Decreased Resting-State Functional Complexity in Elderly with Subjective Cognitive Decline
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADNI Study Design
2.2. Participants
2.3. Neuropsychological Assessments
2.4. Data Preprocessing
2.5. AAPE Algorithm
2.6. Statistical Analysis
3. Results
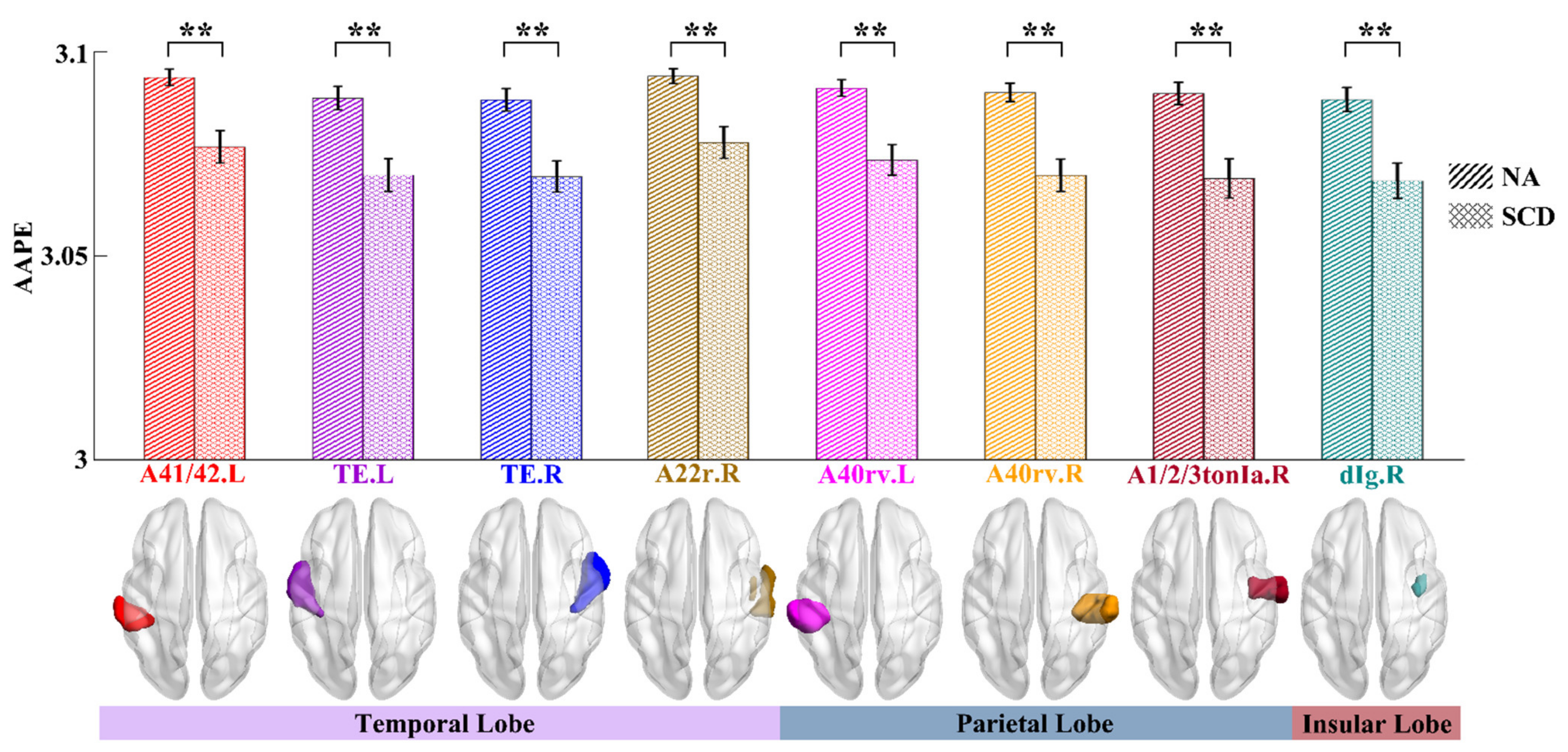
3.1. Complexity Differences of ROIs between NA and SCD Groups
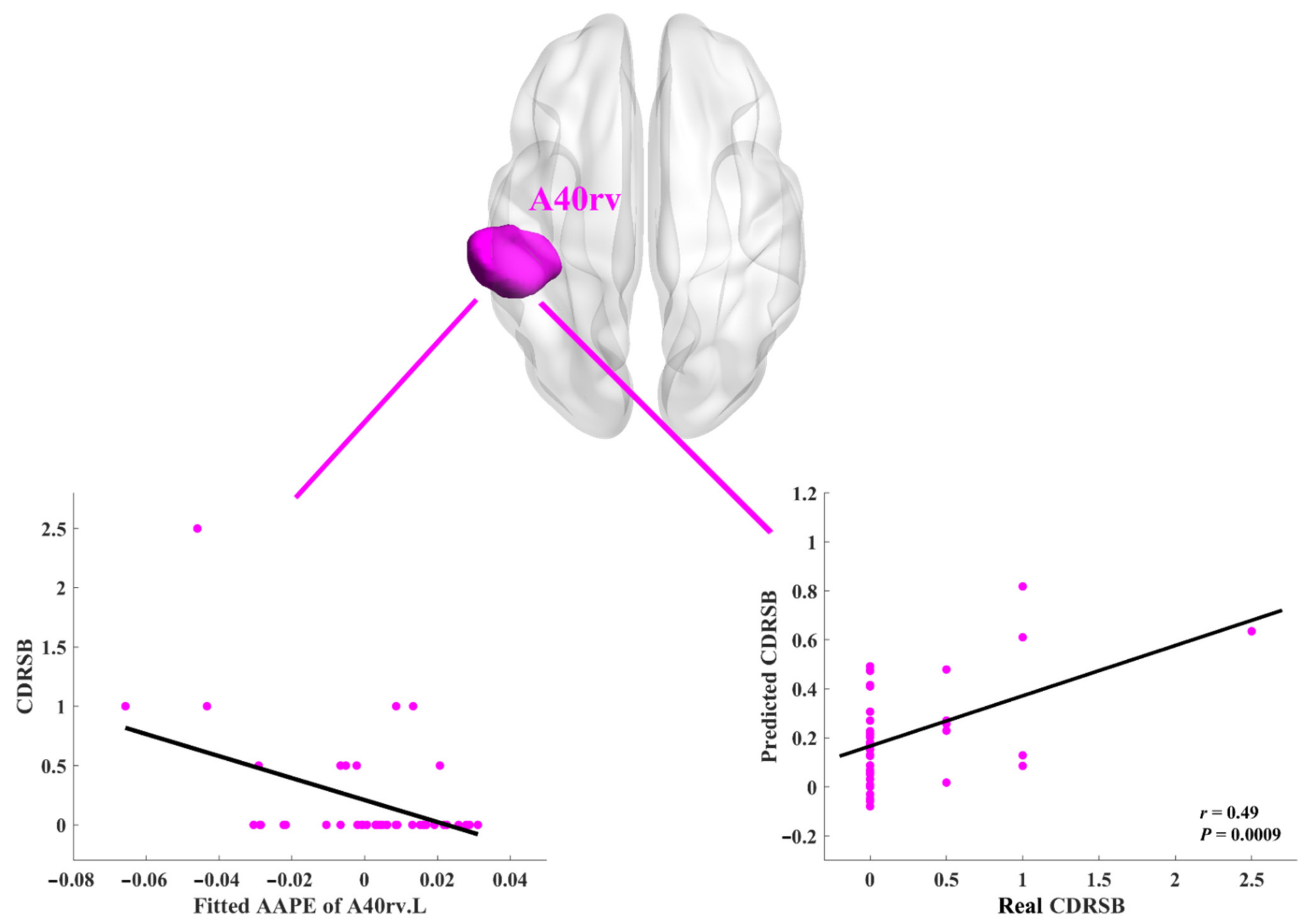
3.2. Relationships between AAPE and Neuropsychological Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabin, L.A.; Smart, C.M.; Amariglio, R.E. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu. Rev. Clin. Psychol. 2017, 13, 369–396. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Huang, W.; Su, L.; Xing, Y.; Jessen, F.; Sun, Y.; Shu, N.; Han, Y. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 1–27. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Shan, P.-Y.; Jiang, W.-J.; Sheng, C.; Ma, L. Subjective cognitive decline: Preclinical manifestation of Alzheimer’s disease. Neurol. Sci. 2018, 40, 41–49. [Google Scholar] [CrossRef]
- Viviano, R.P.; Damoiseaux, J.S. Functional neuroimaging in subjective cognitive decline: Current status and a research path forward. Alzheimer’s Res. Ther. 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillen, K.N.; Jacobs, H.; Kukolja, J.; von Reutern, B.; Richter, N.; Onur, O.; Dronse, J.; Langen, K.-J.; Fink, G.R. Aberrant functional connectivity differentiates retrosplenial cortex from posterior cingulate cortex in prodromal Alzheimer’s disease. Neurobiol. Aging 2016, 44, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Initiative, F.T.A.D.N.; Luo, X.; Zeng, Q.; Jiaerken, Y.; Xu, X.; Huang, P.; Shen, Z.; Xu, J.; Wang, C.; et al. Aberrant functional connectivity network in subjective memory complaint individuals relates to pathological biomarkers. Transl. Neurodegener. 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Liu, T.; Ai, J.; Chen, D.; Zhuo, Y.; Zhao, G.; He, S.; Wu, J.; Han, Y.; Yan, T. Changes in centrality frequency of the default mode network in individuals with subjective cognitive decline. Front. Aging Neurosci. 2019, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Sui, D.; Qiao, K.; Dong, H.-M.; Chen, L.; Han, Y. Impaired functional criticality of human brain during Alzheimer’s disease progression. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Dai, Z.; Li, Y.; Sheng, C.; Li, H.; Wang, X.; Chen, X.; He, Y.; Han, Y. Subjective cognitive decline: Mapping functional and structural brain changes—A combined resting-state functional and structural MR imaging Study. Radiology 2016, 281, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, Y.; Wang, Y.; Hu, X.; Lu, J.; Chan, P.; Yan, T.; Han, Y. gradual disturbances of the amplitude of low-frequency fluctuations (ALFF) and Fractional ALFF in Alzheimer spectrum. Front. Neurosci. 2018, 12, 975. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Zhou, L.; Ning, X.; Wang, L. Exploring multifractal-based features for mild Alzheimer’s disease classification. Magn. Reson. Med. 2016, 76, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, R.; Laumann, T.O.; Snyder, A.Z.; Zhou, J.; Yeo, B.T. Interpreting temporal fluctuations in resting-state functional connectivity MRI. NeuroImage 2017, 163, 437–455. [Google Scholar] [CrossRef]
- Xue, S.-W.; Yu, Q.; Guo, Y.; Song, D.; Wang, Z. Resting-state brain entropy in schizophrenia. Compr. Psychiatry 2019, 89, 16–21. [Google Scholar] [CrossRef]
- Shi, L.; Beaty, R.E.; Chen, Q.; Sun, J.; Wei, D.; Yang, W.; Qiu, J. Brain entropy is associated with divergent thinking. Cereb. Cortex 2019, 30, 708–717. [Google Scholar] [CrossRef]
- Wang, B.; Niu, Y.; Miao, L.; Cao, R.; Yan, P.; Guo, H.; Liwen, M.; Guo, Y.; Yan, T.; Wu, J.; et al. decreased complexity in Alzheimer’s disease: Resting-State fMRI evidence of brain entropy mapping. Front. Aging Neurosci. 2017, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Sokunbi, M.O.; Staff, R.T.; Waiter, G.; Ahearn, T.S.; Fox, H.C.; Deary, I.J.; Starr, J.M.; Whalley, L.J.; Murray, A.D. Inter-individual Differences in fMRI Entropy Measurements in Old Age. IEEE Trans. Biomed. Eng. 2011, 58, 3206–3214. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, G.; Richards, D.A. Predictability analysis of absence seizures with permutation entropy. Epilepsy Res. 2007, 77, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Cuesta–Frau, D. Permutation entropy: Influence of amplitude information on time series classification performance. Math. Biosci. Eng. 2019, 16, 6842–6857. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Escudero, J. Amplitude-aware permutation entropy: Illustration in spike detection and signal segmentation. Comput. Methods Programs Biomed. 2016, 128, 40–51. [Google Scholar] [CrossRef]
- Saykin, A.J.; Wishart, H.A.; Rabin, L.A.; Santulli, R.B.; Flashman, L.A.; West, J.D.; McHugh, T.L.; Mamourian, A.C. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006, 67, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.C. The clinical dementia rating (cdr): Current version and. Young 1991, 41, 1588–1592. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.-K.; Weiner, M.W.; Aisen, P.S. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017, 317, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.C.; Sperling, R.A.; Salmon, D.P.; Rentz, D.M.; Raman, R.; Thomas, R.G.; Weiner, M.; Aisen, P.S. The preclinical alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol. 2014, 71, 961–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitan, R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Yan, C.-G.; Wang, X.-D.; Zuo, X.-N.; Zang, Y.-F. DPABI: Data processing & analysis for (Resting-State) brain imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Yan, C. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.-P.; Frith, C.D.; Frackowiak, R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Parkes, L.; Fulcher, B.; Yücel, M.; Fornito, A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage 2018, 171, 415–436. [Google Scholar] [CrossRef]
- Fan, L.; Lingzhong, F.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.; et al. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- Riedl, M.; Müller, A.; Wessel, N. Practical considerations of permutation entropy—A tutorial review. Eur. Phys. J. Spéc. Top. 2013, 222, 249–262. [Google Scholar] [CrossRef]
- Amigó, J.M.; Zambrano, S.; Sanjuán, M.A.F. Combinatorial detection of determinism in noisy time series. EPL Europhys. Lett. 2008, 83, 60005. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- De Brabanter, K.; Suykens, J.A.K.; De Moor, B. Nonparametric regression via StatLSSVM. J. Stat. Softw. 2013, 55, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Rentz, D.M.; Rodriguez, M.A.P.; Amariglio, R.; Stern, Y.; Sperling, R.A.; Ferris, S.H. Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimer’s Res. Ther. 2013, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Si, T.; Xing, G.; Han, Y. Subjective cognitive decline and related cognitive deficits. Front. Neurol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Jia, F.; Li, Y.; Li, M.; Cao, F. Subjective cognitive decline, cognitive reserve indicators, and the incidence of dementia. J. Am. Med. Dir. Assoc. 2021, 22, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A. Dynamics of Stability: The Physiologic basis of functional health and frailty. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2002, 57, B115–B125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsitz, L.A. Physiological complexity, aging, and the path to frailty. Sci. Aging Knowl. Environ. 2004, 2004, pe16. [Google Scholar] [CrossRef]
- Yang, Y.; Zha, X.; Zhang, X.; Ke, J.; Hu, S.; Wang, X.; Su, Y.; Hu, C. Dynamics and concordance abnormalities among indices of intrinsic brain activity in individuals with subjective cognitive decline: A temporal dynamics resting-state functional magnetic resonance imaging analysis. Front. Aging Neurosci. 2021, 12, 4863. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Choi, M.; Youn, H.; Suh, S.; Jeong, H.-G.; Han, C.E. Diffusion tensor imaging reveals abnormal brain networks in elderly subjects with subjective cognitive deficits. Neurol. Sci. 2019, 40, 2333–2342. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Li, W.; Li, H.; Xu, B.; Zhang, M.; Yue, L.; Wang, P.; Xiao, S. Morphological, structural, and functional networks highlight the role of the cortical-subcortical circuit in individuals with subjective cognitive decline. Front. Aging Neurosci. 2021, 13, 688113. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Pievani, M.; Testa, C.; Sabattoli, F.; Bresciani, L.; Bonetti, M.; Beltramello, A.; Hayashi, K.M.; Toga, A.W.; Thompson, P. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain 2007, 130, 720–730. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Parker, A.; Parker, A.; Derrington, A.; Derrington, A.; Blakemore, C.; Blakemore, C. The neural basis of the blood–oxygen–level–dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1003–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Q.-Y.; Li, T.-R.; Jiang, X.-Y.; Wang, X.-N.; Han, Y.; Jiang, J.-H. Glucose metabolism in the right middle temporal gyrus could be a potential biomarker for subjective cognitive decline: A study of a Han population. Alzheimer’s Res. Ther. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Mosconi, L.; De Santi, S.; Brys, M.; Tsui, W.H.; Pirraglia, E.; Glodzik-Sobanska, L.; Rich, K.E.; Switalski, R.; Mehta, P.D.; Pratico, D.; et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein e e4 carriers with subjective memory complaints. Biol. Psychiatry 2008, 63, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Kielb, S.; Rogalski, E.; Weintraub, S.; Rademaker, A. Objective features of subjective cognitive decline in a United States national database. Alzheimer’s Dement. 2017, 13, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
| NA | SCD | p Value | |
|---|---|---|---|
| Number of participants | 43 | 43 | - |
| Years of age | 73.57 ± 3.27 | 75.48 ± 5.66 | 0.0584 a,c |
| Sex (Male/Female) | 17/26 | 14/29 | 0.5005 b,c |
| Years of education | 16.33 ± 2.35 | 16.37 ± 2.90 | 0.9351 a,c |
| CDRSB | 0.15 ± 0.55 | 0.21 ± 0.48 | 0.6029 a,c |
| MMSE | 28.98 ± 1.14 | 29.14 ± 0.97 | 0.4778 a,c |
| ADAS13 | 9.26 ± 5.32 | 7.91 ± 4.58 | 0.2138 a,c |
| ADAS-Word | 2.88 ± 1.94 | 2.00 ± 1.48 | 0.0199 a,d |
| FAQ | 0.23 ± 0.84 | 0.48 ± 0.89 | 0.1980 a,c |
| GDS | 1.12 ± 1.89 | 1.30 ± 1.12 | 0.5809 a,c |
| CCI | - | 24.91 ± 2.06 | - |
| PACC-DSST | −0.18 ± 4.06 | 0.53 ± 2.90 | 0.3531 a,c |
| PACC-LogTMTB | −0.14 ± 3.64 | 0.34 ± 2.74 | 0.4909 a,c |
| Gyrus | ROI | CDRSB (r, p) | MMSE (r, p) | PACC-DSST (r, p) | PACC-LogTMTB (r, p) |
|---|---|---|---|---|---|
| STG | A41/42.L | 0.39, 0.0099 * | 0.25, 0.1086 | 0.05, 0.7445 | 0.15, 0.3466 |
| TE.L | 0.28, 0.0728 | 0.24, 0.116 | 0.02, 0.9041 | 0.01, 0.9314 | |
| TE.R | 0.29, 0.0633 | 0.34, 0.028 * | 0.02, 0.8882 | 0.01, 0.9273 | |
| A22r.R | 0.20, 0.1935 | 0.15, 0.335 | 0.03, 0.8589 | 0.07, 0.6457 | |
| IPL | A40rv.L | 0.49, 0.0009 ** | 0.26, 0.0878 | 0.08, 0.6174 | 0.16, 0.3178 |
| A40rv.R | 0.43, 0.0043 * | 0.1, 0.5174 | 0.04, 0.8164 | 0.08, 0.6197 | |
| PoG | A1/2/3tonIa.R | 0.18, 0.261 | 0.2, 0.1902 | 0.01, 0.9342 | 0.05, 0.7556 |
| INS | dIg.R | 0.11, 0.5008 | 0.4, 0.0075 * | 0.11, 0.4815 | 0.12, 0.4289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Song, Z.; Liang, L.; Xing, Q.; Qin, J.; Wu, X. Decreased Resting-State Functional Complexity in Elderly with Subjective Cognitive Decline. Entropy 2021, 23, 1591. https://doi.org/10.3390/e23121591
Ni H, Song Z, Liang L, Xing Q, Qin J, Wu X. Decreased Resting-State Functional Complexity in Elderly with Subjective Cognitive Decline. Entropy. 2021; 23(12):1591. https://doi.org/10.3390/e23121591
Chicago/Turabian StyleNi, Huangjing, Zijie Song, Lei Liang, Qiaowen Xing, Jiaolong Qin, and Xiaochuan Wu. 2021. "Decreased Resting-State Functional Complexity in Elderly with Subjective Cognitive Decline" Entropy 23, no. 12: 1591. https://doi.org/10.3390/e23121591
APA StyleNi, H., Song, Z., Liang, L., Xing, Q., Qin, J., & Wu, X. (2021). Decreased Resting-State Functional Complexity in Elderly with Subjective Cognitive Decline. Entropy, 23(12), 1591. https://doi.org/10.3390/e23121591





