1.2. Thermodynamic Invariants from Reciprocal Experiments
Since 2011 new and original types of chemical invariants were described [
3,
4,
5]. These invariants of thermodynamic origin are closely related to Onsager’s famous reciprocal relations [
6,
7]. The experimental procedure, real or computational, consists of two dual experiments performed from different initial conditions of the reacting mixture, called the “dual experiments”. The simplest of these invariants is related to the single reversible reaction A ⇄ B, in a batch reactor (BR):
In both cases, the time-dependent concentrations of A and B are measured, A(t) and B(t), respectively. A special attention was paid to symmetric concentration profiles: the dependences “B produced from pure A”, B
A(t), from the first experiment, and “A produced from pure B”, A
B(t), from the second experiment. Explicit formulas for these concentration profiles are shown in
Table 1, assuming that both the forward and backward reaction as first-order, monomolecular reactions, with kinetic coefficients k
+ and k
−, respectively.
The notation of the concentration profiles is as follows: the first capital letter denotes the substance, whereas the subscript letter denotes the single component primed in the reactor, in this case: pure A or pure B. The concentration profiles shown in
Table 1 are plotted in
Figure 1.
As seen in
Table 1, the ratio of the symmetric concentration profiles B
A(t)/A
B(t) is constant, equal to the equilibrium constant of the reversible reaction K
eq, K
eq = k
+/k
−. The equality B
A(t)/A
B(t) = K
eq is valid for t > 0, i.e., throughout the course of the reaction. Clearly, this invariant expression is different from other linear invariances such as mass conservation balances. The new invariant expression is used as follows: knowing the thermodynamic characteristic—the equilibrium constant—and one concentration profile, say, A
B(t), we can find another, unknown, concentration profile, for instance B
A(t) = A
B(t)K
eq [
8].
This result is valid also for a steady-state plug flow reactor (PFR) and a steady-state continuously stirred tank reactor (CSTR), if the astronomic time t is replaced by the space time τ, defined as the reactor volume divided by the volumetric flow rate [
1,
2].
It is reasonable to define this ratio of concentration profiles, B
A(t)/A
B(t), as a thermodynamic invariant, since it is equal to a thermodynamic parameter such as the equilibrium constant K
eq. This type of invariant can be observed in more complicated, reversible linear mechanisms, calculated from the ratio of concentration profiles of any arbitrary chemical species connected via any number of reversible reactions, as long as these concentration profiles are obtained from dual experiments [
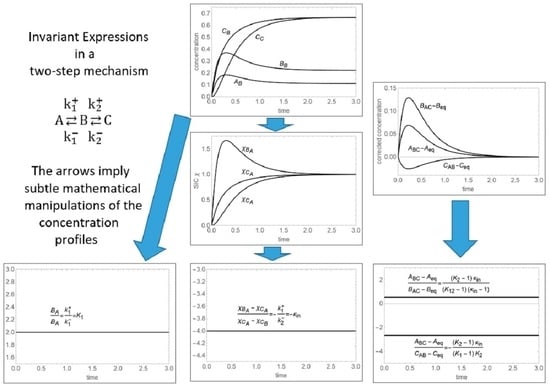
4]. The thermodynamic invariants obtained for complex multistep mechanisms are two-fold:
Pure equilibrium constants, obtained from the ratio of concentration profiles of chemical species connected via a single step reaction within a complex chemical mechanism.
Apparent equilibrium constants, consisting of products of equilibrium constants of elementary reactions, obtained from the ratio of concentration profiles of chemical species connected via multiple step reactions in a complex chemical mechanism.
The theoretical basis of these invariants within the thermodynamic theory of irreversible processes is given elsewhere [
4]. In 1931, Onsager [
6,
7] presented the foundations and generalizations of the reciprocal relations introduced in the 19
th century by Lord Kelvin and Helmholtz. In his historical papers, Onsager mentioned also the close connection between these relations and the detailed balance of elementary processes: at equilibrium, each elementary transaction must be equilibrated by its inverse transaction. For linear or linearized kinetics with microreversibility, x’ = K x, where x is the vector of the solution, the kinetic operator K is symmetric in the entropic inner product. This form on Onsager’s reciprocal relations implies that the shift in time, e
Kt, is also a symmetric operator. This feature generates the reciprocal relations between the kinetic curves; this is the fundamental basis of our thermodynamic invariants [
4].
In a more general setting, duality between experiments must be defined using the entropic inner product [
4]. Let J denote the vector of fluxes and X that of thermodynamic forces, then by Onsager’s relations J = L X, where L is a symmetric matrix. In isolated systems, X is the gradient of the entropy
, and the linear(ized) kinetic equation is
, where
is the product of two symmetric matrices, which need not be symmetric (for the standard inner product). If, however, we use the entropic inner product instead, defined by
symmetry is obtained in the sense that
. Integrated over time, this means that
The general requirement for two trajectories to be dual is then that their initial values be orthogonal in this entropic inner product.
Even some simple non-linear mechanisms may show similar invariants, calculated from the ratio of selected concentration profiles. For instance, for the elementary reaction A + B ⇄ C + D it can be demonstrated that (C
A(t) D
C(t))/(A
C(t) B
A(t)) = K
eq, where C
A(t) and B
A(t) are concentration profiles obtained when the initial concentration of C is zero, and A
C(t) and D
C(t) are concentration profiles obtained when the initial concentration of A is zero [
5].
With this knowledge, it is possible to predict unknown kinetic dependences based on the chemical equilibrium description and known kinetic dependences [
8]. Additionally, we are able to confirm our experimental data validating them via the new invariants. Design of special batteries of kinetic experiments, virtual and/or real, can be considered a new step towards understanding the behavior of complex chemical reactions, and to gain insights on the intrinsic kinetic features of complex mechanisms.