Experimental and Theoretical Analysis of Metal Complex Diffusion through Cell Monolayer
Abstract
1. Introduction
2. Materials and Methods
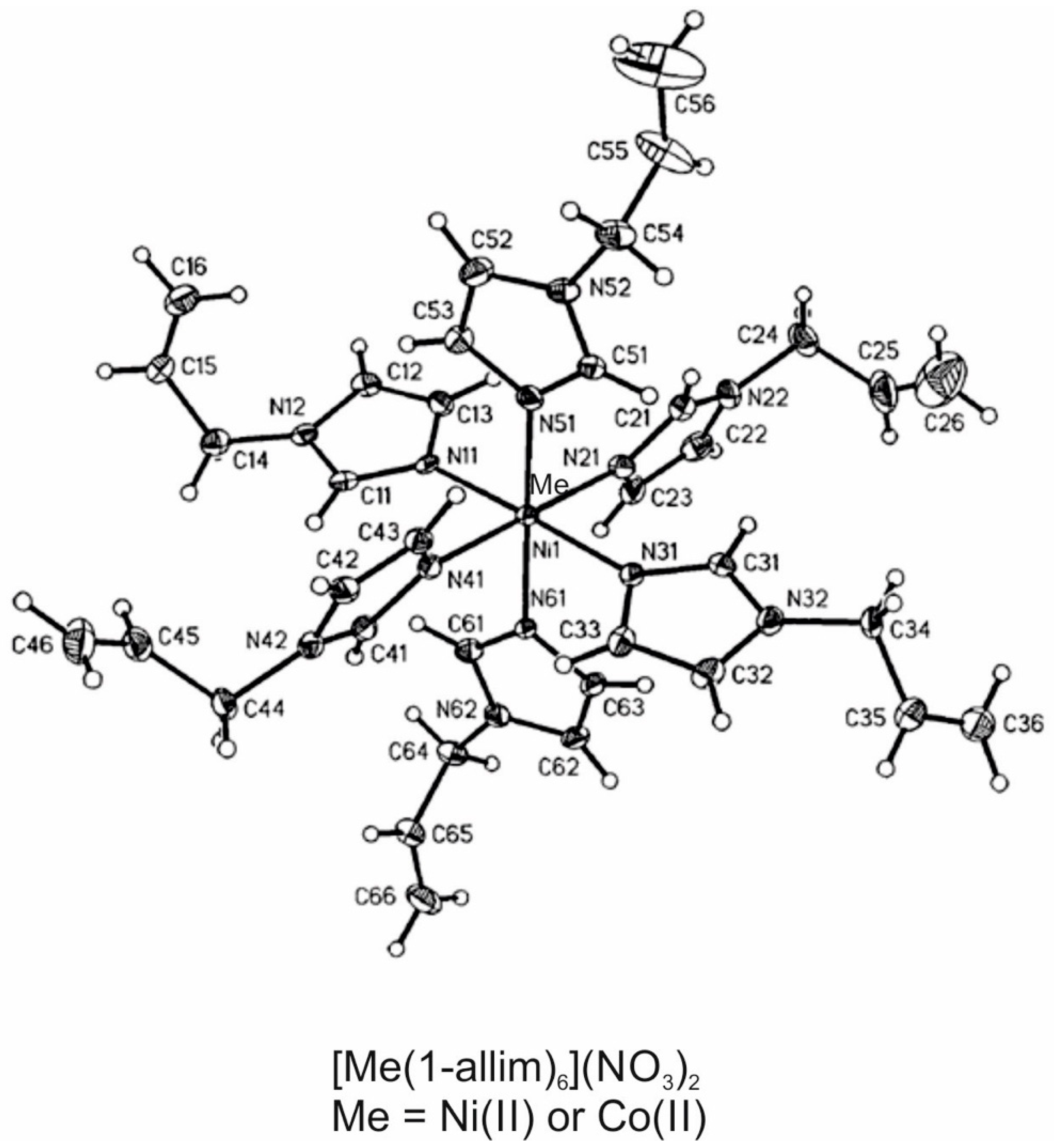
2.1. Metal Complexes
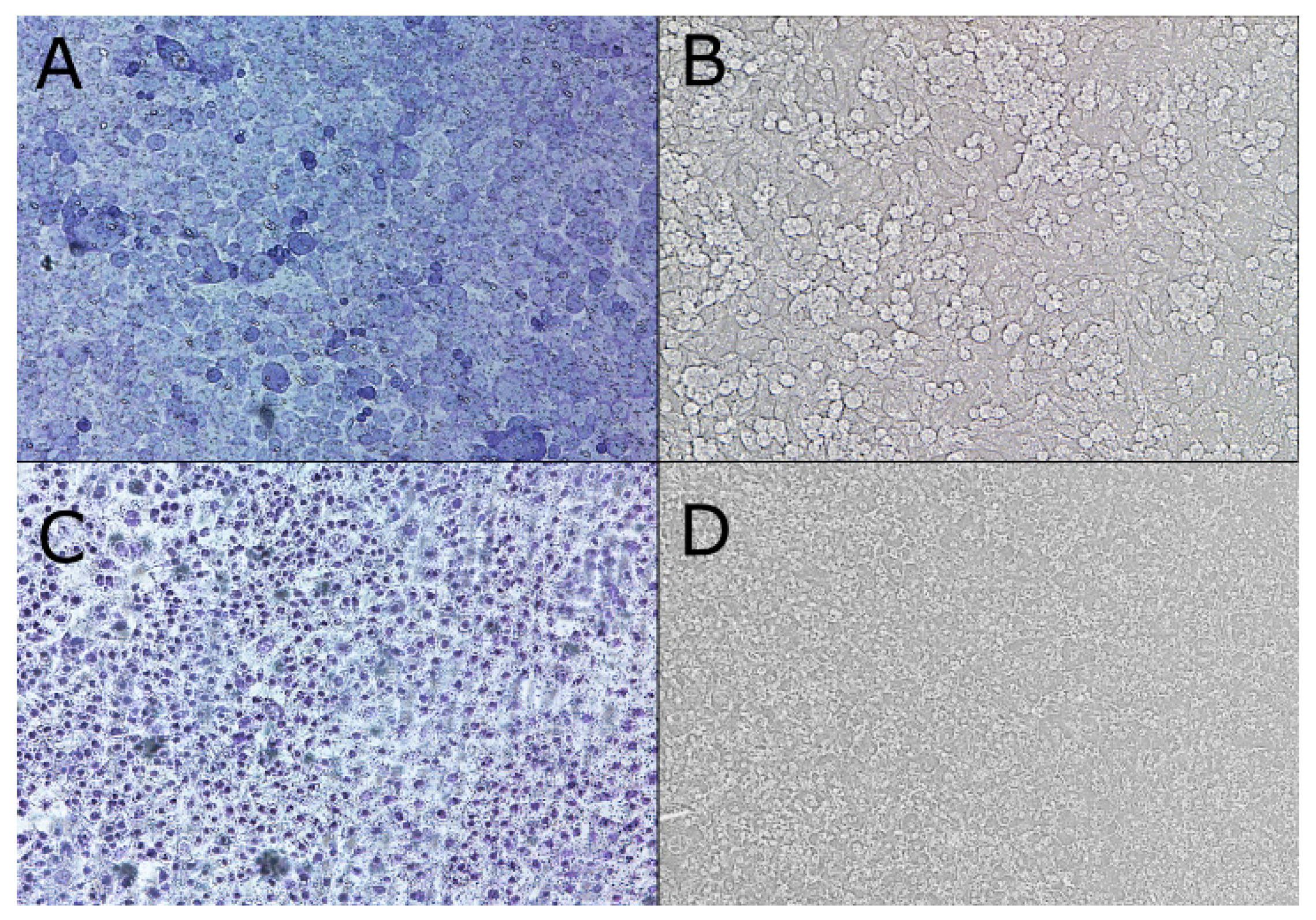
2.2. Cell Monolayer Construction
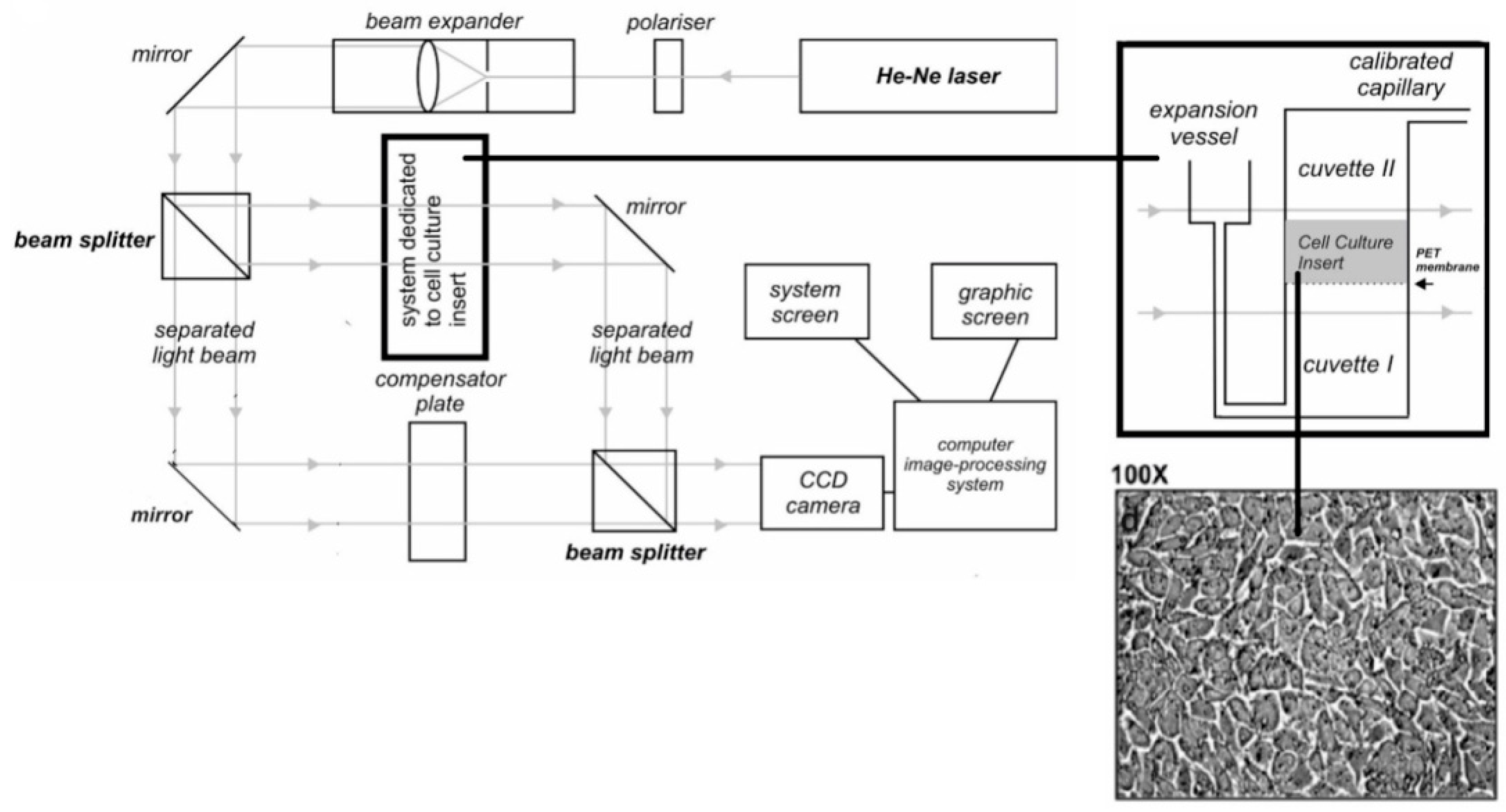
2.3. Laser Interferometry
3. Results
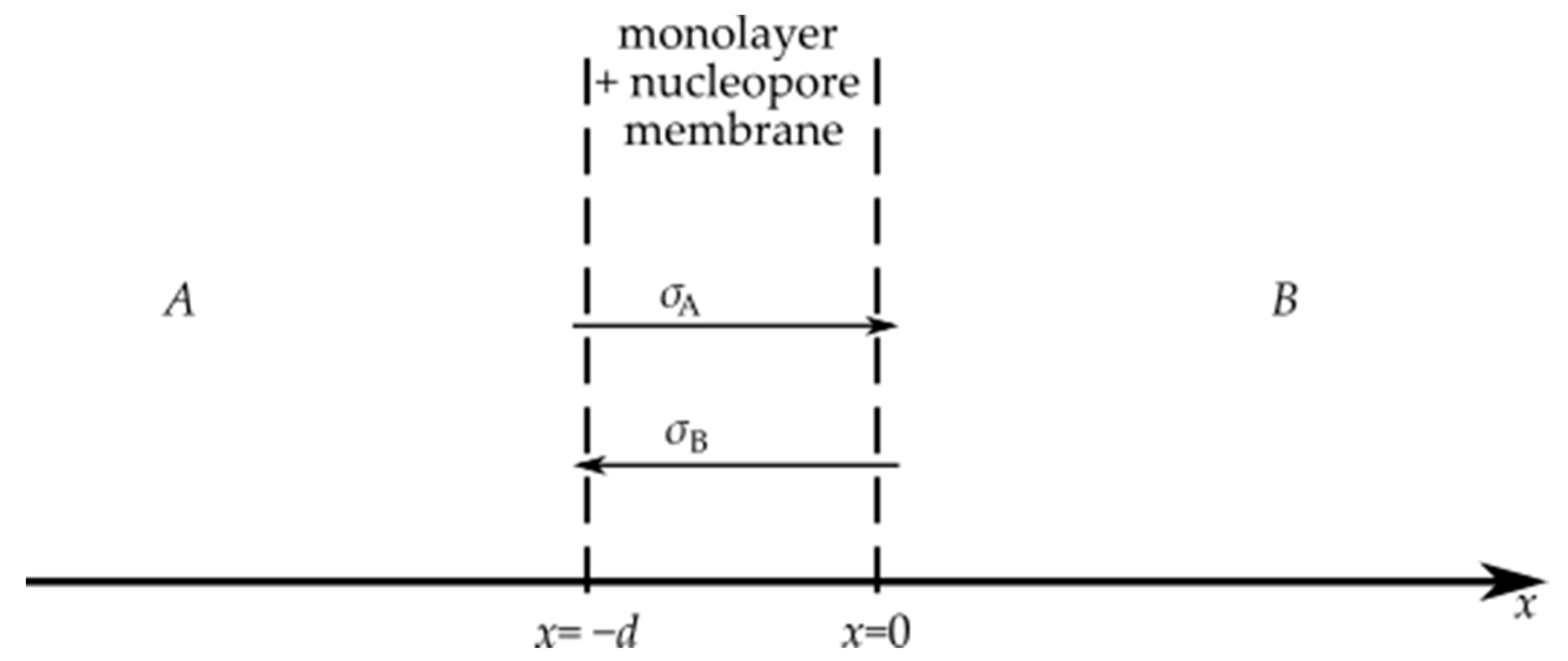
3.1. Diffusion of Metal Complexes Through a Eukaryotic Cell Monolayer
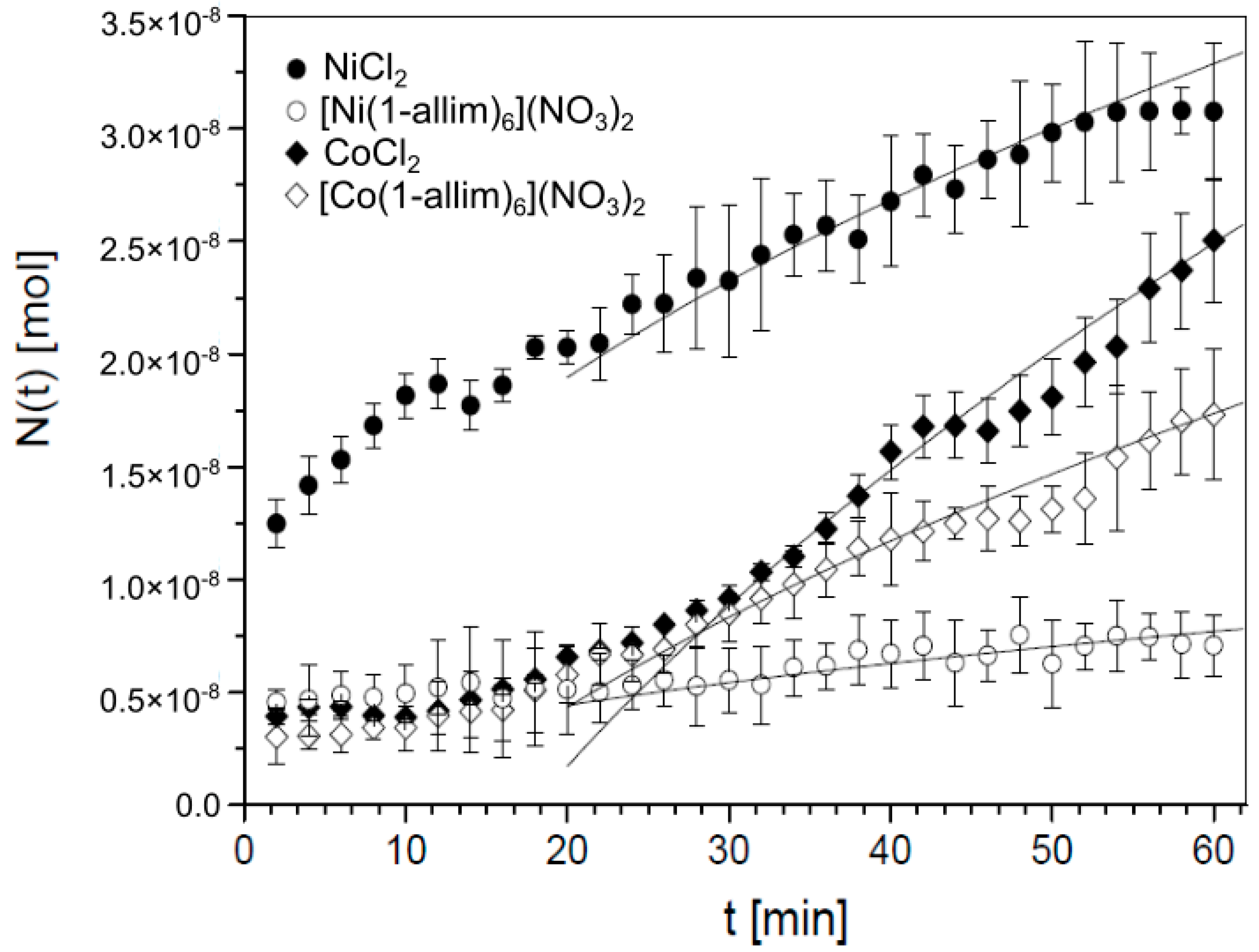
3.1.1. Experiment
3.1.2. Theory
- for NiCl2, a = 4.25 × 10−9 mol/ and b = 0,
- for [Ni(1-allim)6](NO3)2, a = 0.97 × 10−9 mol/ and b = 0,
- for CoCl2, a = 5.70 × 10−9 mol/, b = 3.3 × 10−8 mol and κ = 0.14 1/min,
- for [Co(1-allim)6](NO3)2, a = 2.80 × 10−9 mol/, b = 4.0 × 10−8 mol and κ = 0.14 1/min.
Calculations for Nickel Compounds
Calculations for Cobalt Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Site-Specif. Protein Label. Methods Protoc. 2015, 1266, 29–53. [Google Scholar] [CrossRef]
- Gravelle, S.; Joly, L.; Detcheverry, F.; Ybert, C.; Cottin-Bizonne, C.; Bocquet, L. Optimizing water permeability through the hourglass shape of aquaporins. Proc. Natl. Acad. Sci. USA 2013, 110, 16367–16372. [Google Scholar] [CrossRef]
- Pagliara, S.; Dettmer, S.L.; Keyser, U.F. Channel-facilitated diffusion boosted by particle binding at the channel entrance. Phys. Rev. Lett. 2014, 113, 1–5. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Danelon, C.; Winterhalter, M.; Bezrukov, S.M. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 2002, 99, 9789–9794. [Google Scholar] [CrossRef]
- Łapińska, U.; Glover, G.; Capilla-Lasheras, P.; Young, A.J.; Pagliara, S. Bacterial ageing in the absence of external stressors. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180442. [Google Scholar] [CrossRef]
- Thurber, G.M.; Yang, K.S.; Reiner, T.; Kohler, R.H.; Sorger, P.; Mitchison, T.; Weissleder, R. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 2013, 4, 4–13. [Google Scholar] [CrossRef]
- Pagliara, S.; Schwall, C.; Keyser, U.F. Optimizing diffusive transport through a synthetic membrane channel. Adv. Mater. 2013, 25, 844–849. [Google Scholar] [CrossRef]
- Bezrukov, S.M.; Berezhkovskii, A.M.; Szabo, A. Diffusion model of solute dynamics in a membrane channel: Mapping onto the two-site model and optimizing the flux. J. Chem. Phys. 2007, 127, 1–9. [Google Scholar] [CrossRef]
- Bauer, W.R.; Nadler, W. Molecular transport through channels and pores: Effects of in-channel interactions and blocking. Proc. Natl. Acad. Sci. USA 2006, 103, 11446–11451. [Google Scholar] [CrossRef]
- Dagdug, L.; Vazquez, M.V.; Berezhkovskii, A.M.; Bezrukov, S.M. Unbiased diffusion in tubes with corrugated walls. J. Chem. Phys. 2010, 133, 127–130. [Google Scholar] [CrossRef]
- Kolomeisky, A.B. Channel-facilitated molecular transport across membranes: Attraction, repulsion, and asymmetry. Phys. Rev. Lett. 2007, 98, 1–4. [Google Scholar] [CrossRef]
- Dai, X.; Hou, C.; Xu, Z.; Yang, Y.; Zhu, G.; Chen, P.; Huang, Z. Entropic Effects in Polymer Nanocomposites. Entropy 2019, 21, 186. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, L.; Chen, P.; Yan, L.-T. Diffusive transport of nanoscale objects through cell membranes: A computational perspective. Soft Matter. 2020, 16, 3869–3881. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Palencia, G.; Zuñiga, S.; Rodríguez-Ropon, A.; Osorio-Rico, L.; Torres Luvia, S.; Gracia-Mora, I.; Marquez-Rosado, L.; Sánchez, A.; Moreno-García, M.E.; et al. Cas Ilgly Induces Apoptosis in Glioma C6 Cells In Vitro and In Vivo through Caspase-Dependent and Caspase-Independent Mechanisms. Neoplasia 2005, 7, 563–574. [Google Scholar] [CrossRef]
- Wąsik, S.; Arabski, M.; Dworecki, K.; Janoska, J.; Semaniak, J.; Szary, K.; Ślęzak, A. Laser interferometric analysis of glucose and sucrose diffusion in agarose gel. Gen. Physiol. Biophys. 2014, 33, 383–391. [Google Scholar] [CrossRef]
- Kosztołowicz, T.; Dworecki, K.; Mrówczyński, S. How to measure subdiffusion parameters. Phys. Rev. Lett. 2005, 94, 6–9. [Google Scholar] [CrossRef]
- Kosztołowicz, T.; Metzler, R.; Wa̧sik, S.; Arabski, M. Model of ciprofloxacin subdiffusion in Pseudomonas aeruginosa biofilm formed in artificial sputum medium. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Kosztołowicz, T.; Wasik, S.; Lewandowska, K.D. How to determine a boundary condition for diffusion at a thin membrane from experimental data. Phys. Rev. E 2017, 96, 1–4. [Google Scholar] [CrossRef]
- Arabski, M.; Wąsik, S.; Dworecki, K.; Kaca, W. Laser interferometric determination of ampicillin and colistin transfer through cellulose biomembrane in the presence of Proteus vulgaris O25 lipopolysaccharide. J. Membr. Sci. 2007, 299, 268–275. [Google Scholar] [CrossRef]
- Gałczyńska, K.; Kurdziel, K.; Adamus-Białek, W.; Wąsik, S.; Szary, K.; Drabik, M.; Węgierek-Ciuk, A.; Lankoff, A.; Arabski, M. The effects of nickel(II) complexes with imidazole derivatives on pyocyanin and pyoverdine production by Pseudomonas aeruginosa strains isolated from cystic fibrosis. Acta Biochim. Pol. 2015, 62, 739–745. [Google Scholar] [CrossRef]
- Arabski, M.; Lisowska, H.; Lankoff, A.; Davydova, V.N.; Drulis-Kawa, Z.; Augustyniak, D.; Yermak, I.M.; Molinaro, A.; Kaca, W. The properties of chitosan complexes with smooth and rough forms of lipopolysaccharides on CHO-K1 cells. Carbohydr. Polym. 2013, 97, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Danis-Wlodarczyk, K.; Arabski, M.; Gula, G.; Maciejewska, B.; Wasik, S.; Lood, C.; Higgins, G.; Harvey, B.; Lavigne, R.; et al. Pseudomonas aeruginosa PA5oct jumbo phage impacts planktonic and biofilm population and reduces its host virulence. Viruses 2019, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Danis-Wlodarczyk, K.; Vandenheuvel, D.; Jang, H.B.; Briers, Y.; Olszak, T.; Arabski, M.; Wasik, S.; Drabik, M.; Higgins, G.; Tyrrell, J.; et al. A proposed integrated approach for the preclinical evaluation of phage therapy in Pseudomonas infections. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Danis-Wlodarczyk, K.; Olszak, T.; Arabski, M.; Wasik, S.; Majkowska-Skrobek, G.; Augustyniak, D.; Gula, G.; Briers, Y.; Jang, H.B.; Vandenheuvel, D.; et al. Characterization of the newly isolated lytic bacteriophages KTN6 and KT28 and their efficacy against Pseudomonas aeruginosa biofilm. PLoS ONE 2015, 10, e127603. [Google Scholar] [CrossRef]
- Kosztołowicz, T.; Metzler, R. Diffusion of antibiotics through a biofilm in the presence of diffusion and absorption barriers. Phys. Rev. E 2020, 102, 1–11. [Google Scholar] [CrossRef]
- Gałczyńska, K.; Ciepluch, K.; Madej, Ł.; Kurdziel, K.; Maciejewska, B.; Drulis-Kawa, Z.; Węgierek-Ciuk, A.; Lankoff, A.; Arabski, M. Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kurdziel, K.; Glowiak, T. X-ray and spectroscopic characterisation of octahedral cobalt(II) and nickel(II) complexes with 1-allylimidazole in the solid state and electron-donor properties of the latter in aqueous solution. Polyhedron 2000, 19, 2183–2188. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Arabski, M.; Wa̧sik, S.; Zych, M.; Łakomiec, W.; Kaca, W. Analysis of ciprofloxacin and gentamicin diffusion in Proteus mirabilis O18 biofilm by laser interferometry method. Acta Biochim. Pol. 2013, 60, 707–711. [Google Scholar] [CrossRef]
- Kosztołowicz, T. Model of anomalous diffusion-absorption process in a system consisting of two different media separated by a thin membrane. Phys. Rev. E 2019, 99, 1–16. [Google Scholar] [CrossRef]
- Kosztołowicz, T. Subdiffusion in a system consisting of two different media separated by a thin membrane. Int. J. Heat Mass Transf. 2017, 111, 1322–1333. [Google Scholar] [CrossRef]
- Kosztołowicz, T. Random walk model of subdiffusion in a system with a thin membrane. Phys. Rev. E 2015, 91, 1–9. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Gomes, J.C.S.; Barros, M.C.F.; Lobo, V.M.M.; Esteso, M.A. Diffusion coefficients of nickel chloride in aqueous solutions of lactose at T = 298.15 K and T = 310.15 K. J. Chem. Thermodyn. 2011, 43, 270–274. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Lobo, V.M.M.; Natividade, J.J.S. Diffusion Coefficients in Aqueous Solutions of Cobalt Chloride at 298.15 K. J. Chem. Eng. Data 2002, 47, 539–541. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Ruthenium anticancer compounds: Myths and realities of the emerging metal-based drugs. Dalt. Trans. 2011, 40, 7817–7823. [Google Scholar] [CrossRef]
- Kisova, A.; Zerzankova, L.; Habtemariam, A.; Sadler, P.J.; Brabec, V.; Kasparkova, J. Differences in the cellular response and signaling pathways between cisplatin and monodentate organometallic Ru(II) antitumor complexes containing a terphenyl ligand. Mol. Pharm. 2011, 8, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg Chem. 2008, 13, 1149–1155. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Kausar, S. Synthesis, characterization and biological properties of tridentate NNO, NNS and NNN donor thiazole-derived furanyl, thiophenyl and pyrrolyl Schiff bases and their Co(II), Cu(II), Ni(II) and Zn(II) metal chelates. Met. Drugs 2000, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ikram, M.; Rehman, S.; Shahnawaz, A. Synthesis, characterization and antimicrobial studies of Trnsition complexes of Imidazole derivatives. Bull. Chem. Soc. Ethiop. 2010, 24, 201–207. [Google Scholar] [CrossRef]
- Arjmand, F.; Mohani, B.; Ahmad, S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem. 2005, 40, 1103–1110. [Google Scholar] [CrossRef]
- Rodríguez-Argüelles, M.C.; López-Silva, E.C.; Sanmartín, J.; Pelagatti, P.; Zani, F. Copper complexes of imidazole-2-, pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: Inhibitory activity against fungi and bacteria. J. Inorg. Biochem. 2005, 99, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Sharma, P.; Kumar, N. Imidazole and its biological activities: A review. Chem. Sin 2010, 1, 36–47. [Google Scholar]
- Congiu, C.; Cocco, M.T.; Onnis, V. Design, synthesis, and in vitro antitumor activity of new 1,4-diarylimidazole-2-ones and their 2-thione analogues. Bioorg. Med. Chem. Lett. 2008, 18, 989–993. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Agarwal, A.; Abe, T.; Ushirogochi, H.; Ado, M.; Tsuyoshi, T.; Dos Santos, O.; Li, Z.; Francisco, G.; Lin, Y.I.; et al. 5,5,6-Fused tricycles bearing imidazole and pyrazole 6-methylidene penems as broad-spectrum inhibitors of β-lactamases. Bioorg. Med. Chem. 2008, 16, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kakinuma, H.; Umemiya, H.; Amada, H.; Miyata, N.; Taniguchi, K.; Bando, K.; Sato, M. Imidazole derivatives as new potent and selective 20-HETE synthase inhibitors. Bioorganic Med. Chem. Lett. 2004, 14, 333–336. [Google Scholar] [CrossRef]
- Han, M.S.; Kim, D.H. Effect of zinc ion on the inhibition of carboxypeptidase A by imidazole-bearing substrate analogues. Bioorg. Med. Chem. Lett. 2001, 11, 1425–1427. [Google Scholar] [CrossRef]
- Roman, G.; Riley, J.G.; Vlahakis, J.Z.; Kinobe, R.T.; Brien, J.F.; Nakatsu, K.; Szarek, W.A. Heme oxygenase inhibition by 2-oxy-substituted 1-(1H-imidazol-1-yl)-4-phenylbutanes: Effect of halogen substitution in the phenyl ring. Bioorg. Med. Chem. 2007, 15, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Nantermet, P.G.; Barrow, J.C.; Lindsley, S.R.; Young, M.; Mao, S.S.; Carroll, S.; Bailey, C.; Bosserman, M.; Colussi, D.; McMasters, D.R.; et al. Imidazole acetic acid TAFIa inhibitors: SAR studies centered around the basic P′1group. Bioorg. Med. Chem. Lett. 2004, 14, 2141–2145. [Google Scholar] [CrossRef]
- Adams, J.L.; Boehm, J.C.; Gallagher, T.F.; Kassis, S.; Webb, E.F.; Hall, R.; Sorenson, M.; Garigipati, R.; Griswold, D.E.; Lee, J.C. Pyrimidinylimidazole inhibitors of p38: Cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg. Med. Chem. Lett. 2001, 11, 2867–2870. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałczyńska, K.; Rachuna, J.; Ciepluch, K.; Kowalska, M.; Wąsik, S.; Kosztołowicz, T.; Lewandowska, K.D.; Semaniak, J.; Kurdziel, K.; Arabski, M. Experimental and Theoretical Analysis of Metal Complex Diffusion through Cell Monolayer. Entropy 2021, 23, 360. https://doi.org/10.3390/e23030360
Gałczyńska K, Rachuna J, Ciepluch K, Kowalska M, Wąsik S, Kosztołowicz T, Lewandowska KD, Semaniak J, Kurdziel K, Arabski M. Experimental and Theoretical Analysis of Metal Complex Diffusion through Cell Monolayer. Entropy. 2021; 23(3):360. https://doi.org/10.3390/e23030360
Chicago/Turabian StyleGałczyńska, Katarzyna, Jarosław Rachuna, Karol Ciepluch, Magdalena Kowalska, Sławomir Wąsik, Tadeusz Kosztołowicz, Katarzyna D. Lewandowska, Jacek Semaniak, Krystyna Kurdziel, and Michał Arabski. 2021. "Experimental and Theoretical Analysis of Metal Complex Diffusion through Cell Monolayer" Entropy 23, no. 3: 360. https://doi.org/10.3390/e23030360
APA StyleGałczyńska, K., Rachuna, J., Ciepluch, K., Kowalska, M., Wąsik, S., Kosztołowicz, T., Lewandowska, K. D., Semaniak, J., Kurdziel, K., & Arabski, M. (2021). Experimental and Theoretical Analysis of Metal Complex Diffusion through Cell Monolayer. Entropy, 23(3), 360. https://doi.org/10.3390/e23030360






