Entropic Balance Conditions and Optimization of Distillation Column System
Abstract
:1. Introduction
- How to distribute raw material and heat flows in the parallel columns system so that for a given flow rate of separated mixture and composition of product flows, the total heat consumption would be minimal?
- What is the maximum capacity of the parallel columns for given compositions of raw materials and product flows at their outlets?
- In which order should the cascade of serial columns separate the flow of raw materials into fractions of a given composition with minimum total heat consumption?
- Given the design parameters of the columns, which of them is reasonable to use in the first stage in a serial separation?
- What is the maximum target product throughput of a cascade of consecutive columns?
2. Limitations of a Binary Distillation Column
2.1. Key Assumptions
- -
- Mass transfer is equimolar
- -
- In each section, pressures and temperatures of steam and liquid flows are close to each other (they change from section to section)
- -
- Effects of diffusion between adjacent sections are negligibly small
- -
- Heat from the outgoing flows is transferred to the incoming flows, and irreversibility of this heat exchange can be neglected
- -
- The flow of the separated mixture as a liquid is fed at the boiling point to the column section with the reflux composition close to the composition of the flow
- -
- Properties of the mixture are close to those of ideal solutions and the separation is close to clear.
2.2. Relationship between Heat Consumption and Column Capacity
2.3. Irreversible Energy Losses
- in the top section of the column
- in bottom section
2.4. Calculation of Irreversibility Factor and Reversible Efficiency Based on Measurement Results
2.5. Dependence of Reflux Ratio on Column Load and Characteristic Factors
3. The Limits of the Parallel Structure
3.1. Reversible Efficiency and Maximum Performance
3.2. Optimal Distribution of Heat Flows
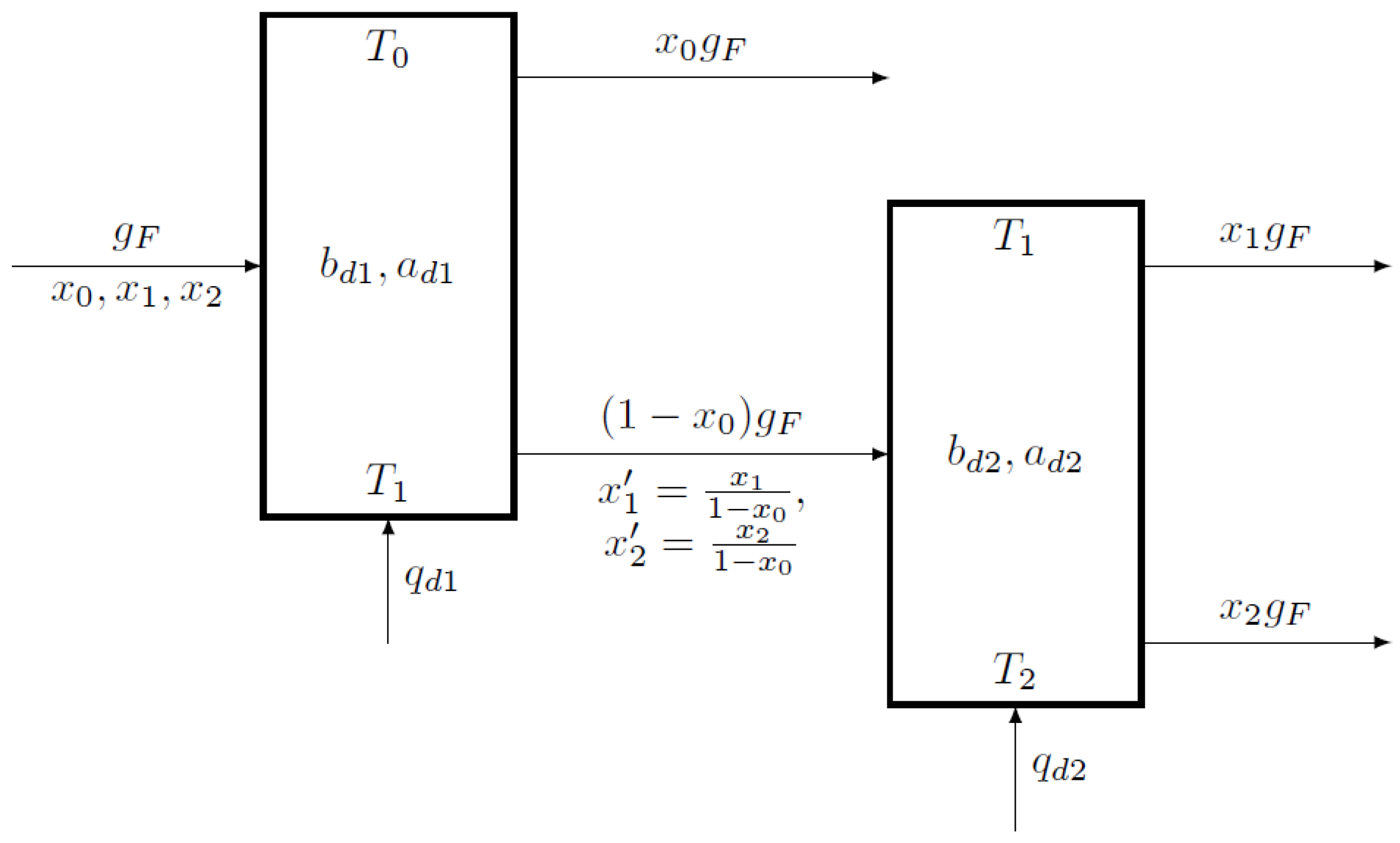
4. Limits of the Serial Structure
4.1. The Possible Modes and Choice of Sequence of Separation of a Three-Component Mixture
4.2. Selection of the Separation Order in the Reversible Approximation
4.3. The Realizable Modes of Cascade Separation of Three-Component Mixture
- for direct order
- for reverse order
4.4. Example of Selecting a Separation Order
- 1.
- Source data.
- 2.
- Let us calculate the characteristic parameters for each column.
- 3.
- Equation (25), which defines the separation order in the reversible approximation, is accurate up to a constant multiplier 105:It is fulfilled; hence the direct order is preferable.
- 4.
- Maximum cascade performance for direct and reverse separation order by Equations (26) and (27):
- 5.
- For each option, the total heat consumption by Equations (28) and (29) for = 1 mol/s are:
4.5. Generalization for a Multi-Component Mixture
4.6. Case of n Consecutive Columns
- For the last two columns, all possible fractions of the three components with close boiling points (n − 2) are combed through. For each such fraction, using Equations (23)–(26), one finds the optimal separation order and the corresponding minimum heat consumption.
- One adds to this cascade the third to last column and for the resulting cascade chooses all fractions from the four components having similar boiling points (n − 3). Of the two possible options for direct and reverse order of separation of each such fraction, the best one is selected, taking into account that each order of separation corresponds to the optimal performance of the cascade of the last two columns. Thus, each mixture of four closely related components is assigned the order of its separation and the minimum required heat consumption.
- The fourth to last column of the cascade is added, and all five-component fractions are screened in the same way.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| a | irreversibility factor; |
| b | reversible efficiency; |
| A | separation work; |
| g | flow rate, mol/s; |
| h | molar enthalpy, J/mol; |
| k | effective mass transfer factor, mol/(s·m); |
| q | heat flow, W; |
| R | reflux ratio, universal gas constant, J/(mol·K); |
| s | molar entropy, J/(mol·K); |
| T | temperature, K; |
| temperatures of coolant in the still and refrigerant in the dephlegmator, respectively, K; | |
| L | reflux flow rate, mol/s; |
| V | steam flow rate, mol/s; |
| x | molar concentration of low-boiling component in the liquid phase, mol/mol; |
| y | molar concentration of low-boiling component in the vapor phase, mol/mol; |
| y0 | equilibrium concentration of the low-boiling component in the vapor phase, mol/mol; |
| r | molar heat of vaporization, J/mol; |
| β | heat exchange rate, W/K; |
| ε | relative selection of distillate; |
| µ | chemical potential, J/mol; |
| ηc | thermal efficiency of the column; |
| σ | entropy production, W/K. |
| Indices | Indices |
| B | for variables related to the cube; |
| D | for variables related to the dephlegmator; |
| F | for variables related to the initial mixture; |
| i | distillation column number; |
| d | direct separation order; |
| r | reverse separation order. |
References
- Gel’perin, N.I. Osnovnye Processy i Apparaty Himicheskoj Tekhnologii; Chimiya: Moscow, Russia, 1981. (In Russian) [Google Scholar]
- Kafarov, V.V.; Meshalkin, V.P.; Perov, V.L. Matematicheskie Osnovy Avtomatizirovannogo Proektirovaniya Himicheskih Proizvodstv; Chimiya: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Holland, C.D. Fundamentals of Multicomponent Distillation; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Kister, H.Z. Distillation Design; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Tsirlin, A.M.; Grigorevskiy, I.N.; Schwalbe, K. Thermodynamical estimation of the bounds on performance of irreversible binary distillation. Int. J. Heat Mass Transf. 2018, 118, 289–296. [Google Scholar] [CrossRef]
- Liu, G.B.; Yu, K.T.; Yuan, X.G.; Liu, C.J. A numerical method for predicting the performance of a randomly packed distillation column. Int. J. Heat Mass Transf. 2009, 52, 5300–5338. [Google Scholar] [CrossRef]
- Balunov, A.I.; Tsirlin, A.M. Heat and mass exchange and feasible mode sets of irreversible separation processes. Int. J. Heat Mass Transf. 2018, 121, 119–126. [Google Scholar] [CrossRef]
- Tsirlin, A.M.; Balunov, A.I.; Sukin, I.A. Estimates of energy consumption and selection of optimal distillation sequence for multicomponent distillation. Theor. Found. Chem. Eng. 2016, 50, 250–259. [Google Scholar] [CrossRef]
- Balunov, A.I.; Tsirlin, A.M. Estimation of the maximum output of the process of rectification of a continuous mixture with regard for the irreversibility of the heat and mass Transfer in it. J. Eng. Phys. Thermophys. 2020, 93, 261–270. [Google Scholar] [CrossRef]
- Tsirlin, A.M.; Sukin, I.A.; Balunov, A.I. Mathematical model of rectification process and selection of the separation sequence for multicomponent mixtures. Math. Models Comput. Simul. 2021, 13, 483–491. [Google Scholar] [CrossRef]
- Kjelstrup, S.; Koeijer, G.M. Transport equations for distillation of ethanol and water from the entropy production rate. Chem. Eng. Sci. 2003, 58, 1147–1161. [Google Scholar] [CrossRef]
- Røsjorde, A.; Kjelstrup, S. The second law optimal state of adiabatic binary tray distillation column. Chem. Eng. Sci. 2003, 58, 1199–1210. [Google Scholar]
- Mendoza, D.F.; Kjelstrup, S. Modeling a non-equilibrium distillation stage using irreversible thermodynamics. Chem. Eng. Sci. 2011, 66, 2713–2722. [Google Scholar] [CrossRef]
- Mullins, O.C.; Berry, R.S. Minimization of entropy production in distillation. J. Phys. Chem. 1984, 88, 723–728. [Google Scholar] [CrossRef]
- Tsirlin, A.M.; Kazakov, V.A.; Zubov, D.V. Finite-time thermodynamics: Limiting possibilities of irreversible separation processes. J. Phys. Chem. A 2002, 106, 10926–10936. [Google Scholar] [CrossRef]
- Orlov, V.N.; Berry, R.S. Estimation of minimal heat consumption for heat-driven separation processes via methods of finite-time thermodynamics. J. Phys. Chem. 1991, 95, 5624–5628. [Google Scholar] [CrossRef]
- Schaller, M.; Hoffmann, K.H.; Rivero, R.; Andresen, B.; Salamon, P. The influence of heat transfer irreversibilities on the optimal performance of diabatic distillation columns. J. Non-Equilib. Thermodyn. 2002, 27, 257–269. [Google Scholar] [CrossRef]
- Koeijer, G.D.; Rosjorde, A.; Kjelstrup, S. Distribution of heat exchange in optimum diabatic distillation columns. Energy 2004, 29, 2425–2440. [Google Scholar] [CrossRef]
- Shu, L.W.; Chen, L.G.; Sun, F.R. Performance optimization of a diabatic distillation column by allocating sequential heat exchanger inventory. Appl. Energy 2007, 84, 893–903. [Google Scholar] [CrossRef]
- Shu, L.W.; Chen, L.G.; Sun, F.R. The minimal average heat consumption for heat-driven binary separation process with linear phenomenological heat transfer law. Sci. China Ser. B Chem. 2009, 52, 1154–1163. [Google Scholar] [CrossRef]
- Berry, R.S.; Kasakov, V.A.; Sieniutycz, S.; Szwast, Z.; Tsirlin, A.M. Thermodynamic Optimization of Finite Time Processes; John Wiley and Sons: Chichester, UK, 1999. [Google Scholar]
- Tsirlin, A.M.; Grigorevsky, I.N. Thermodynamical estimation of the limit capacity of irreversible binary distillation. J. Non-Equilib. Thermodyn. 2010, 35, 213–233. [Google Scholar] [CrossRef]
- Tsirlin, A.M.; Sukin, I.A. Finite-time thermodynamics: The maximal productivity of binary distillation and selection of optimal separation sequence for an ideal ternary mixture. J. Non-Equilib. Thermodyn. 2014, 39, 13–25. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Ge, Y.; Feng, H. Optimizing power and thermal efficiency of an irreversible variable-temperature heat reservoir Lenoir cycle. Appl. Sci. 2021, 11, 7171. [Google Scholar] [CrossRef]
- Masser, R.; Hoffmann, K.H. Optimal control for a hydraulic recuperation system using endoreversible thermodynamics. Appl. Sci. 2021, 11, 5001. [Google Scholar] [CrossRef]
- Feidt, M. The history and perspectives of efficiency at maximum power of the Carnot engine. Entropy 2017, 19, 369. [Google Scholar] [CrossRef]
- Feidt, M.; Costea, M. Progress in Carnot and Chambadal modeling of thermomechnical engine by considering entropy and heat transfer entropy. Entropy 2019, 21, 1232. [Google Scholar] [CrossRef] [Green Version]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Bellman, R.; Dreyfus, S. Applied Dynamic Programming; Princeton University Press: Princeton, NJ, USA, 1962. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balunov, A.; Sukin, I.; Tsirlin, A. Entropic Balance Conditions and Optimization of Distillation Column System. Entropy 2021, 23, 1468. https://doi.org/10.3390/e23111468
Balunov A, Sukin I, Tsirlin A. Entropic Balance Conditions and Optimization of Distillation Column System. Entropy. 2021; 23(11):1468. https://doi.org/10.3390/e23111468
Chicago/Turabian StyleBalunov, Alexander, Ivan Sukin, and Anatoly Tsirlin. 2021. "Entropic Balance Conditions and Optimization of Distillation Column System" Entropy 23, no. 11: 1468. https://doi.org/10.3390/e23111468
APA StyleBalunov, A., Sukin, I., & Tsirlin, A. (2021). Entropic Balance Conditions and Optimization of Distillation Column System. Entropy, 23(11), 1468. https://doi.org/10.3390/e23111468






