Vernic, C.; Dumbuleu, C.M.; Nartita, R.; Sandesc, D. New perspectives of volemic resuscitation in polytrauma patients: A review. Burn. Trauma 2016, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bedreag, O.H.; Rogobete, A.F.; Sarandan, M.; Cradigati, A.C.; Papurica, M.; Dumbuleu, M.C.; Chira, A.M.; Rosu, O.M.; Sandesc, D. Oxidative stress in severe pulmonary trauma in critical ill patients. Antioxidant therapy in patients with multiple trauma—A review. Anaesthesiol. Intensive Ther. 2015, 47, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Papurica, M.; Rogobete, A.F.; Sandesc, D.; Cradigati, C.A.; Sarandan, M.; Crisan, D.C.; Horhat, F.G.; Boruga, O.; Dumache, R.; Nilima, K.R.; et al. The Expression of Nuclear Transcription Factor Kappa B (NF-kappaB) in the Case of Critically Ill Polytrauma Patients with Sepsis and Its Interactions with microRNAs. Biochem. Genet. 2016, 54, 337–347. [Google Scholar] [CrossRef]
- Horhat, F.G.; Gundogdu, F.; David, L.V.; Boia, E.S.; Pirtea, L.; Horhat, R.; Cucui-Cozma, A.; Ciuca, I.; Diaconu, M.; Nitu, R.; et al. Early Evaluation and Monitoring of Critical Patients with Acute Respiratory Distress Syndrome (ARDS) Using Specific Genetic Polymorphisms. Biochem. Genet. 2017, 55, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.R.; Rogobete, A.F.; Bratu, T.; Popovici, S.E.; Bedreag, O.H.; Papurica, M.; Bratu, L.M.; Sandesc, D. Cannabis Sativa Revisited—Crosstalk between microRNA Expression, Inflammation, Oxidative Stress, and Endocannabinoid Response System in Critically Ill Patients with Sepsis. Cells. 2020, 54, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, M.A.; Cavalheiro, B.T.; Filho, G.R.D.O. Laparoscopic cholecystectomy under neuraxial anesthesia compared with general anesthesia: Systematic review and meta-analyses. J. Clin. Anesth. 2017, 41, 48–54. [Google Scholar] [CrossRef]
- Fleming, N.; Cockerham, R. General anaesthesia for operative obstetrics. Anaesth. Intensive Care Med. 2019, 20, 495–499. [Google Scholar] [CrossRef]
- Cumpanas, A.A.; Bardan, R.; Ferician, O.C.; Latcu, S.C.; Duta, C. Does previous open surgical experience have any influence on robotic surgery simulation exercises? Videosurg. Other Miniinvasive Tech. 2017, 12, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vãrcuæ, F.; Duåã, C.; Dobrescu, A.; Lazãr, F.; Papurica, M.; Tarta, C. Laparoscopic Repair of Inguinal Hernia TEP versus TAPP. Chirurgia 2016, 111, 308–312. [Google Scholar]
- Papurica, M.; Rogobete, A.F.; Sandesc, D.; Dumache, R.; Nartita, R.; Sarandan, M.; Cradigati, A.C.; Luca, L.; Vernic, C.; Bedreag, O.H. Redox Changes Induced by General Anesthesia in Critically Ill Patients with Multiple Traumas. Mol. Biol. Int. 2015, 2015, 238586. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Huang, G.H.; Rebmann, C.S.; Ellery, C. Performance of entropy and Bispectral Index as measures of anaesthesia effect in children of different ages. Br. J. Anaesth. 2005, 95, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgaia, A.O.; Irimie, A.; Sandesc, D.; Vlad, C.; Lisencu, C.; Rogobete, A.; Achimas-Cadariu, P. The role of Ketamine in the treatment of chronic cancer pain. Clujul Med. 2015, 88, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Hans, P.; Dewandre, P.; Brichant, J.F.; Bonhomme, V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br. J. Anaesth. 2005, 94, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.C.; Canosa-Mas, C.E.; Parr, A.D.; Pierce, J.M.T.; Wayne, R.P. Tropospheric lifetimes of halogenated anaesthetics. Nature 1989, 341, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Yasny, J.S.; White, J. Environmental implications of anesthetic gases. Anesth. Prog. 2012, 59, 154–158. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Group A (N = 43) | Group B (N = 25) | 95% Confidence Interval | Statistical p Value |
|---|---|---|---|---|
| Age; years, mean ± SD | 51 ± 16.51 | 52.20 ± 13.79 | −6.620–9.020 | > 0.05 |
| Weight; kg, mean ± SD | 87 ± 2.71 | 91 ± 1.99 | −5.445–7.012 | > 0.05 |
| Gender; M, N (%) | 7 (16.28) | 6 (24) | −10.8233%–28.7947% | > 0.05 |
| ASA Score; I, N (%) | 10 (23) | 3 (12) | −0.5716%–27.4520% | > 0.05 |
| ASA Score; II, N (%) | 24 (56) | 17 (68) | −11.9231%–32.8672% | > 0.05 |
| ASA Score; III, N (%) | 6 (14) | 5 (20) | −11.3628%–26.5172% | > 0.05 |
| HR at M0; bpm, mean ± SD | 78.48 ± 13.87 | 75.32 ± 14.28 | −10.46–3.616 | > 0.05 |
| SAB at M0; bpm, mean ± SD | 136.5 ± 22.47 | 134 ± 17.51 | −12.97–7.917 | > 0.05 |
| Group A (N = 43) | Group B (N = 25) | |||||
|---|---|---|---|---|---|---|
| No. Hemodynamic Events | No. Hemodynamic Events/Patient | % of Hemodynamic Events | No. Hemodynamic Events | No. Hemodynamic Events/Patient | % of Hemodynamic Events | |
| No. Hypertensions | 17 | 0.4 | 25.4 | 21 | 0.84 | 29.6 |
| No. Hypotensions | 19 | 0.5 | 28.4 | 14 | 0.56 | 19.7 |
| No. Tachycardia | 12 | 0.3 | 17.9 | 21 | 0.84 | 29.6 |
| No. Bradycardia | 19 | 0.5 | 28.4 | 15 | 0.6 | 21.1 |
| Total | 67 | 1.6 | 71 | 2.84 | ||
| HR (bpm) | ||||||||||||||
| M0 | M15 | M30 | M45 | M60 | M75 | M90 | M105 | M120 | M135 | M156 | M165 | Mext | ||
| Group A | MEAN | 75.9 | 75.4 | 76.1 | 77.3 | 79.3 | 81.8 | 82.1 | 82.6 | 82.7 | 76.0 | 74.0 | 72.0 | 82.9 |
| SD | 13.-9 | 11.7 | 12.1 | 11.3 | 13.4 | 12.5 | 12.2 | 16.8 | 10.8 | 0.0 | 0.0 | 0.0 | 9.8 | |
| p | M15/M0 | M30/M0 | M45/M0 | M60/M0 | M75/M0 | M90/M0 | M105/M0 | M120/M0 | M135/M0 | M150/M0 | M165/M0 | Mext/M0 | ||
| < 0.05 | < 0.05 | > 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | - | - | - | < 0.05 | |||
| Group B | MEAN | 74.9 | 69.3 | 67.6 | 69.9 | 70.0 | 75.0 | 76.4 | 73.4 | 75.8 | 80.5 | 89.0 | 89.0 | 94.6 |
| SD | 14.4 | 6.3 | 7.7 | 9.6 | 8.9 | 9.1 | 8.3 | 8.8 | 9.5 | 10.9 | 11.1 | 9.9 | 18.2 | |
| p | M15/M0 | M30/M0 | M45/M0 | M60/M0 | M75/M0 | M90/M0 | M105/M0 | M120/M0 | M135/M0 | M150/M0 | M165/M0 | Mext/M0 | ||
| < 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | < 0.05 | > 0.05 | < 0.05 | |||
| SAP (mmHg) | ||||||||||||||
| Group A | MEAN | 136.6 | 121.4 | 122.2 | 122.7 | 119.3 | 118.8 | 130.2 | 122.7 | 127.0 | 0.0 | 0.0 | 0.0 | 136.6 |
| SD | 23.9 | 24.1 | 19.8 | 17.9 | 14.6 | 15.1 | 23.8 | 4.6 | 5.2 | 0.0 | 0.0 | 0.0 | 15.7 | |
| p | M15/M0 | M30/M0 | M45/M0 | M60/M0 | M75/M0 | M90/M0 | M105/M0 | M120/M0 | M135/M0 | M150/M0 | M165/M0 | Mext/M0 | ||
| < 0.05 | < 0.05 | < 0.05 | 0< 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | - | - | - | > 0.05 | |||
| Group B | MEAN | 134.0 | 109.9 | 112.0 | 118.5 | 117.7 | 113.9 | 112.6 | 116.6 | 115.0 | 114.3 | 117.8 | 105.0 | 129.4 |
| SD | 17.5 | 16.3 | 21.0 | 18.6 | 19.7 | 15.5 | 14.3 | 11.8 | 12.5 | 14.5 | 19.9 | 11.3 | 12.0 | |
| p | M15/M0 | M30/M0 | M45/M0 | M60/M0 | M75/M0 | M90/M0 | M105/M0 | M120/M0 | M135/M0 | M150/M0 | M165/M0 | Mext/M0 | ||
| < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | > 0.05 | > 0.05 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, A.R.; Rogobete, A.F.; Popovici, S.E.; Bedreag, O.H.; Papurica, M.; Dumbuleu, C.M.; Velovan, R.R.; Toma, D.; Georgescu, C.M.; Trache, L.I.; et al. Impact of General Anesthesia Guided by State Entropy (SE) and Response Entropy (RE) on Perioperative Stability in Elective Laparoscopic Cholecystectomy Patients—A Prospective Observational Randomized Monocentric Study. Entropy 2020, 22, 356. https://doi.org/10.3390/e22030356
Dinu AR, Rogobete AF, Popovici SE, Bedreag OH, Papurica M, Dumbuleu CM, Velovan RR, Toma D, Georgescu CM, Trache LI, et al. Impact of General Anesthesia Guided by State Entropy (SE) and Response Entropy (RE) on Perioperative Stability in Elective Laparoscopic Cholecystectomy Patients—A Prospective Observational Randomized Monocentric Study. Entropy. 2020; 22(3):356. https://doi.org/10.3390/e22030356
Chicago/Turabian StyleDinu, Anca Raluca, Alexandru Florin Rogobete, Sonia Elena Popovici, Ovidiu Horea Bedreag, Marius Papurica, Corina Maria Dumbuleu, Raluca Ramona Velovan, Daiana Toma, Corina Maria Georgescu, Lavinia Ioana Trache, and et al. 2020. "Impact of General Anesthesia Guided by State Entropy (SE) and Response Entropy (RE) on Perioperative Stability in Elective Laparoscopic Cholecystectomy Patients—A Prospective Observational Randomized Monocentric Study" Entropy 22, no. 3: 356. https://doi.org/10.3390/e22030356
APA StyleDinu, A. R., Rogobete, A. F., Popovici, S. E., Bedreag, O. H., Papurica, M., Dumbuleu, C. M., Velovan, R. R., Toma, D., Georgescu, C. M., Trache, L. I., Barsac, C., Luca, L., Buzzi, B., Maghiar, A., Sandesc, M. A., Rimawi, S., Vaduva, M. M., Bratu, L. M., Luminosu, P. M., & Sandesc, D. (2020). Impact of General Anesthesia Guided by State Entropy (SE) and Response Entropy (RE) on Perioperative Stability in Elective Laparoscopic Cholecystectomy Patients—A Prospective Observational Randomized Monocentric Study. Entropy, 22(3), 356. https://doi.org/10.3390/e22030356






