Zipf’s Law of Vasovagal Heart Rate Variability Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Head-Up Tilt Table Test
2.3. Signal Analysis
2.4. Vasovagal Events
2.5. Statistics
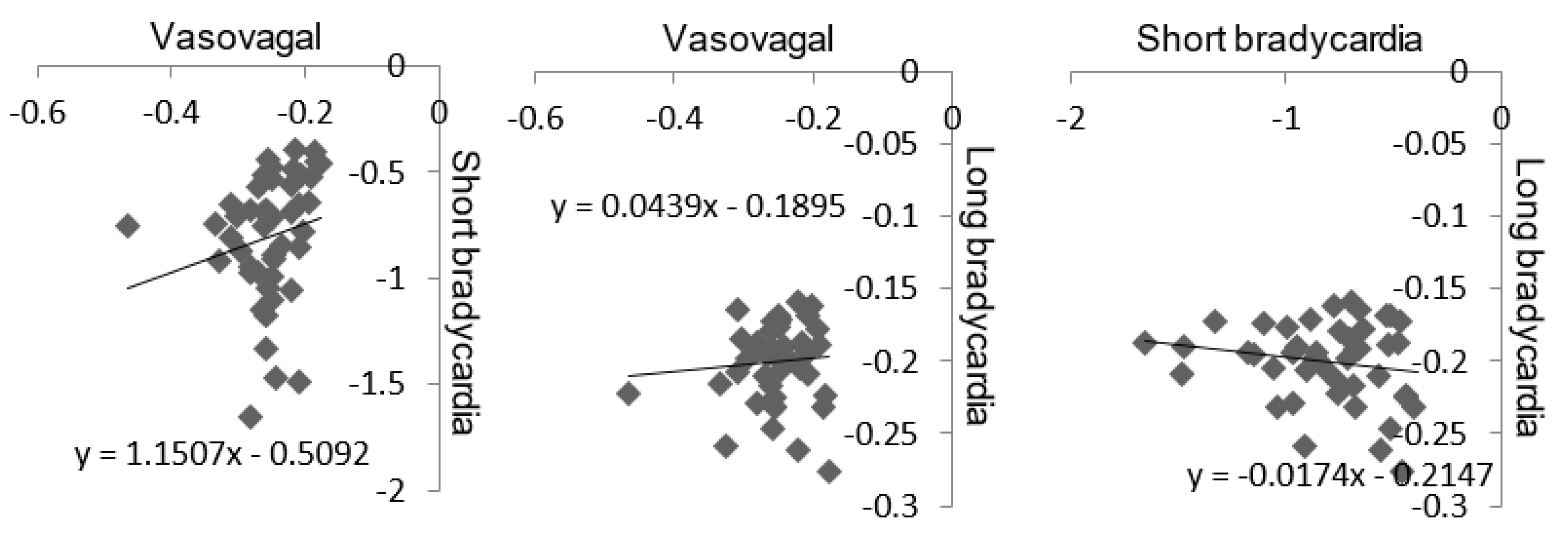
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Struzik, Z.R. Is heart rate variability dynamics poised at criticality? In Proceedings of the 8th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Trento, Italy, 25–28 May 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Muñoz, M.A. Colloquium: Criticality and dynamical scaling in living systems. Rev. Mod. Phys. 2018, 90, 031001. [Google Scholar] [CrossRef]
- Goldstein, D.S. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. 2019, 316, R301–R317. [Google Scholar] [CrossRef] [PubMed]
- Fortrat, J.O.; Gharib, C. Self-Organization of Blood Pressure Regulation: Clinical Evidence. Front. Physiol. 2016, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Fortrat, J.O.; Levrard, T.; Courcinous, S.; Victor, J. Self-Organization of Blood Pressure Regulation: Experimental Evidence. Front. Physiol. 2016, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Grubb, B.P. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005, 111, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Bak, P. How Nature Works: The Science of Self-Organised Criticality; Copernicus Press: New York, NY, USA, 1996; pp. 1–212. [Google Scholar]
- Mora, T.; Bialek, W. Are biological systems poised at criticality? J. Stat. Phys. 2011, 144, 268–302. [Google Scholar] [CrossRef]
- Tria, F.; Loreto, V.; Servedio, V.D.P. Zipf’s, Heaps’ and Taylor’s laws are determined by the expansion into the adjacent possible. Entropy 2018, 20, 752. [Google Scholar] [CrossRef]
- Kalda, J.; Sakki, M.; Vainu, M.; Laan, M. Zipf’s law in human heart beat dynamics. arXiv 2001, arXiv:physics/0110075v1. [Google Scholar]
- Yang, A.C.; Hseu, S.S.; Yien, H.W.; Goldberger, A.L.; Peng, C.K. Linguistic analysis of the human heartbeat using frequency and rank order statistics. Phys. Rev. Lett. 2003, 90, 108103. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Prieto, S.; Correa, C.; Mendoza, F.; Weiz, G.; Soracipa, Y.; Velásquez, N.; Pardo, J.; Martínez, M.; Barrios, F. Physical mathematical evaluation of the cardiac dynamic applying the Zipf-Mandelbrot law. J. Mod. Phys. 2015, 6, 1881–1888. [Google Scholar] [CrossRef]
- Blons, E.; Arsac, L.M.; Gilfriche, P.; Deschodt-Arsac, V. Multiscale Entropy of Cardiac and Postural Control Reflects a Flexible Adaptation to a Cognitive Task. Entropy 2019, 21, 1024. [Google Scholar] [CrossRef]
- Ravé, G.; Fortrat, J.O. Heart rate variability in the standing position reflects training adaptation in professional soccer players. Eur. J. Appl. Physiol. 2016, 116, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Rompelman, O. Accuracy aspects in ECG preprocessing for the study of heart rate variability. In The Beat-to-Beat Investigation of Cardiovascular Function; Kitney, R.I., Rompelman, O., Eds.; Oxford University Press: Oxford, UK, 1986; pp. 103–125. [Google Scholar]
- Fortrat, J.O.; Ravé, G. Autonomic influences on heart rate variability self-organized criticality: Insights of Zipf’s law. In Proceedings of the European Study Group on Cardiovascular Oscillations Pisa 2020, Pisa, Italy, 27–29 April 2020. [Google Scholar]
- Laude, D.; Elghozi, J.L.; Girard, A.; Bellard, E.; Bouhaddi, M.; Castiglioni, P.; Cerutti, C.; Cividjian, A.; Di Rienzo, M.; Fortrat, J.O.; et al. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study). Am. J. Physiol. 2004, 286, R226–R231. [Google Scholar] [CrossRef] [PubMed]
- Fortrat, J.O.; Formet, C.; Frutoso, J.; Gharib, C. Even slight movements disturb analysis of cardiovascular dynamics. Am. J. Physiol. 1999, 277, H261–H267. [Google Scholar] [CrossRef] [PubMed]
- Fortrat, J.O.; de Germain, V.; Custaud, M.A. Holter heart rate variability: Are we measuring physical activity? Am. J. Cardiol. 2010, 106, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Graff, B.; Graff, G.; Makowiec, D.; Kaczkowska, A.; Wejer, D.; Budrejko, S.; Kozłowski, D.; Narkiewicz, K. Entropy Measures in the Assessment of Heart Rate Variability in Patients with Cardiodepressive Vasovagal Syncope. Entropy 2015, 17, 1007–1022. [Google Scholar] [CrossRef]
- Fortrat, J.O.; Baum, C.; Jeanguillaume, C.; Custaud, M.A. Noisy fluctuation of heart rate indicates cardiovascular system instability. Eur. J. Appl. Physiol. 2013, 113, 2253–2261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rowell, L.B. Passive effect of gravity. In Human Cardiovascular Control; Rowell, L.B., Ed.; Oxford University Press: New York, NY, USA, 1993; pp. 3–36. [Google Scholar]




© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortrat, J.-O. Zipf’s Law of Vasovagal Heart Rate Variability Sequences. Entropy 2020, 22, 413. https://doi.org/10.3390/e22040413
Fortrat J-O. Zipf’s Law of Vasovagal Heart Rate Variability Sequences. Entropy. 2020; 22(4):413. https://doi.org/10.3390/e22040413
Chicago/Turabian StyleFortrat, Jacques-Olivier. 2020. "Zipf’s Law of Vasovagal Heart Rate Variability Sequences" Entropy 22, no. 4: 413. https://doi.org/10.3390/e22040413
APA StyleFortrat, J.-O. (2020). Zipf’s Law of Vasovagal Heart Rate Variability Sequences. Entropy, 22(4), 413. https://doi.org/10.3390/e22040413




