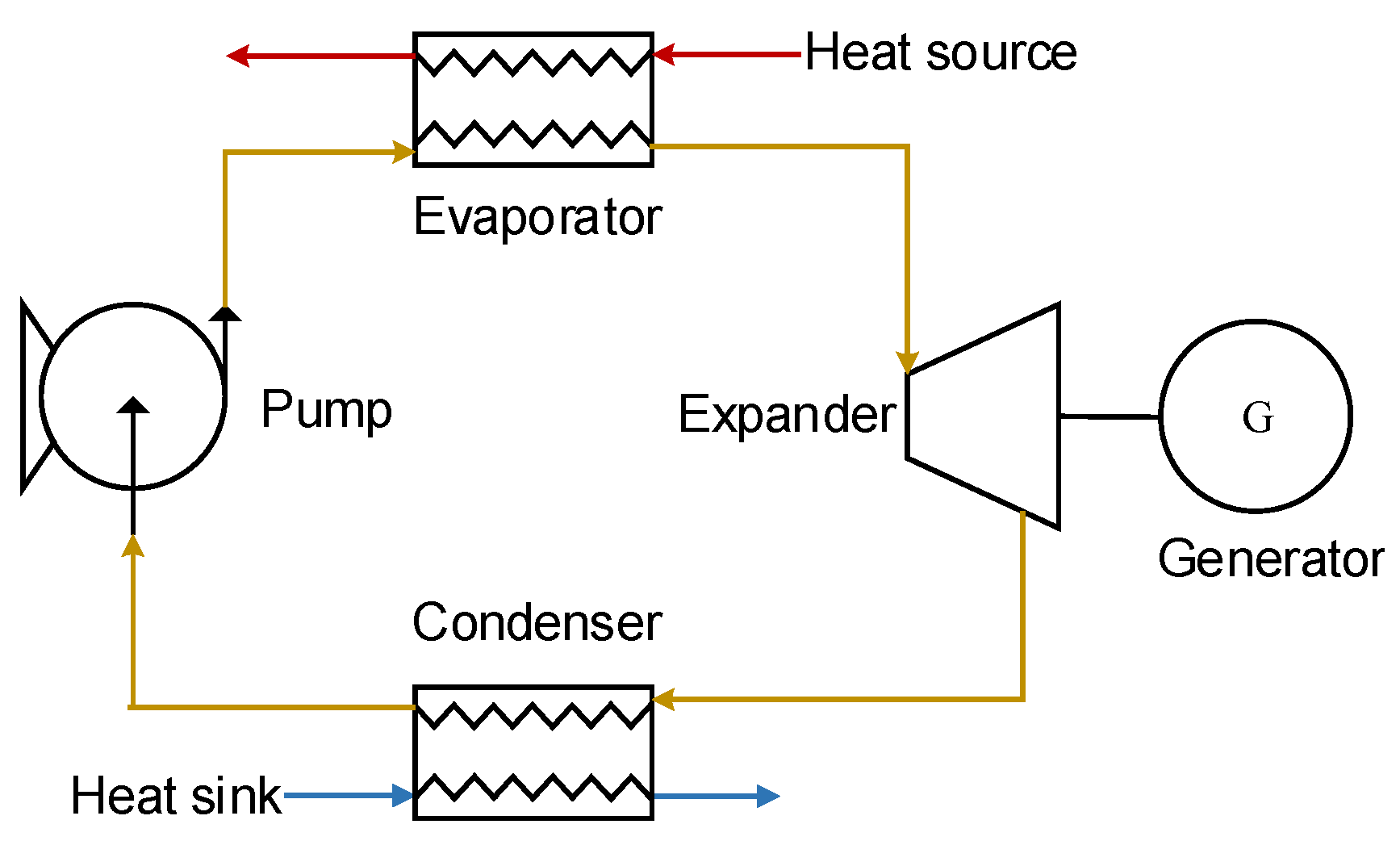
Figure 1.
Basic Organic Rankine Cycle configuration.
Figure 1.
Basic Organic Rankine Cycle configuration.
Figure 2.
Three varying configurations of the Multi-Loop Organic Rankine Cycle. (a) Series type. (b) Parallel type. (c) Series–parallel type.
Figure 2.
Three varying configurations of the Multi-Loop Organic Rankine Cycle. (a) Series type. (b) Parallel type. (c) Series–parallel type.
Figure 3.
T-s diagrams of three various Multi-Loop Organic Rankine Cycle. (a) Series type. (b) Parallel type. (c) Series–parallel type.
Figure 3.
T-s diagrams of three various Multi-Loop Organic Rankine Cycle. (a) Series type. (b) Parallel type. (c) Series–parallel type.
Figure 4.
Probable circumstances of PPTD in series MLORC. (a) The pinch points may appear at the super-cooling zone, liquid saturation point, and the outlet of the next working fluid. (b) The pinch points may appear at the two-phase zone, and the outlet of the next working fluid. (c) The pinch points may appear at the super heated zone, and the outlet of the next working fluid.
Figure 4.
Probable circumstances of PPTD in series MLORC. (a) The pinch points may appear at the super-cooling zone, liquid saturation point, and the outlet of the next working fluid. (b) The pinch points may appear at the two-phase zone, and the outlet of the next working fluid. (c) The pinch points may appear at the super heated zone, and the outlet of the next working fluid.
Figure 5.
Probable circumstances of PPTD in series–parallel MLORC. (a) The next working fluid is preheated by the previous working fluid and exhaust gas, evaporated and superheated by the exhaust gas. (b) The next working fluid is preheated by the previous working fluid, evaporated by the previous working fluid and exhaust gas, and superheated by the exhaust gas. (c) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas. (d) The next working fluid is preheated by the previous working fluid, evaporated by the previous working fluid and exhaust gas, and superheated by the exhaust gas. (e) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas. (f) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas.
Figure 5.
Probable circumstances of PPTD in series–parallel MLORC. (a) The next working fluid is preheated by the previous working fluid and exhaust gas, evaporated and superheated by the exhaust gas. (b) The next working fluid is preheated by the previous working fluid, evaporated by the previous working fluid and exhaust gas, and superheated by the exhaust gas. (c) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas. (d) The next working fluid is preheated by the previous working fluid, evaporated by the previous working fluid and exhaust gas, and superheated by the exhaust gas. (e) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas. (f) The next working fluid is preheated and evaporated by the previous working fluid, and superheated by the previous working fluid and exhaust gas.
Figure 6.
The flow chart of the implantation in MATLAB.
Figure 6.
The flow chart of the implantation in MATLAB.
Figure 7.
The effect of second loop’s evaporating temperature on the thermoeconomic performance of the series MLORC. (a) Effect on thermal and exergy efficiency. (b) Effect on power output and LCOE.
Figure 7.
The effect of second loop’s evaporating temperature on the thermoeconomic performance of the series MLORC. (a) Effect on thermal and exergy efficiency. (b) Effect on power output and LCOE.
Figure 8.
The effect of loop number on the thermoeconomic performance with the series MLORC. (a) The effect on thermal and exergy efficiency. (b) The effect on power output and LCOE.
Figure 8.
The effect of loop number on the thermoeconomic performance with the series MLORC. (a) The effect on thermal and exergy efficiency. (b) The effect on power output and LCOE.
Figure 9.
Effect of the distribution of heat energy on the thermoeconomic parallel dual-loop ORC under various outlet temperatures and inlet temperatures of the exhaust gas. (a) Inlet and outlet temperature of the heat source are higher than the working fluid critical temperature. (b) . (c) .
Figure 9.
Effect of the distribution of heat energy on the thermoeconomic parallel dual-loop ORC under various outlet temperatures and inlet temperatures of the exhaust gas. (a) Inlet and outlet temperature of the heat source are higher than the working fluid critical temperature. (b) . (c) .
Figure 10.
Composite Curves for single loop ORC and dual-loop ORC in the scenario . (a) Single loop ORC. (b) Heat proportion of the second cycle is 10%. (c) Heat proportion of the second cycle is 50%.
Figure 10.
Composite Curves for single loop ORC and dual-loop ORC in the scenario . (a) Single loop ORC. (b) Heat proportion of the second cycle is 10%. (c) Heat proportion of the second cycle is 50%.
Figure 11.
Composite Curves for dual-loop ORC in the scenario . (a) Heat proportion of the second cycle is 90%. (b) Heat proportion of the second cycle is 50%.
Figure 11.
Composite Curves for dual-loop ORC in the scenario . (a) Heat proportion of the second cycle is 90%. (b) Heat proportion of the second cycle is 50%.
Figure 12.
Effect of the second loop’s evaporating temperature on the thermoeconomic performance of the series MLORC. (a) Effect on power output under . (b) Effect on LCOE under . (c) Effect on power output under . (d) Effect on LCOE under .
Figure 12.
Effect of the second loop’s evaporating temperature on the thermoeconomic performance of the series MLORC. (a) Effect on power output under . (b) Effect on LCOE under . (c) Effect on power output under . (d) Effect on LCOE under .
Figure 13.
Effects of heat proportion and evaporating temperature of the second loop on power output and thermal efficiency with series–parallel configuration. (a) Power output. (b) Thermal efficiency.
Figure 13.
Effects of heat proportion and evaporating temperature of the second loop on power output and thermal efficiency with series–parallel configuration. (a) Power output. (b) Thermal efficiency.
Figure 14.
Pareto Frontiers of the series and parallel dual-loop ORC with the highest grey relation grade R.
Figure 14.
Pareto Frontiers of the series and parallel dual-loop ORC with the highest grey relation grade R.
Figure 15.
Pareto Frontiers of the series and parallel triple-loop ORC with the highest grey relation grade R.
Figure 15.
Pareto Frontiers of the series and parallel triple-loop ORC with the highest grey relation grade R.
Figure 16.
Pareto Frontiers of the series and parallel quadruple-loop ORC with the highest grey relation grade R.
Figure 16.
Pareto Frontiers of the series and parallel quadruple-loop ORC with the highest grey relation grade R.
Figure 17.
Pareto Frontiers of the parallel MLORC with different number of loops under the circumstance .
Figure 17.
Pareto Frontiers of the parallel MLORC with different number of loops under the circumstance .
Figure 18.
Pareto Frontiers of the parallel MLORC with different numbers of loops under the circumstance .
Figure 18.
Pareto Frontiers of the parallel MLORC with different numbers of loops under the circumstance .
Figure 19.
Pareto Frontiers of the series MLORC with different numbers of loops .
Figure 19.
Pareto Frontiers of the series MLORC with different numbers of loops .
Table 1.
The parameters of the exhaust gas [
20].
Table 1.
The parameters of the exhaust gas [
20].
| Parameter | Unit | Value |
|---|
| Exhaust gas temperature (after turbine) | K | 535.75 |
| Exhaust gas mass flow (after turbine) | kg/s | 10.33 |
Table 2.
Composition of exhaust gas (%mol).
Table 2.
Composition of exhaust gas (%mol).
| Parameter | Value |
|---|
| 13.75 |
| 75.812 |
| 4.703 |
| 4.771 |
| 0.902 |
| 0.062 |
Table 3.
The thermodynamic properties of the four candidate working fluids.
Table 3.
The thermodynamic properties of the four candidate working fluids.
| Item | R134a [23] | R245fa [24] | R141b [10] | Cyclohexane [25] |
|---|
| Molar mass (kg/kmol) | 102.03 | 134.05 | 116.95 | 84.16 |
| Boiling temperature (K) | 247.1 | 288.3 | 305.2 | 353.9 |
| Critical temperature (K) | 374.21 | 427.16 | 477.5 | 553.6 |
| Critical pressure (kPa) | 4059 | 3651 | 4210 | 4081 |
| ODP | 0 | 0 | 0.12 | 0 |
| GWP | 1430 | 1030 | 20 | 20 |
Table 4.
Constants in the Kandlikar correlation.
Table 4.
Constants in the Kandlikar correlation.
| Constant | Convective Region | Nucleate Boiling Region |
|---|
| 1.1360 | 0.6683 |
| −0.9 | −0.2 |
| 667.2 | 1058.0 |
| 0.7 | 0.7 |
| * | 0.3 | 0.3 |
Table 5.
Coefficients in the module cost evaluation equations [
20].
Table 5.
Coefficients in the module cost evaluation equations [
20].
| Equipment Type | | | | | | | | | |
|---|
| Plate heat exchanger | 4.6561 | −0.2947 | 0.2207 | 0 | 0 | 0 | 0.96 | 1.21 | 1 |
| Shell and tube heat exchanger | 4.3247 | −0.3030 | 0.1634 | 0.0381 | −0.11272 | 0.08183 | 1.63 | 1.66 | 1.2 |
| Condenser | 4.6561 | −0.2947 | 0.2207 | 0 | 0 | 0 | 0.96 | 1.21 | 1 |
| Expander | 2.2476 | 1.4965 | −0.1618 | 0 | 0 | 0 | / | / | 3.8 |
| Working pump | 3.3892 | 0.0536 | 0.1538 | −0.3935 | 0.3957 | −0.00226 | 1.89 | 1.35 | 1.6 |
Table 6.
Critical parameters of the NSGA II method.
Table 6.
Critical parameters of the NSGA II method.
| Items | Value |
|---|
| Population size | 100 |
| Maximum iterations | 100 |
| Crossover probability | 0.75 [35] |
| Mutation probability | 0.25 [35] |
Table 7.
Main parameters of the thermodynamic model and decision boundaries.
Table 7.
Main parameters of the thermodynamic model and decision boundaries.
| Items | Value | Unit |
|---|
| Pump isentropic efficiency [40] | 75 | % |
| Expander isentropic efficiency [41] | 80 | % |
| Environment temperature [23] | 25 | °C |
| Exhaust gas outlet temperature | | °C |
| Seawater temperature | 20 | °C |
Table 8.
Comparison results of the thermodynamic model with the previous article [
43].
Table 8.
Comparison results of the thermodynamic model with the previous article [
43].
| Items | W (kW) | (-) | (kg/s) | (kPa) | (K) | (kPa) | (K) |
|---|
| R134a [43] | 147.5 | 0.0852 | 8.9667 | 3723.4 | 369.9 | 883.3 | 308 |
| R134a (Present) | 148.9 | 0.0853 | 8.967 | 3724.1 | 369.9 | 883.2 | 308 |
| R11 [43] | 290.3 | 0.166 | 7.487 | 3835.94 | 461 | 147.9 | 308 |
| R11 (Present) | 291.5 | 0.167 | 7.488 | 3837.4 | 461 | 147.9 | 308 |
Table 9.
Comparison results of the economic model with the previous article [
23].
Table 9.
Comparison results of the economic model with the previous article [
23].
| Items | W (kW) (K) | (K) | (K) | (K) | (K) | LCOE ($/kWh) | (%) |
|---|
| R717 [23] | 296.32 | 284.20 | 1.54 | 297.89 | 282.78 | 0.341 | 28.17 |
| R717 (Present) | 296.32 | 284.20 | 1.54 | 297.89 | 282.78 | 0.332 | 28.25 |
| R134a [23] | 296.05 | 284.45 | 2.26 | 297.87 | 283.36 | 0.549 | 26.88 |
| R134a (Present) | 296.05 | 284.45 | 2.26 | 297.87 | 283.36 | 0.538 | 26.96 |
Table 10.
The series dual-loop ORC’ optimal parameters calculated by the TOPSIS method.
Table 10.
The series dual-loop ORC’ optimal parameters calculated by the TOPSIS method.
| Fluid Pairs | Cy\Cy | Cy\R141b | Cy\R245fa | R141b\Cy | Unit |
|---|
| 484.90 | 487.17 | 494.51 | 474.50 | K |
| 5.00 | 5.00 | 5.00 | 49.88 | K |
| 447.69 | 447.65 | 447.67 | 447.65 | K |
| 371.19 | 361.59 | 317.42 | 392.73 | K |
| 5.08 | 5.04 | 5.00 | 5.00 | K |
| 305.15 | 305.15 | 305.15 | 305.15 | K |
| 10.78 | 10.71 | 11.00 | 10.92 | K |
| 62.18 | 61.64 | 56.56 | 61.14 | % |
| LCOE | 0.1509 | 0.1510 | 0.1417 | 0.1540 | $/kWh |
Table 11.
The parallel dual-loop ORC’s optimal parameters calculated by the TOPSIS method.
Table 11.
The parallel dual-loop ORC’s optimal parameters calculated by the TOPSIS method.
| Fluid Pairs | Cy\R141b | Cy\Cy | Cy\R245fa | R141b\Cy | Unit |
|---|
| 496.69 | 496.68 | 520.67 | 473.60 | K |
| 5.00 | 5.00 | 5.00 | 49.13 | K |
| 449.57 | 449.41 | 533.77 | 533.79 | K |
| 305.15 | 305.15 | 305.15 | 305.15 | K |
| 10.98 | 11.00 | 5.01 | 5.03 | K |
| 434.36 | 424.11 | 494.15 | 493.85 | K |
| 5.00 | 15.05 | 5.00 | 5.00 | K |
| 447.65 | 447.65 | 447.65 | 447.65 | K |
| 305.15 | 305.15 | 305.15 | 305.15 | K |
| 5.02 | 5.00 | 11.00 | 10.80 | K |
| 449.57 | 449.41 | 449.42 | 533.79 | K |
| 56.89 | 56.90 | 56.77 | 56.79 | % |
| LCOE | 0.1391 | 0.1398 | 0.1395 | 0.1403 | $/kWh |
Table 12.
The series triple-loop ORC’ optimal parameters calculated by the TOPSIS method.
Table 12.
The series triple-loop ORC’ optimal parameters calculated by the TOPSIS method.
| Fluid Pairs | Cy\R245fa\Cy | Cy\Cy\Cy | Cy\R245fa\R141b | Cy\R141b\Cy | Unit |
|---|
| 481.75 | 484.92 | 485.10 | 482.14 | K |
| 5.00 | 5.00 | 5.00 | 5.00 | K |
| 447.65 | 447.65 | 447.65 | 447.66 | K |
| 382.35 | 368.24 | 370.44 | 380.99 | K |
| 5.00 | 5.00 | 5.00 | 5.00 | K |
| 372.57 | 362.17 | 362.97 | 364.91 | K |
| 5.01 | 5.00 | 5.00 | 5.00 | K |
| 305.15 | 305.15 | 305.15 | 305.15 | K |
| 11.00 | 11.00 | 11.00 | 10.96 | K |
| 61.30 | 60.41 | 60.60 | 61.97 | % |
| LCOE | 0.1588 | 0.1580 | 0.1581 | 0.1632 | $/kWh |
Table 13.
The series quadruple-loop ORC’ optimal parameters calculated by the TOPSIS method.
Table 13.
The series quadruple-loop ORC’ optimal parameters calculated by the TOPSIS method.
| Fluid pairs | Cy\R245fa\R245fa\Cy | Cy\R245fa\R245fa\R141b | Cy\R245fa\R134a\Cy | Cy\R245fa\Cy\Cy | Unit |
|---|
| 480.97 | 481.50 | 484.28 | 480.09 | K |
| 5.00 | 5.00 | 5.00 | 5.00 | K |
| 447.65 | 447.65 | 447.65 | 447.65 | K |
| 385.00 | 383.38 | 373.68 | 387.54 | K |
| 5.01 | 5.04 | 5.07 | 5.00 | K |
| 376.20 | 374.65 | 364.70 | 380.84 | K |
| 5.00 | 5.00 | 5.00 | 5.00 | K |
| 365.77 | 364.79 | 358.62 | 358.23 | K |
| 5.00 | 5.04 | 5.00 | 5.00 | K |
| 305.15 | 305.15 | 305.15 | 305.15 | K |
| 10.97 | 10.88 | 11.00 | 10.92 | K |
| 60.16 | 59.86 | 59.35 | 61.08 | % |
| LCOE | 0.1663 | 0.1667 | 0.1655 | 0.1714 | $/kWh |