Recent Advances in W-Containing Refractory High-Entropy Alloys—An Overview
Abstract
:1. Introduction
1.1. Development of RHEAs
1.2. The Constituent Elements of RHEAs
1.3. Design Methods for HEAs
2. The Fabrication and Processing Techniques of W-Containing RHEAs
2.1. Vacuum Arc Melting (VAM)
2.2. Powder Metallurgy Method
2.3. Mechanical Alloying (MA)
2.4. Gas Atomization Process
2.5. Magnetron Sputtering (MS)
2.6. Additive Manufacturing (AM)
3. Microstructure of W-Containing RHEAs
3.1. Single-Phase W-Containing RHEAs
3.2. Multi-Phase W-Containing RHEAs
4. Mechanical Properties of W-Containing RHEAs
4.1. Room-Temperature Mechanical Properties
4.2. Mechanical Properties at Elevated Temperatures
5. Functional Properties of W-Containing RHEAs
5.1. Oxidation Resistance
5.2. Corrosion Resistance
5.3. Irradiation Resistance
5.4. Wear Resistance
6. Conclusions and Future Directions
- Although more than 150 W-containing RHEAs have been reported, the number of reported W-containing RHEAs is far less than the theoretically predicted systems. The effects of different elements on the phase structure, mechanical properties and functional characteristics of RHEAs is also not been fully understood. It is still challenging to develop new W-containing RHEA systems with high-end properties efficiently, where the use of machine learning combined with advanced preparation techniques, such as additive manufacturing, could be helpful.
- Many conventional techniques have been employed to fabricate W-containing RHEAs. However, with unique atomic and physical properties, it is still a key issue to seek advanced techniques which are especially suitable for the preparation of W-containing RHEAs and RHEA products. Further research and explorations are necessary to achieve the practical manufacturing and application of W-containing RHEAs.
- Although the formation of phase structures, the evolution of microstructures and mechanical properties of RHEAs have been widely investigated, theories on the strengthening mechanisms in W-containing RHEAs are still under debate, especially for the formation and evolution of microstructures at high temperatures. Aiming at high-temperature applications, more efforts must be devoted to revealing the strengthening and deformation mechanisms of W-containing RHEAs in harsh environments. To date, most of the W-containing RHEA systems have high-strength but limited plasticity. How to achieve large plasticity in W-containing RHEAs is still challenging, especially under tension. Moreover, it is also important to resolve such conflicts to achieve a better overall mechanical performance.
- Preliminary findings have shown that the W-containing RHEAs indeed have excellent functional properties, such as oxidation, irradiation, corrosion and wear resistances. However, the underlying mechanisms are not yet thoroughly understood, which is worthy of further investigation in the future before their widespread application in industry. Studies have shown that RHEA coatings can demonstrate extremely high-strength and better corrosion resistance than the bulk forms of specimens. The fabrication and application of RHEA films could be a promising direction for functional applications of W-containing RHEAs.
- Due to the characteristics of high-strength, high hardness and limited plasticity, W-containing RHEAs are classified as typical difficult-to-cut materials. The machining of W-containing RHEAs and components with a good surface finish is an unexplored topic, which greatly hinders their engineering applications. It is urgent and necessary to investigate the machining performance of W-containing RHEAs using traditional and non-traditional machining techniques, such as turning, electrical discharge machining (EDM), electrochemical machining (ECM) and laser machining. For example, with non-contact forces, EDM having a high-pulse discharge density could be suitable for the processing of W-containing RHEAs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nechaev, Y.S. The distribution of carbon in steels. Phys.-Uspekhi 2011, 54, 465–471. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Feng, A.H.; Chen, D.L.; Shen, J. Recent advances in friction stir welding/processing of aluminum alloys: Microstructural evolution and mechanical properties. Crit. Rev. Solid State Mater. Sci. 2017, 43, 269–333. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yang, M.; Chen, X. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloy. 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H. Physical properties of high entropy alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Matériaux 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.-P.; Miracle, D.B. From high-entropy alloys to complex concentrated alloys. Comptes Rendus Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.D.; Liu, P.; Guo, S.; Hirata, A.; Fujita, T.; Nieh, T.G.; Liu, C.T.; Chen, M.W. Nanoscale phase separation in a fcc-based CoCrCuFeNiAl0.5 high-entropy alloy. Acta Mater. 2015, 84, 145–152. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Bachani, S.K.; Wang, C.-J.; Lou, B.-S.; Chang, L.-C.; Lee, J.-W. Microstructural characterization, mechanical property and corrosion behavior of VNbMoTaWAl refractory high entropy alloy coatings: Effect of Al content. Surf. Coat. Technol. 2020, 403, 126351. [Google Scholar] [CrossRef]
- Luo, G.; Jiang, S.; Wei, Q.; Kang, K.; Qian, Y.; Zhang, J.; Sun, Y.; Shen, Q. Microstructure and mechanical properties of MoNbW(TaC)x composites. Int. J. Refract. Met. Hard Mater. 2021, 99, 105574. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Q.; Wang, J.; Li, P.; Yuan, J.; Lyu, B.; Wang, J.; Tokunaga, K.; Yao, G.; Luo, L.; et al. Effect of surface quality on hydrogen/helium irradiation behavior in tungsten. Nucl. Eng. Technol. 2022, 54, 1947–1953. [Google Scholar] [CrossRef]
- Ravi Kiran, U.; Panchal, A.; Sankaranarayana, M.; Nageswara Rao, G.V.S.; Nandy, T.K. Effect of alloying addition and microstructural parameters on mechanical properties of 93% tungsten heavy alloys. Mater. Sci. Eng. A 2015, 640, 82–90. [Google Scholar] [CrossRef]
- Rieth, M.; Dudarev, S.L.; Gonzalez de Vicente, S.M.; Aktaa, J.; Ahlgren, T.; Antusch, S.; Armstrong, D.E.J.; Balden, M.; Baluc, N.; Barthe, M.F.; et al. Recent progress in research on tungsten materials for nuclear fusion applications in Europe. J. Nucl. Mater. 2013, 432, 482–500. [Google Scholar] [CrossRef] [Green Version]
- Beiersdorfer, P.; Clementson, J.; Safronova, U. Tungsten data for current and future uses in fusion and plasma science. Atoms 2015, 3, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Neu, R.; Hopf, C.; Kallenbach, A.; Pütterich, T.; Dux, R.; Greuner, H.; Gruber, O.; Herrmann, A.; Krieger, K.; Maier, H.; et al. Operational conditions in a W-clad tokamak. J. Nucl. Mater. 2007, 367–370, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Travere, J.M.; Aumeunier, M.H.; Joanny, M.; Loarer, T.; Firdaouss, M.; Gauthier, E.; Martin, V.; Moncada, V.; Marot, L.; Chabaud, D.; et al. Imaging challenges for ITER plasma-facing component protection. Fusion Sci. Technol. 2017, 64, 735–740. [Google Scholar] [CrossRef]
- Wurster, S.; Baluc, N.; Battabyal, M.; Crosby, T.; Du, J.; García-Rosales, C.; Hasegawa, A.; Hoffmann, A.; Kimura, A.; Kurishita, H.; et al. Recent progress in R&D on tungsten alloys for divertor structural and plasma facing materials. J. Nucl. Mater. 2013, 442, S181–S189. [Google Scholar] [CrossRef] [Green Version]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloy. Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Ikeuchi, D.; King, D.J.M.; Laws, K.J.; Knowles, A.J.; Aughterson, R.D.; Lumpkin, G.R.; Obbard, E.G. Cr-Mo-V-W: A new refractory and transition metal high-entropy alloy system. Scr. Mater. 2019, 158, 141–145. [Google Scholar] [CrossRef]
- Jia, Y.J.; Chen, H.N.; Liang, X.D. Microstructure and wear resistance of CoCrNbNiW high-entropy alloy coating prepared by laser melting deposition. Rare Met. 2019, 38, 1153–1159. [Google Scholar] [CrossRef]
- Liang, H.; Gao, B.Y.; Li, Y.N.; Nie, Q.X.; Cao, Z.Q. Microstructures and wear resistance of Al1.5CrFeNiTi0.5 and Al1.5CrFeNiTi0.5W0.5 high entropy alloy coatings manufactured by laser cladding. Mater. Sci. Forum 2019, 956, 154–159. [Google Scholar] [CrossRef]
- Alvi, S.; Waseem, O.A.; Akhtar, F. High temperature performance of spark plasma sintered W0.5(TaTiVCr)0.5 alloy. Metals 2020, 10, 1512. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloy. Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hung, S.B.; Wang, C.J.; Wei, W.C.; Lee, J.W. High temperature electrical properties and oxidation resistance of V-Nb-Mo-Ta-W high entropy alloy thin films. Surf. Coat. Technol. 2019, 375, 854–863. [Google Scholar] [CrossRef]
- Sheng, H.; Zihao, C.; Xinglixiang, W.; Dichen, L.I.; Hang, Z.; Qingyu, L.I. Manufacture of WNbMoTa high performance high-entropy alloy by laser additive manufacturing. J. Mech. Eng. 2019, 55, 15. [Google Scholar] [CrossRef] [Green Version]
- Postolnyi, B.; Buranich, V.; Smyrnova, K.; Araújo, J.P.; Rebouta, L.; Pogrebnjak, A.; Rogoz, V. Multilayer and high-entropy alloy-based protective coatings for solving the issue of critical raw materials in the aerospace industry. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1024, 012009. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Helium ions irradiation analysis of W0.5(TaTiVCr)0.5 for application as a future fusion plasma-facing material. Mater. Chem. Phys. 2021, 260, 124198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing methods, microstructural and mechanical properties evolutions of high-entropy alloys: A review. Met. Mater. Int. 2019, 26, 1099–1133. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Szost, B.A.; Battezzati, L. Search for high entropy alloys in the X-NbTaTiZr systems (X=Al, Cr, V, Sn). J. Alloy. Compd. 2015, 620, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.K.; Sanchez, F.; Ramana, C.V. Microstructures in a Nb-Cr-V-W-Ta high entropy alloy during annealing. J. Mater. Sci. Technol. 2020, 53, 66–72. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Chen, R.R.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Hot deformation characteristics and dynamic recrystallization of the MoNbHfZrTi refractory high-entropy alloy. Mater. Sci. Eng. A 2016, 651, 698–707. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Lilensten, L.; Danard, Y.; Brozek, C.; Mantri, S.; Castany, P.; Gloriant, T.; Vermaut, P.; Sun, F.; Banerjee, R.; Prima, F. On the heterogeneous nature of deformation in a strain-transformable beta metastable Ti-V-Cr-Al alloy. Acta Mater. 2019, 162, 268–276. [Google Scholar] [CrossRef]
- Wei, S.; Kim, S.J.; Kang, J.; Zhang, Y.; Zhang, Y.; Furuhara, T.; Park, E.S.; Tasan, C.C. Natural-mixing guided design of refractory high-entropy alloys with as-cast tensile ductility. Nat. Mater. 2020, 19, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Maiti, S.; Steurer, W.; Spolenak, R. Size-dependent plasticity in an Nb25Mo25Ta25W25 refractory high-entropy alloy. Acta Mater. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Liu, Y.; Li, T.; Fu, A.; Fang, Q.; Nie, Y. Microstructures and mechanical properties of ductile NbTaTiV refractory high entropy alloy prepared by powder metallurgy. J. Alloy. Compd. 2019, 776, 428–436. [Google Scholar] [CrossRef]
- Srikanth, M.; Annamalai, A.R.; Muthuchamy, A.; Jen, C.-P. A review of the latest developments in the field of refractory high-entropy alloys. Crystals 2021, 11, 612. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Powder metallurgy processing of a WxTaTiVCr high-entropy alloy and Its derivative alloys for fusion material applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Pan, J.; Dai, T.; Lu, T.; Ni, X.; Dai, J.; Li, M. Microstructure and mechanical properties of Nb25Mo25Ta25W25 and Ti8Nb23Mo23Ta23W23 high entropy alloys prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2018, 738, 362–366. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, C.; Shen, Y.; Feng, X. Microstructure and corrosion property of CrMnFeCoNi high entropy alloy coating on Q235 substrate via mechanical alloying method. Surf. Interfaces 2019, 15, 135–140. [Google Scholar] [CrossRef]
- Ding, P.; Mao, A.; Zhang, X.; Jin, X.; Wang, B.; Liu, M.; Gu, X. Preparation, characterization and properties of multicomponent AlCoCrFeNi2.1 powder by gas atomization method. J. Alloy. Compd. 2017, 721, 609–614. [Google Scholar] [CrossRef]
- Yang, M.; Dai, Y.; Song, C.; Zhai, Q. Microstructure evolution of grey cast iron powder by high pressure gas atomization. J. Mater. Process. Technol. 2010, 210, 351–355. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef]
- Li, X. Additive manufacturing of advanced multi-component alloys: Bulk metallic glasses and high entropy alloys. Adv. Eng. Mater. 2018, 20, 1700874. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive manufacturing of high entropy alloys: A practical review. J. Mater. Sci. Technol. 2021, 77, 131–162. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.; Goune, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Wakai, A.; Moridi, A. Materials and manufacturing renaissance: Additive manufacturing of high-entropy alloys. J. Mater. Res. 2020, 35, 1963–1983. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Torralba, J.M.; Campos, M. High entropy alloys manufactured by additive manufacturing. Metals 2020, 10, 639. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Xu, Y.; Lu, Z.; Li, D. The thermal-mechanical behavior of WTaMoNb high-entropy alloy via selective laser melting (SLM): Experiment and simulation. Int. J. Adv. Manuf. Technol. 2018, 96, 461–474. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. First demonstration of promising selective electron beam melting method for utilizing high-entropy alloys as engineering materials. Mater. Lett. 2015, 159, 12–15. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Xiao, B.; Jia, W.; Tang, H.; Wang, J.; Zhou, L. Microstructure and mechanical properties of WMoTaNbTi refractory high-entropy alloys fabricated by selective electron beam melting. J. Mater. Sci. Technol. 2022, 108, 54–63. [Google Scholar] [CrossRef]
- Han, Z.D.; Chen, N.; Zhao, S.F.; Fan, L.W.; Yang, G.N.; Shao, Y.; Yao, K.F. Effect of Ti additions on mechanical properties of NbMoTaW and VNbMoTaW refractory high entropy alloys. Intermetallics 2017, 84, 153–157. [Google Scholar] [CrossRef]
- Bartkowski, D.; Młynarczak, A.; Piasecki, A.; Dudziak, B.; Gościański, M.; Bartkowska, A. Microstructure, microhardness and corrosion resistance of Stellite-6 coatings reinforced with WC particles using laser cladding. Opt. Laser Technol. 2015, 68, 191–201. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Yu, X.; Li, J. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
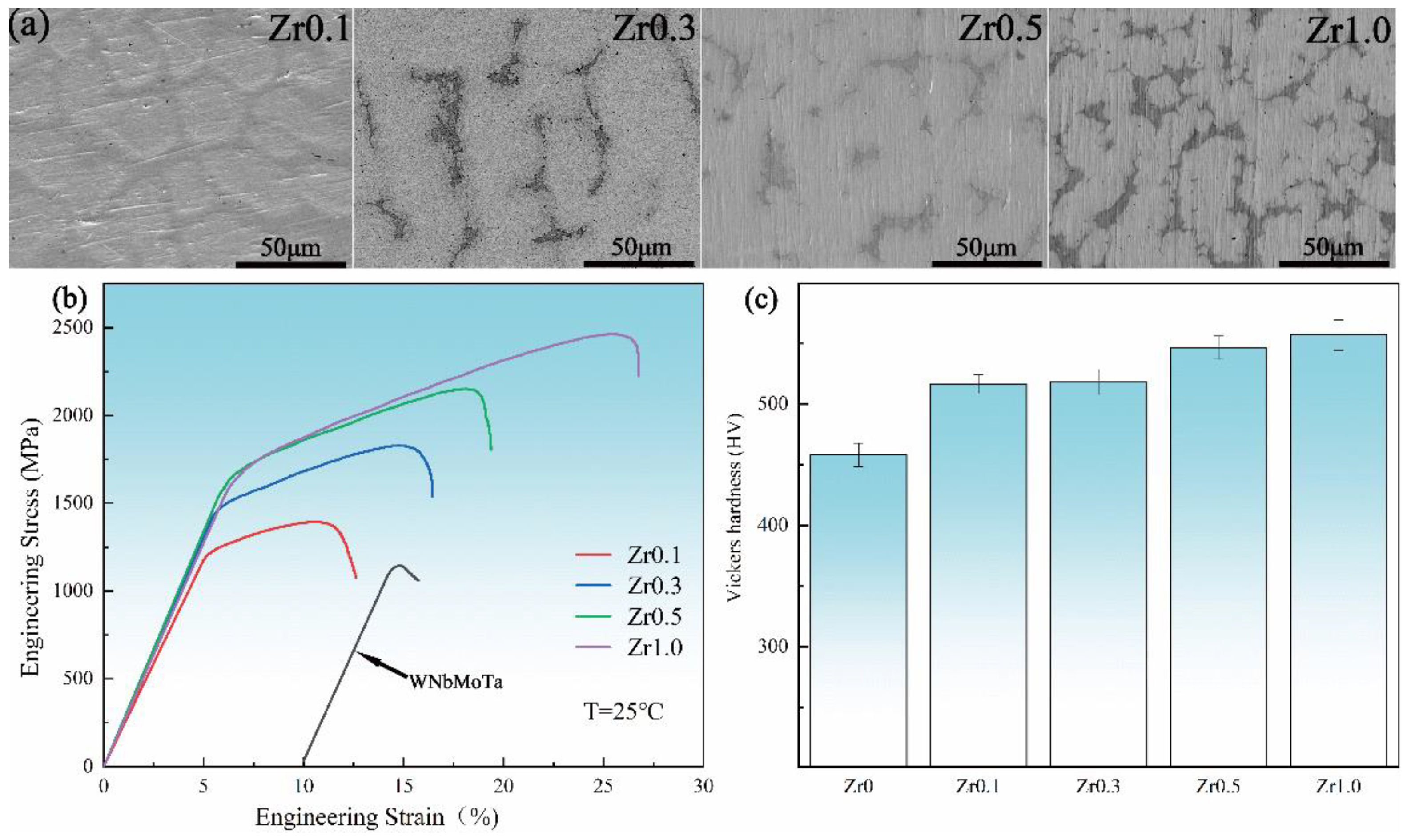
- Chen, S.H.; Zhang, J.S.; Guan, S.; Li, T.; Liu, J.Q.; Wu, F.F.; Wu, Y.C. Microstructure and mechanical properties of WNbMoTaZrx (x = 0.1, 0.3, 0.5, 1.0) refractory high entropy alloys. Mater. Sci. Eng. A 2022, 835, 142701. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.H.; Wu, Z.W.; Zhang, Z.F.; Wu, Y.C. Development of high-strength WNbMoTaVZrx refractory high entropy alloys. J. Mater. Res. 2022, 37, 1664–1678. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of TixNbMoTaW refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Wu, Y.C. The routes and mechanism of plasma facing tungsten materials to Improve ductility. Acta Metall. Sin. 2019, 55, 171–180. [Google Scholar] [CrossRef]
- Long, Y.; Liang, X.; Su, K.; Peng, H.; Li, X. A fine-grained NbMoTaWVCr refractory high-entropy alloy with ultra-high strength: Microstructural evolution and mechanical properties. J. Alloy. Compd. 2019, 780, 607–617. [Google Scholar] [CrossRef]
- Waseem, O.A.; Lee, J.; Lee, H.M.; Ryu, H.J. The effect of Ti on the sintering and mechanical properties of refractory high-entropy alloy TixWTaVCr fabricated via spark plasma sintering for fusion plasma-facing materials. Mater. Chem. Phys. 2018, 210, 87–94. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling laves phase stability. Intermetallics 2004, 12, 713–720. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloy. Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2016, 33, 17–22. [Google Scholar] [CrossRef]
- Li, T.; Jiao, W.; Miao, J.; Lu, Y.; Guo, E.; Wang, T.; Li, T.; Liaw, P.K. A novel ZrNbMoTaW refractory high-entropy alloy with in-situ forming heterogeneous structure. Mater. Sci. Eng. A 2021, 827, 142061. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.; Xu, Z.; Cheng, X. Microstructures and mechanical properties of HfNbTaTiZrW and HfNbTaTiZrMoW refractory high-entropy alloys. J. Alloy. Compd. 2019, 803, 778–785. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-transformation ductilization of brittle high-entropy alloys via metastability engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef]
- Poletti, M.G.; McCaughey, C.M.; Fiore, G.; Goodall, R.; Battezzati, L. Refractory high entropy alloys: CrMoNbTiVWZr and AlxCryNbMoTiVzZry(x = 0,0.6;y = 0.3,z = 0,0.6). Int. J. Refract. Met. Hard Mater. 2018, 76, 128–133. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Guo, Y.; Lan, H. MoFe1.5CrTiWAlNbx refractory high-entropy alloy coating fabricated by laser cladding. Intermetallics 2019, 115, 106613. [Google Scholar] [CrossRef]
- Wu, S.; Qiao, D.; Zhao, H.; Wang, J.; Lu, Y. A novel NbTaW0.5 (Mo2C)x refractory high-entropy alloy with excellent mechanical properties. J. Alloy. Compd. 2021, 889, 161800. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y. Effects of tungsten addition on the microstructure and mechanical properties of near-eutectic AlCoCrFeNi2 high-entropy alloy. J. Mater. Eng. Perform. 2017, 27, 109–115. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.P. Microstructure and mechanical properties of CoCrFeNi2Al1-xWx high entropy alloys. Arab. J. Sci. Eng. 2019, 44, 803–808. [Google Scholar] [CrossRef]
- Vida, Á.; Chinh, N.Q.; Lendvai, J.; Heczel, A.; Varga, L.K. Microstructures and transition from brittle to ductile behavior of NiFeCrMoW high entropy alloys. Mater. Lett. 2017, 195, 14–17. [Google Scholar] [CrossRef]
- Wei, Q.; Luo, G.; Zhang, J.; Jiang, S.; Chen, P.; Shen, Q.; Zhang, L. Designing high entropy alloy-ceramic eutectic composites of MoNbRe0.5TaW(TiC)x with high compressive strength. J. Alloy. Compd. 2020, 818, 152846. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Lu, Y.P.; Wang, T.M.; Cao, Z.Q.; Li, T.J. Microstructure and mechanical properties of the W-Ni-Co system refractory high-entropy alloys. Mater. Sci. Forum 2015, 816, 324–329. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Li, J. Microstructure and mechanical properties of a refractory CoCrMoNbTi high-entropy alloy. J. Mater. Eng. Perform. 2017, 26, 3657–3665. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Liu, J.; Qing, Z.; Wu, Y. Microstructure and mechanical properties of novel high-strength, low-activation Wx(TaVZr)100−x (x = 5, 10, 15, 20, 25) refractory high entropy alloys. Entropy 2022, 24, 1342. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Xia, L.; Wu, Y. Deformation behavior of bulk metallic glasses and high entropy alloys under complex stress fields: A review. Entropy 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Jiang, L.; Han, K.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effects of tungsten on microstructure and mechanical properties of CrFeNiV0.5Wx and CrFeNi2V0.5Wx high-entropy alloys. J. Mater. Eng. Perform. 2015, 24, 4594–4600. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, M.C.; Zhang, Y.; Guo, S.M. Senary refractory high-entropy alloy CrxMoNbTaVW. Calphad 2015, 51, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gao, M.C.; Zhang, Y.; Yang, S.; Guo, S.M. Senary refractory high entropy alloy MoNbTaTiVW. Mater. Sci. Technol. 2015, 31, 1207–1213. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Huang, T.; Lu, Y.; Wang, T.; Li, T. Microstructures and mechanical properties of Co2MoxNi2VWx eutectic high entropy alloys. Mater. Des. 2016, 109, 539–546. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F.; Zhang, Y. NbTaV-(Ti,W) refractory high-entropy alloys: Experiments and modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Das, S.; Robi, P.S. Mechanical Alloying of W-Mo-V-Cr-Ta High Entropy Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2018, 346, 012047. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liaw, P.K.; Zhang, Y. A novel low-activation VCrFeTaxWx (x = 0.1, 0.2, 0.3, 0.4, and 1) high-entropy alloys with excellent heat-softening resistance. Entropy 2018, 20, 951. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Li, P.; Xi, S.; Zhou, Y.; Li, S.; Yang, X. A new type of high entropy alloy composite Fe18Ni23Co25Cr21Mo8WNb3C2 prepared by mechanical alloying and hot pressing sintering. Mater. Sci. Eng. A 2018, 728, 144–150. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, G.; Liu, B.; Wang, J.; Han, L.; Liu, Y. Microstructures and wear behaviour of (FeCoCrNi)1-x(WC)x high entropy alloy composites. Int. J. Refract. Met. Hard Mater. 2018, 75, 56–62. [Google Scholar] [CrossRef]
- Wei, Q.; Shen, Q.; Zhang, J.; Chen, B.; Luo, G.; Zhang, L. Microstructure and mechanical property of a novel ReMoTaW high-entropy alloy with high density. Int. J. Refract. Met. Hard Mater. 2018, 77, 8–11. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, A.; Han, J.; Meng, J. Effect of Si additions on microstructure and mechanical properties of refractory NbTaWMo high-entropy alloys. J. Mater. Sci. 2019, 54, 5844–5851. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Hung, S.-B.; Wang, C.-J.; Chen, Y.-Y.; Lee, J.-W.; Li, C.-L. Thermal and corrosion properties of V-Nb-Mo-Ta-W and V-Nb-Mo-Ta-W-Cr-B high entropy alloy coatings. Surf. Coat. Technol. 2019, 375, 802–809. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Roh, A.; Son, M.; Ham, M.-H.; Kim, J.-H.; Choi, H. Mechanical and electrical properties of NbMoTaW refractory high-entropy alloy thin films. Int. J. Refract. Met. Hard Mater. 2019, 80, 286–291. [Google Scholar] [CrossRef]
- Ley, N.A.; Segovia, S.; Gorsse, S.; Young, M.L. Characterization and modeling of NbNiTaTiW and NbNiTaTiW-Al refractory high-entropy alloys. Metall. Mater. Trans. A 2019, 50, 4867–4876. [Google Scholar] [CrossRef]
- Lv, S.; Zu, Y.; Chen, G.; Fu, X.; Zhou, W. An ultra-high strength CrMoNbWTi-C high entropy alloy co-strengthened by dispersed refractory IM and UHTC phases. J. Alloy. Compd. 2019, 788, 1256–1264. [Google Scholar] [CrossRef]
- Niu, Z.; Xu, J.; Wang, T.; Wang, N.; Han, Z.; Wang, Y. Microstructure, mechanical properties and corrosion resistance of CoCrFeNiWx (x = 0, 0.2, 0.5) high entropy alloys. Intermetallics 2019, 112, 106550. [Google Scholar] [CrossRef]
- Wei, Q.; Shen, Q.; Zhang, J.; Zhang, Y.; Luo, G.; Zhang, L. Microstructure evolution, mechanical properties and strengthening mechanism of refractory high-entropy alloy matrix composites with addition of TaC. J. Alloy. Compd. 2019, 777, 1168–1175. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Wang, Y.; Qiu, J. Microstructure and mechanical properties of WMoNbCrTi HEAs sintered from the powders milled for different durations. Jom 2019, 71, 2489–2497. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Li, D.; Chen, Z.; Huang, S.; Lu, Z.; Yan, H. WxNbMoTa refractory high-entropy alloys fabricated by laser cladding deposition. Materials 2019, 12, 533. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Bai, L.; Liang, X.; Chen, Y.; Zhang, Z.; Liu, J.; Li, Y.; Hu, Y. Influence of alloying elements on mechanical and electronic properties of NbMoTaWX (X = Cr, Zr, V, Hf and Re) refractory high entropy alloys. Intermetallics 2020, 126, 106928. [Google Scholar] [CrossRef]
- Alvi, S.; Jarzabek, D.M.; Kohan, M.G.; Hedman, D.; Jenczyk, P.; Natile, M.M.; Vomiero, A.; Akhtar, F. Synthesis and mechanical characterization of a CuMoTaWV high-entropy film by magnetron sputtering. ACS Appl. Mater. Interfaces 2020, 12, 21070–21079. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, U.; Zhang, C.; Zeng, C.; Guo, S.; Yang, S. Computational and experimental investigation of refractory high entropy alloy Mo15Nb20Re15Ta30W20. J. Mater. Res. Technol. 2020, 9, 8929–8936. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Samal, S.; Kumar, V. Microstructure exploration and an artificial neural network approach for hardness prediction in AlCrFeMnNiWx High-Entropy Alloys. J. Alloy. Compd. 2020, 823, 153726. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, T.-D.; Su, C.; Zhang, H.-B.; Han, K.-M.; Qin, S.-X. Microstructure and mechanical behavior of CrFeNi2V0.5Wx (x = 0, 0.25) high-entropy alloys. Acta Metall. Sin. 2020, 33, 1117–1123. [Google Scholar] [CrossRef]
- Kanyane, L.R.; Popoola, A.P.I.; Malatji, N.; Sibisi, P.N. Synthesis and characterization of TixAlSixMoW light-weight high entropy alloys. Mater. Today Proc. 2020, 28, 1231–1238. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Lu, P.; Saal, J.E.; Olson, G.B.; Frankel, G.S.; Scully, J.R.; Ogle, K. Communication—Dissolution and passivation of a Ni-Cr-Fe-Ru-Mo-W high entropy alloy by elementally resolved electrochemistry. J. Electrochem. Soc. 2020, 167, 061505. [Google Scholar] [CrossRef]
- Li, X.; Wei, D.; Vitos, L.; Lizárraga, R. Micro-mechanical properties of new alternative binders for cemented carbides: CoCrFeNiW high-entropy alloys. J. Alloy. Compd. 2020, 820, 153141. [Google Scholar] [CrossRef]
- Moorehead, M.; Bertsch, K.; Niezgoda, M.; Parkin, C.; Elbakhshwan, M.; Sridharan, K.; Zhang, C.; Thoma, D.; Couet, A. High-through put synthesis of Mo-Nb-Ta-W high-entropy alloys via additive manufacturing. Mater. Des. 2020, 187, 108358. [Google Scholar] [CrossRef]
- Naser-Zoshki, H.; Kiani-Rashid, A.-R.; Vahdati-Khaki, J. Design of a low density refractory high entropy alloy in non-equiatomic W–Mo–Cr–Ti–Al system. Vacuum 2020, 181, 109614. [Google Scholar] [CrossRef]
- Regenberg, M.; Hasemann, G.; Wilke, M.; Halle, T.; Krüger, M. Microstructure evolution and mechanical properties of refractory Mo-Nb-V-W-Ti high-entropy alloys. Metals 2020, 10, 1530. [Google Scholar] [CrossRef]
- Roh, A.; Kim, D.; Nam, S.; Kim, D.-I.; Kim, H.-Y.; Lee, K.-A.; Choi, H.; Kim, J.-H. NbMoTaW refractory high entropy alloy composites strengthened by in-situ metal-non-metal compounds. J. Alloy. Compd. 2020, 822, 153423. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Tang, Y.; Luo, L.; Luo, L.; Su, Y.; Guo, J.; Fu, H. Microstructure and mechanical properties of CoCrFeNiW high entropy alloys reinforced by μ phase particles. J. Alloy. Compd. 2020, 843, 155997. [Google Scholar] [CrossRef]
- Wang, N.; Wu, B.; Wu, W.; Li, J.; Ge, C.; Dong, Y.; Zhang, L.; Wang, Y. Microstructure and properties of aluminium-high entropy alloy composites fabricated by mechanical alloying and spark plasma sintering. Mater. Today Commun. 2020, 25, 101366. [Google Scholar] [CrossRef]
- Xing, Q.-w.; Ma, J.; Zhang, Y. Phase thermal stability and mechanical properties analyses of (Cr,Fe,V)-(Ta,W) multiple-based elemental system using a compositional gradient film. Int. J. Miner. Metall. Mater. 2020, 27, 1379–1387. [Google Scholar] [CrossRef]
- Yan, D.; Song, K.; Sun, H.; Wu, S.; Zhao, K.; Zhang, H.; Yuan, S.; Kim, J.T.; Chawake, N.; Renk, O.; et al. Microstructures, mechanical properties, and corrosion behaviors of refractory high-entropy ReTaWNbMo alloys. J. Mater. Eng. Perform. 2020, 29, 399–409. [Google Scholar] [CrossRef]
- Yan, J.; Li, M.; Li, K.; Qiu, J.; Guo, Y. Effects of Cr content on microstructure and mechanical properties of WMoNbTiCr high-entropy alloys. J. Mater. Eng. Perform. 2020, 29, 2125–2133. [Google Scholar] [CrossRef]
- Zhang, C.; Bhandari, U.; Zeng, C.; Ding, H.; Guo, S.; Yan, J.; Yang, S. Carbide formation in refractory Mo15Nb20Re15Ta30W20 alloy under a combined high-pressure and high-temperature condition. Entropy 2020, 22, 718. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, J.; Zhang, Y.; Shao, G.; Wang, H.; Li, M.; Liu, W.; Fan, B.; Xu, H.; Lu, H.; et al. A novel high-entropy monoboride (Mo0.2Ta0.2Ni0.2Cr0.2W0.2)B with superhardness and low thermal conductivity. Ceram. Int. 2020, 46, 26626–26631. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Wei, Q.; Xiao, Y.; Chen, P.; Luo, G.; Shen, Q. Microstructure and mechanical properties of RexNbMoTaW high-entropy alloys prepared by arc melting using metal powders. J. Alloy. Compd. 2020, 827, 154301. [Google Scholar] [CrossRef]
- Ma, H.; Shao, Y.; Shek, C.H. Microstructure, grain growth behavior and mechanical properties of W-CoCuFeNi tungsten heavy alloys prepared by infiltration. Int. J. Refract. Met. Hard Mater. 2021, 98, 105572. [Google Scholar] [CrossRef]
- Pegues, J.W.; Melia, M.A.; Rodriguez, M.A.; Babuska, T.F.; Gould, B.; Argibay, N.; Greco, A.; Kustas, A.B. In situ synchrotron X-ray imaging and mechanical properties characterization of additively manufactured high-entropy alloy composites. J. Alloy. Compd. 2021, 876, 159505. [Google Scholar] [CrossRef]
- Soni, V.K.; Sanyal, S.; Sinha, S.K. Influence of tungsten on microstructure evolution and mechanical properties of selected novel FeCoCrMnWx high entropy alloys. Intermetallics 2021, 132, 107161. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ma, Y.J.; Han, X.T.; Chen, J.H.; Li, G.J.; Shi, H.Q.; Wang, L.; Cao, Z.H.; Meng, X.K. Ultrahigh strengthening effect induced by element addition in nanostructural (TiVCr)100−xWx medium entropy alloy. J. Alloy. Compd. 2022, 899, 163329. [Google Scholar] [CrossRef]
- Pole, M.; Sadeghilaridjani, M.; Shittu, J.; Ayyagari, A.; Mukherjee, S. High temperature wear behavior of refractory high entropy alloys based on 4-5-6 elemental palette. J. Alloy. Compd. 2020, 843, 156004. [Google Scholar] [CrossRef]
- The Periodic Table of the Elements by WebElements. Available online: https://www.webelements.com/ (accessed on 14 October 2022).
- Wu, Y.C. Research progress in irradiation damage behavior of tungsten and Its alloys for nuclear fusion reactor. Acta Metall. Sin. 2019, 55, 939–950. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hou, Q.Q.; Luo, L.M.; Zan, X.; Zhu, X.Y. Preparation of ultrafine-grained/nanostructured tungsten materials: An overview. J. Alloy. Compd. 2019, 779, 926–941. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Zong, H.; Ding, X.; Sun, J.; Ma, E. Unusual activated processes controlling dislocation motion in body-centered-cubic high-entropy alloys. Proc. Natl. Acad. Sci. USA 2020, 117, 16199–16206. [Google Scholar] [CrossRef]
- Maresca, F.; Curtin, W.A. Mechanistic origin of high strength in refractory BCC high entropy alloys up to 1900K. Acta Mater. 2020, 182, 235–249. [Google Scholar] [CrossRef]
- Maresca, F.; Curtin, W.A. Theory of screw dislocation strengthening in random BCC alloys from dilute to “high-entropy” alloys. Acta Mater. 2020, 182, 144–162. [Google Scholar] [CrossRef]
- Wirth, B.D.; Odette, G.R.; Marian, J.; Ventelon, L.; Young-Vandersall, J.A.; Zepeda-Ruiz, L.A. Multiscale modeling of radiation damage in Fe-based alloys in the fusion environment. J. Nucl. Mater. 2004, 329–333, 103–111. [Google Scholar] [CrossRef]
- Kohyama, A.; Hishinuma, A.; Gelles, D.S.; Klueh, R.L.; Dietz, W.; Ehrlich, K. Low-activation ferritic and martensitic steels for fusion application. J. Nucl. Mater. 1996, 233–237A, 138–147. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloy. Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-temperature oxidation behavior of refractory high-entropy alloys: Effect of alloy composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Gorr, B.; Schellert, S.; Müller, F.; Christ, H.-J.; Kauffmann, A.; Heilmaier, M. Current status of research on the oxidation behavior of refractory high entropy alloys. Adv. Eng. Mater. 2021, 23, 202001047. [Google Scholar] [CrossRef]
- DiStefano, J.R.; Pint, B.A.; DeVan, J.H. Oxidation of refractory metals in air and low pressure oxygen gas. Int. J. Refract. Met. Hard Mater. 2000, 18, 237–243. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Xie, Z.-H.; Munroe, P. Titanium bipolar plates augmented by nanocrystalline TiZrHfMoW coatings for application in proton exchange membrane fuel cells. Appl. Surf. Sci. 2022, 591, 153200. [Google Scholar] [CrossRef]
- Alamdari, A.A.; Unal, U.; Motallebzadeh, A. Investigation of microstructure, mechanical properties, and biocorrosion behavior of Ti1.5ZrTa0.5Nb0.5W0.5 refractory high-entropy alloy film doped with Ag nanoparticles. Surf. Interfaces 2022, 28, 101617. [Google Scholar] [CrossRef]
- Ron, T.; Leon, A.; Popov, V.; Strokin, E.; Eliezer, D.; Shirizly, A.; Aghion, E. Synthesis of refractory high-entropy alloy WTaMoNbV by powder bed fusion process using mixed elemental alloying powder. Materials 2022, 15, 4043. [Google Scholar] [CrossRef]
- Si, Y.; Wang, G.; Wen, M.; Tong, Y.; Wang, W.; Li, Y.; Yan, L.; Yu, W.; Zhang, S.; Ren, P. Corrosion and friction resistance of TiVCrZrWNx high entropy ceramics coatings prepared by magnetron sputtering. Ceram. Int. 2022, 48, 9342–9352. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, F.; Chen, H.; Gao, M.; Zhang, H. Improvement of the mechanical properties and corrosion resistance of CSS-42L steel with a novel TiAlMoNbW nitrid film deposition. Coatings 2022, 12, 1048. [Google Scholar] [CrossRef]
- Zhang, W.-R.; Liao, W.-B.; Liaw, P.K.; Ren, J.-L.; Brechtl, J.; Zhang, Y. Effects of transient thermal shock on the microstructures and corrosion properties of a reduced activation high-entropy alloy. J. Alloy. Compd. 2022, 918, 165762. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yao, W.; Liang, X. Microstructure, mechanical properties and corrosion resistance of high-level hard Nb-Ta-W and Nb-Ta-W-Hf multi-principal element alloy thin films. J. Alloy. Compd. 2022, 920, 166000. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.; Lee, K. Thermodynamic parameters, microstructure, and electrochemical properties of equiatomic TiMoVWCr and TiMoVNbZr high-entropy alloys prepared by vacuum arc remelting. Int. J. Refract. Met. Hard Mater. 2021, 99, 105595. [Google Scholar] [CrossRef]
- Philipps, V. Tungsten as material for plasma-facing components in fusion devices. J. Nucl. Mater. 2011, 415, S2–S9. [Google Scholar] [CrossRef]
- Tan, X.; Luo, L.; Chen, H.; Zhu, X.; Zan, X.; Luo, G.; Chen, J.; Li, P.; Cheng, J.; Liu, D.; et al. Mechanical properties and microstructural change of W-Y2O3 alloy under helium irradiation. Sci. Rep. 2015, 5, 12755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinkle, S.J.; Blanchard, J.P.; Callis, R.W.; Kessel, C.E.; Kurtz, R.J.; Lee, P.J.; McCarthy, K.A.; Morley, N.B.; Najmabadi, F.; Nygren, R.E.; et al. Fusion materials science and technology research opportunities now and during the ITER era. Fusion Eng. Des. 2014, 89, 1579–1585. [Google Scholar] [CrossRef]
- Budaev, V.P.; Martynenko, Y.V.; Karpov, A.V.; Belova, N.E.; Zhitlukhin, A.M.; Klimov, N.S.; Podkovyrov, V.L.; Barsuk, V.A.; Putrik, A.B.; Yaroshevskaya, A.D.; et al. Tungsten recrystallization and cracking under ITER-relevant heat loads. J. Nucl. Mater. 2015, 463, 237–240. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wrobel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 2019, 5, 9. [Google Scholar] [CrossRef]
- Patel, D.; Richardson, M.D.; Jim, B.; Akhmadaliev, S.; Goodall, R.; Gandy, A.S. Radiation damage tolerance of a novel metastable refractory high entropy alloy V2.5Cr1.2WMoCo0.04. J. Nucl. Mater. 2020, 531, 152005. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A. Dry-sliding wear response of MoTaWNbV high entropy alloy. Adv. Eng. Mater. 2017, 19, 1600535. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry-sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. 2018, 210, 126–135. [Google Scholar] [CrossRef]
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and wear behavior of a single-phase equiatomic TiZrHfNb high-entropy alloy studied using a nanoscratch technique. Acta Mater. 2018, 147, 78–89. [Google Scholar] [CrossRef]



















| Element | Crystal Structure at RT | Crystal Structure at Tm | R (pm) | Tm (K) | Density (g/cm3) |
|---|---|---|---|---|---|
| W | BCC | BCC | 136.70 | 3695 | 19.41 |
| Ta | BCC | BCC | 143.00 | 3290 | 16.68 |
| Mo | BCC | BCC | 136.26 | 2896 | 10.23 |
| Nb | BCC | BCC | 142.90 | 2750 | 8.58 |
| Hf | HCP | BCC | 157.75 | 2506 | 13.28 |
| V | BCC | BCC | 131.60 | 2183 | 6.12 |
| Cr | BCC | BCC | 124.91 | 2180 | 7.19 |
| Zr | HCP | BCC | 160.25 | 2128 | 6.51 |
| Ti | HCP | BCC | 146.15 | 1941 | 4.50 |
| Preparation Method | Sample Form | Advantages | Disadvantages |
|---|---|---|---|
| VAM | bulk |
|
|
| Powder metallurgy | bulk |
|
|
| MA | powder |
|
|
| Gas atomization | powder |
|
|
| MS | film |
|
|
| SLM | bulk |
|
|
| SEBM | bulk |
|
|
| LC | coating |
|
|
| Alloy | Year | Phase Structure | Preparation | ρ/(g/cm3) | Hardness/HV | σ0.2/MPa | σmax/MPa | ɛ (%) | σ0.2 at 1200 °C/MPa | σ0.2 at 1600 °C/MPa | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WNbMoTa | 2010 | BCC | VAM | 13.75 | 4455 ± 185 Mpa | — | — | — | — | — | [18] |
| WNbMoTaV | 2010 | BCC | VAM | 12.36 | 5250 ± 281 Mpa | — | — | — | — | — | [18] |
| W25Nb25Mo25Ta25 | 2011 | BCC | VAM | — | — | 1058 | 1211 | 2.1 | 506 | 405 | [19] |
| W20Nb20Mo20Ta20V20 | 2011 | BCC | VAM | — | — | 1246 | 1270 | 1.7 | 735 | 477 | [19] |
| WMoCrTiAl | 2015 | BCC + BCC2 | VAM | — | 802 ± 10 | — | — | — | — | — | [29] |
| CrFeNiV0.5W0.25 | 2015 | FCC + σ | VAM | — | 869 | — | 1113 | 3.28 | — | — | [100] |
| CrFeNiV0.5W0.50 | 2015 | BCC + FCC + σ | VAM | — | — | — | 1306 | 4.49 | — | — | [100] |
| CrFeNiV0.5W0.75 | 2015 | BCC + FCC + σ | VAM | — | — | — | 1599 | 6 | — | — | [100] |
| CrFeNiV0.5W1.00 | 2015 | BCC + FCC + σ | VAM | — | 633 | — | 2240 | 7.28 | — | — | [100] |
| CrFeNi2V0.5W0.25 | 2015 | FCC + σ | VAM | — | 226 | — | — | >70 | — | — | [100] |
| CrFeNi2V0.5W0.50 | 2015 | FCC + σ | VAM | — | — | — | — | >70 | — | — | [100] |
| CrFeNi2V0.5W0.75 | 2015 | BCC + FCC + σ | VAM | — | — | — | — | >70 | — | — | [100] |
| CrFeNi2V0.5W1.00 | 2015 | BCC + FCC + σ | VAM | — | 305 | — | — | >70 | — | — | [100] |
| W0.5Ni2Co2VMo0.5 | 2015 | FCC + BCC + Laves | VAM | 9.86 | 376.1 | 1000 | — | — | — | — | [96] |
| W0.5Ni2Co2VCr0.5 | 2015 | FCC + Laves | VAM | 9.31 | 255.88 | 750 | — | — | — | — | [96] |
| W0.5Ni2Co2CrMo0.5 | 2015 | FCC + μ+σ | VAM | 9.88 | 306.8 | 250 | — | — | — | — | [96] |
| Cr0.5MoNbTaVW | 2015 | BCC1 | VAM | 11.5 | 675.5 ± 27.5 | — | — | — | — | — | [101] |
| CrMoNbTaVW | 2015 | BCC1 | VAM | 11.4 | 704.6 ± 17.4 | — | — | — | — | — | [101] |
| Cr2.0MoNbTaVW | 2015 | BCC1 + BCC2 | VAM | 11.2 | 754.9 ± 43.1 | — | — | — | — | — | [101] |
| MoNbTaTiVW | 2015 | BCC | VAM | 11.7 | 4954 ± 198 MPa | — | — | — | — | — | [102] |
| WNbMoTa | 2015 | BCC | MS | — | — | ~10 GPa | — | >30 | — | — | [60] |
| Co2Mo0.5Ni2VW0.5 | 2016 | FCC + μ | VAM | — | 376 | 925 | — | >50 | — | — | [103] |
| Co2Mo0.6Ni2VW0.6 | 2016 | FCC + μ | VAM | — | 560 | 1411 | 2108 | 11.8 | — | — | [103] |
| Co2Mo0.8Ni2VW0.8 | 2016 | FCC + μ | VAM | — | 577 | 1431 | 2364 | 14.4 | — | — | [103] |
| Co2Mo1.0Ni2VW1.0 | 2016 | BCC + FCC + μ | VAM | — | 510 | 1371 | 2208.6 | 16 | — | — | [103] |
| Co2Mo1.5Ni2VW1.5 | 2016 | BCC + FCC + μ | VAM | — | 583 | 1320 | 2133 | 13.9 | — | — | [103] |
| Co2Mo1.75Ni2VW1.75 | 2016 | BCC + FCC + μ | VAM | — | 667 | 1607 | 2313 | 9.4 | — | — | [103] |
| NbTaVW | 2016 | BCC | VAM | — | 4830 ± 29 MPa | 1530 | — | 12 | — | — | [104] |
| NbTaTiVW | 2016 | BCC | VAM | — | 5380 ± 34 MPa | 1420 | — | 20 | — | — | [104] |
| WNbMoTaTi | 2017 | BCC | VAM | — | 498.7 ± 8.3 | 1343 | 2005 | 14.1 | 586 | — | [71] |
| WNbMoTaVTi | 2017 | BCC | VAM | — | 510.3 ± 6.0 | 1515 | 2135 | 10.6 | 659 | — | [71] |
| W32Ta18Ti18V20Cr19 | 2017 | BCC | MA + SPS | 13.4 | 768 | 2265 | — | — | — | — | [53] |
| W42Ta15Ti14V14Cr14 | 2017 | BCC | MA + SPS | 13.6 | 793 | 2314 | — | — | — | — | [53] |
| W56Ta15Ti9V11Cr9 | 2017 | BCC | MA + SPS | 14.5 | 788 | 2144 | — | — | — | — | [53] |
| W63Ta9Ti19V9Cr9 | 2017 | BCC | MA + SPS | 14.9 | 767 | 2187 | — | — | — | — | [53] |
| W71Ta4Ti7V7Cr7 | 2017 | BCC + Laves | MA + SPS | 15.7 | 671 | 1473 | — | — | — | — | [53] |
| W77Ta5Ti7V5Cr6 | 2017 | BCC + Laves | MA + SPS | 16.5 | 517 | 1208 | — | — | — | — | [53] |
| W90Ta3Ti2V3Cr2 | 2017 | BCC + Laves | MA + SPS | 16.5 | 486 | 1206 | — | — | — | — | [53] |
| WNbMoTaV | 2017 | BCC | MA + SPS | — | — | 2612 | 3472 | 8.8 | — | — | [47] |
| AlCoCrFeNi2 * | 2017 | BCC + FCC | VAM | — | 293 ± 10.1 | 575.5 | 2583.8 | 42.7 | — | — | [92] |
| AlCoCrFeNi2W0.1 * | 2017 | BCC + FCC | VAM | — | 307.5 ± 7.5 | 560.8 | 2543 | 41.4 | — | — | [92] |
| AlCoCrFeNi2W0.2 * | 2017 | BCC + FCC | VAM | — | 325.8 ± 4.8 | 619.5 | 2785.9 | 42 | — | — | [92] |
| AlCoCrFeNi2W0.3 * | 2017 | BCC + FCC | VAM | — | 356.2 ± 12 | 651.5 | 2255.6 | 31 | — | — | [92] |
| NiFeCrMoW | 2017 | BCC + FCC | VAM | — | — | 1.3 GPa | — | — | — | — | [94] |
| Ni35Fe30Cr20Mo10W5 | 2017 | BCC + FCC | VAM | — | — | 0.4 GPa | — | — | — | — | [94] |
| W23Nb23Mo23Ta23Ti8 | 2018 | BCC | MA + SPS | — | 7.35 GPa | 2377 | 3340 | 26.3 | — | — | [55] |
| W25Nb25Mo25Ta25 | 2018 | BCC | MA + SPS | — | 7.78 GPa | 2460 | 3016 | 16.8 | — | — | [55] |
| MoFe1.5CrTiWAlNb3 | 2018 | BCC + FCC + HCP | LC | — | 910 | — | — | — | — | — | [89] |
| WMoVCrTa | 2018 | BCC | MA | — | — | — | — | — | — | — | [105] |
| VCrFeTa0.1W0.1 | 2018 | BCC1 | VAM | — | 564 | 1341 | 2917 | >42.2 | 1019 (800 °C) | 371 (1000 °C) | [106] |
| VCrFeTa0.2W0.2 | 2018 | BCC1 | VAM | — | 673 | 1742 | 3265 | >35.7 | 1033 (800 °C) | 182 (1000 °C) | [106] |
| VCrFeTa0.3W0.3 | 2018 | BCC1 + BCC2 | VAM | — | 726 | — | 701 | — | — | — | [106] |
| VCrFeTa0.4W0.4 | 2018 | BCC1 + BCC2 + Laves | VAM | — | 886 | 1580 | 1767 | — | — | — | [106] |
| VCrFeTaW | 2018 | BCC1 + BCC2 + Laves | VAM | — | 1135 | — | 1501 | — | — | — | [106] |
| WNbMoTaTi0.25 | 2018 | BCC | VAM | — | — | 1109 | 1197 | 2.5 | — | — | [76] |
| WNbMoTaTi0.5 | 2018 | BCC | VAM | — | — | 1211 | 1578 | 5.9 | — | — | [76] |
| WNbMoTaTi0.75 | 2018 | BCC | VAM | — | — | 1304 | 1593 | 8.4 | — | — | [76] |
| WNbMoTaTi | 2018 | BCC | VAM | — | — | 1455 | 1910 | 11.5 | — | — | [76] |
| CrMoNbTiVWZr | 2018 | BCC + HCP + Laves | VAM | — | 727 ± 18 | — | — | — | — | — | [88] |
| Ti0WTaVCr | 2018 | BCC + Laves | MA + SPS | — | 714 | 2327 | — | 1–2% | 979 | — | [79] |
| Ti4WTaVCr | 2018 | BCC + Laves | MA + SPS | — | — | — | — | 1–2% | — | — | [79] |
| Ti7WTaVCr | 2018 | BCC | MA + SPS | — | — | — | — | 1–2% | 586 | — | [79] |
| CoCrFeNi2Al | 2018 | BCC + FCC | VAM | — | 293 ± 10.1 | 575.5 | 2583.8 | 42.7 | — | — | [93] |
| CoCrFeNi2Al0.9W0.1 | 2018 | FCC + BCC | VAM | — | 288.8 ± 7.2 | 395 | — | >50 | — | — | [93] |
| CoCrFeNi2Al0.8W0.2 | 2018 | FCC + BCC | VAM | — | 270.4 ± 5.4 | 359.5 | — | >50 | — | — | [93] |
| CoCrFeNi2Al0.7W0.3 | 2018 | FCC + BCC | VAM | — | 265.5 ± 7.6 | 333.5 | — | >50 | — | — | [93] |
| Fe18Ni23Co25Cr21Mo8 WNb3C2 | 2018 | — | MA + HPS | — | — | 1452 | — | 3.9 | — | — | [107] |
| WNbMoTa | 2018 | BCC | SLM | — | 475.1 MPa | — | — | — | — | — | [67] |
| (FeCoCrNi)0.97(WC)0.03* | 2018 | FCC+ Carbide | MA + SPS | — | 603 | — | — | — | — | — | [108] |
| (FeCoCrNi)0.95(WC)0.05* | 2018 | FCC+ Carbide | MA + SPS | — | — | — | — | — | — | — | [108] |
| (FeCoCrNi)0.93(WC)0.07 * | 2018 | FCC+ Carbide | MA + SPS | — | — | — | — | — | — | — | [108] |
| (FeCoCrNi)0.91(WC)0.09 * | 2018 | FCC+ Carbide | MA + SPS | — | — | — | — | — | — | — | [108] |
| (FeCoCrNi)0.89(WC)0.11 * | 2018 | FCC+ Carbide | MA + SPS | — | 768 | — | — | — | — | — | [108] |
| ReMoTaW | 2018 | BCC + HCP + Ta-rich phase | VAM | — | 640 | 1451 | — | 5.69 | — | — | [109] |
| W21.6Nb19.4Mo20.3Ta19.5V19.2 | 2019 | BCC | MS | — | — | — | — | — | — | — | [35] |
| WNbMoTaSi0 | 2019 | BCC + Silicids | MA + SPS | 13.44 | 504.5 | 1217 | 1499 | 3.8 | — | — | [110] |
| WNbMoTaSi0.25 | 2019 | BCC + Silicids | MA + SPS | 12.92 | 567 | 1826 | 2548 | 10.5 | 926 | — | [110] |
| WNbMoTaSi0.5 | 2019 | BCC + Silicids | MA + SPS | 12.65 | 697 | 1883 | 2454 | 5.8 | — | — | [110] |
| WNbMoTaSi0.75 | 2019 | BCC + Silicids | MA + SPS | 12.23 | 682.6 | 2483 | 2732 | 1.6 | — | — | [110] |
| (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2 | 2019 | — | HEBM + SPS | — | 11.6 ± 0.5 GPa | — | — | — | — | — | [111] |
| V10.41Nb10.52Mo10.55Ta11.23W10.53Cr16.26B28.64 | 2019 | — | MS | — | 18.4 ± 0.5 GPa | — | — | — | — | — | [112] |
| CrMoVW | 2019 | BCC | — | — | — | — | — | — | — | — | [30] |
| CoCrNbNiW | 2019 | FCC + BCC | LMD | — | 515.4 | — | — | — | — | — | [31] |
| WNbMoTa | 2019 | BCC | MS | — | 12 GPa | — | — | — | — | — | [113] |
| NbNiTaTiW | 2019 | BCC + μ | VAM | — | 410 ± 6 | — | — | — | — | — | [114] |
| Nb18Ni18Ta18Ti18W18Al10 | 2019 | BCC + μ+L21 | VAM | — | 578 ± 17 | — | — | — | — | — | [114] |
| Al1.5CrFeNiTi0.5W0.5 | 2019 | BCC + Laves | LC | — | 848.34 | — | — | — | — | — | [32] |
| WNbMoTaVCr | 2019 | BCC + Laves | MA + SPS | 11.16 | 9908 MPa | 3416 | 3834 | 5.3 | — | — | [78] |
| CrMoNbWTi-C | 2019 | BCC + Laves + Carbide | MA + HPS | 9.654 | 8.26 GPa | — | 3094 | — | — | — | [115] |
| CoCrFeNiW0.2 * | 2019 | FCC | VAM | — | — | 335 | 1552 | 52.0 | — | — | [116] |
| CoCrFeNiW0.5 * | 2019 | FCC + μ | VAM | — | 357.9 | 556 | 1037 | 35.6 | — | — | [116] |
| HfNbTaTiZrW | 2019 | BCC1 + BCC2 | VAM | — | — | 1550 | 2322 | 26.3 | 345 | — | [86] |
| HfNbTaTiZrMoW | 2019 | BCC1 + BCC2 | VAM | — | — | 1637 | — | 15.5 | 703 | — | [86] |
| MoNbRe0.5W | 2019 | BCC | VAM | — | — | 896 ± 13 | 1232 ± 25 | 7.08 ± 0.33 | — | — | [117] |
| MoNbRe0.5W(TaC)0.2 | 2019 | BCC+ Carbide | VAM | — | — | 1074 ± 11 | 1700 ±13 | 8.33 ± 0.52 | — | — | [117] |
| MoNbRe0.5W(TaC)0.4 | 2019 | BCC+ Carbide | VAM | — | — | 1144 ± 33 | 1833 ± 34 | 8.81 ± 0.26 | — | — | [117] |
| MoNbRe0.5W(TaC)0.5 | 2019 | BCC+ Carbide | VAM | — | — | 1202 ± 15 | 2067 ± 46 | 10.25 ± 0.41 | — | — | [117] |
| MoNbRe0.5W(TaC)0.6 | 2019 | BCC + MC | VAM | — | — | 1241 ± 26 | 2351 ± 33 | 9.64 ± 0.50 | — | — | [117] |
| WMoNbCrTi | 2019 | BCC1 + BCC2 + Laves | MA + SPS | — | 10.40 Gpa | 2492 | 2765 | 9.8 | — | — | [118] |
| W0.16NbMoTa | 2019 | BCC | LCD | 10.572 | 476.0 ± 12.9 | — | 840 | — | — | — | [119] |
| W0.33NbMoTa | 2019 | BCC | LCD | 10.634 | 485.3 ± 8.7 | — | 895 | — | — | — | [119] |
| W0.53NbMoTa | 2019 | BCC | LCD | 11.044 | 497.6 ± 5.6 | — | 890 | — | — | — | [119] |
| WNbMoTaCr | 2020 | BCC | VAM | — | — | 1056 | 1104 | 4.6 | — | — | [120] |
| WNbMoTaZr | 2020 | BCC | VAM | — | — | 1480 | 1822 | 15.9 | — | — | [120] |
| WNbMoTaV | 2020 | BCC | VAM | — | — | 1460 | 1520 | 8.8 | — | — | [120] |
| WNbMoTaHf | 2020 | BCC | VAM | — | — | 1252 | 1252 | 5.7 | — | — | [120] |
| WNbMoTaRe | 2020 | BCC | VAM | — | — | 1062 | 1147 | 4.2 | — | — | [120] |
| CuMoTaWV | 2020 | BCC | MS | — | 19 ± 2.3 GPa | 10 ± 0.8 GPa | — | — | — | — | [121] |
| W0.5(TaTiVCr)0.5 | 2020 | BCC | MA + SPS | 14.1 | 788 | 2100 | — | — | 830 | 425 (1400 °C) | [33] |
| Mo15Nb20Re15Ta30W20 | 2020 | BCC | VAM | 15.64 | 6.45 GPa | — | — | — | — | — | [122] |
| AlCrFeMnNiW0.05 * | 2020 | BCC1 + BCC2 | VAM | — | 552.7 ± 22 | — | — | — | — | — | [123] |
| CrFeNi2V0.5W0.25 * | 2020 | FCC + σ | VAM | — | — | 278 | — | >70 | — | — | [124] |
| TiAlMoSiW * | 2020 | BCC + FCC | MA + SPS | 7.1994 | 802.01 | — | — | — | — | — | [125] |
| Ti0.25AlMoSi0.25W0.1 * | 2020 | BCC + FCC | MA + SPS | 6.2274 | 750.25 | — | — | — | — | — | [125] |
| Ti0.3AlMoSi0.3W0.1 * | 2020 | BCC + FCC | MA + SPS | 5.9812 | 764.63 | — | — | — | — | — | [125] |
| NiCrFeRuMoW | 2020 | — | — | — | — | — | — | — | — | — | [126] |
| (CoCrFeNi)0.96W0.04 * | 2020 | FCC | VAM | 9.0348 | 171.2 | — | — | — | — | — | [127] |
| WNbMoTa | 2020 | BCC | AM | — | — | — | — | — | — | — | [128] |
| W10Mo27Cr21Ti22Al20 | 2020 | BCC | VAM | 7.48 | 5110 MPa | 1245 | 1310 | 7.7 | 105 | — | [129] |
| MoNbVWTi | 2020 | BCC | VAM | — | 5 ± 0.1 GPa | 1289 ± 42 | — | — | — | — | [130] |
| NbMoVW | 2020 | BCC | VAM | — | 6 ± 0.2 GPa | 1243 ± 49 | — | — | — | — | [130] |
| WNbMoTa | 2020 | BCC + FCC | MA + SPS | 10.667 | 892.38 | — | — | — | — | — | [131] |
| CoCrFeNiW0.2 * | 2020 | FCC | VAM | — | — | 254.9 | 475.8 | 42.0 | — | — | [132] |
| CoCrFeNiW0.4 * | 2020 | FCC + μ | VAM | — | — | 315.6 | 690.7 | 33.1 | — | — | [132] |
| CoCrFeNiW0.6 * | 2020 | FCC + μ | VAM | — | — | — | — | — | — | — | [132] |
| (CuZrNiAlTiW)10Al90 * | 2020 | BCC | MA + SPS | 3.20 ± 0.03 | — | 258 ± 12 | 344 ± 2 | 7.23 | — | — | [133] |
| (CuZrNiAlTiW)20Al80 * | 2020 | BCC | MA + SPS | 3.72 ± 0.04 | — | — | 544 ± 2 | 6.57 | — | — | [133] |
| (CuZrNiAlTiW)30Al70 * | 2020 | BCC | MA + SPS | 4.26 ± 0.02 | — | — | 270 ± 2 | 3.09 | — | — | [133] |
| (Cr0.33Fe0.33V0.33)33(Ta0.5W0.5)67 | 2020 | BCC | MS | — | 20.96 GPa | — | — | — | — | — | [134] |
| WNbMoTaRe | 2020 | BCC | VAM | — | 8.1–10.5 Gpa | 1000 ± 50 | 1320 ± 50 | 1.7 ± 0.5 | — | — | [135] |
| (WMoNbTi)95Cr5 | 2020 | BCC +Laves | MA + SPS | 10.3 | 7.35 GPa | — | 808 | 3.4 | — | — | [136] |
| (WMoNbTi)90Cr10 | 2020 | BCC +Laves | MA + SPS | 10.17 | 8.02 GPa | — | 887 | 3.6 | — | — | [136] |
| (WMoNbTi)85Cr15 | 2020 | BCC +Laves | MA + SPS | 10.05 | 9.07 GPa | — | 959 | 4.6 | — | — | [136] |
| WMoNbTiCr | 2020 | BCC +Laves | MA + SPS | 9.92 | 9.73 GPa | — | 2116 | 5.1 | — | — | [136] |
| Mo15Nb20Re15Ta30W20 | 2020 | BCC | VAM | — | 6.451 ± 0.140 | — | — | — | — | — | [137] |
| C0.1Mo15Nb20Re15Ta30W20 | 2020 | BCC | VAM | — | 5.826 ± 0.104 | — | — | — | — | — | [137] |
| (Mo0.2Ta0.2Ni0.2Cr0.2W0.2)B | 2020 | — | VAM | 10.55 | 48.51 GPa | — | — | — | — | — | [138] |
| WNbMoTaRe0.5 | 2020 | BCC | VAM | — | 567 ± 9 | 1147 ± 10 | 1465 ± 18 | 7.01 ± 0.30 | — | — | [139] |
| WNbMoTaRe | 2020 | BCC | VAM | — | 536 ± 22 | 1062 ± 15 | 1147 ± 10 | 4.22 ± 0.18 | — | — | [139] |
| W0.5(TaTiVCr)0.5 | 2020 | BCC | MA + SPS | — | 14.9 GPa | — | — | — | — | — | [38] |
| VNbMoTaWAl | 2020 | BCC | MS | — | 18.1 GPa | — | — | — | — | — | [20] |
| MoNbW(TaC)0.2 | 2021 | BCC + FCC | VAM | — | 663 ± 11 | 911 ± 31 | 1655 ± 24 | 9.67 ± 0.34 | — | — | [21] |
| MoNbW(TaC)0.5 | 2021 | BCC + FCC | VAM | — | 760 ± 26 | 1033 ± 43 | 1803 ± 33 | 10.80 ± 0.56 | — | — | [21] |
| MoNbW(TaC)0.7 | 2021 | BCC + FCC | VAM | — | 772 ± 19 | 1367 ± 28 | 2091 ± 22 | 10.27 ± 0.29 | — | — | [21] |
| MoNbW(TaC)0.9 | 2021 | BCC + FCC | VAM | — | 816 ± 37 | 1458 ± 45 | 2779 ± 37 | 7.46 ± 0.43 | — | — | [21] |
| MoNbW(TaC)1.0 | 2021 | BCC + FCC | VAM | — | 831 ± 43 | 1519 ± 27 | 2520 ± 18 | 7.27 ± 0.38 | — | — | [21] |
| MoNbW(TaC)1.2 | 2021 | BCC + FCC | VAM | — | 849 ± 38 | 1638 ± 26 | 2459 ± 35 | 6.13 ± 0.41 | — | — | [21] |
| MoNbW(TaC)1.5 | 2021 | BCC + FCC | VAM | — | 1050 ± 8 | 1742 ± 33 | 2349 ± 41 | 3.40 ±0.35 | — | — | [21] |
| MoNbRe0. aW(TiC)0.2 | 2021 | BCC + FCC | VAM | — | — | 1238 | 1510 | — | — | — | [95] |
| MoNbRe0.5TaW(TiC)0.5 | 2021 | BCC + FCC | VAM | — | — | 1408 | 1734 | — | — | — | [95] |
| MoNbRe0.5TaW(TiC)1.0 | 2021 | BCC + FCC | VAM | — | — | 1438 | 1903 | — | — | — | [95] |
| MoNbRe0.5TaW(TiC)0.9 | 2021 | BCC + FCC | VAM | — | — | 1496 | 1943 | — | — | — | [95] |
| MoNbRe0.5TaW(TiC)1.5 | 2021 | BCC + FCC | VAM | — | — | 1543 | 1680 | — | — | — | [95] |
| W-CoCuFeNi * | 2021 | BCC + FCC | VAM | — | — | 599 ± 8 | 1897 ± 157 | 47 ± 3 | — | — | [140] |
| Wx(CoCrFeMnNi)100−x * | 2021 | BCC + FCC | AM | — | — | — | — | — | — | — | [141] |
| FeCoCrMnW0.2 * | 2021 | FCC + BCC + σ | VAM | — | 617 ± 21.25 | — | 1136 | 12.5 | — | — | [142] |
| FeCoCrMnW0.4 * | 2021 | FCC + BCC + σ | VAM | — | 674 ± 8.60 | — | 1186 | 11.1 | — | — | [142] |
| FeCoCrMnW0.6 * | 2021 | FCC + BCC + σ | VAM | — | 725 ± 14.14 | — | 1370 | 16.3 | — | — | [142] |
| FeCoCrMnW0.8 * | 2021 | FCC + BCC + σ | VAM | — | 774 ± 14.62 | — | 1554 | 16.5 | — | — | [142] |
| FeCoCrMnW * | 2021 | FCC + BCC | VAM | — | 836 ± 16.61 | — | 1771 | 17.1 | — | — | [142] |
| NbTaW0.5 | 2021 | BCC | VAM | — | — | 1005 | 2602 | >50 | 326 (1473k) | 324 (1673k) | [90] |
| NbTaW0.5(Mo2C)0.05 | 2021 | BCC + HCP | VAM | — | — | 1204 | 2722 | 41.5 | — | — | [90] |
| NbTaW0.5(Mo2C)0.1 | 2021 | BCC + HCP | VAM | — | — | 1378 | 2227 | 20.1 | 835 (1473k) | 574 (1673k) | [90] |
| NbTaW0.5(Mo2C)0.15 | 2021 | BCC + HCP | VAM | — | — | 1480 | 2068 | 16.1 | — | — | [90] |
| NbTaW0.5(Mo2C)0.2 | 2021 | BCC + HCP | VAM | — | — | 1615 | 2235 | 13.6 | 1026 (1473k) | 697 (1673k) | [90] |
| NbTaW0.5(Mo2C)0.25 | 2021 | BCC + HCP | VAM | — | — | 1600 | 2301 | 14.9 | — | — | [90] |
| WNbMoTaZr0.1 | 2021 | BCC1 + BCC2 | VAM | — | — | 1354 ± 54 | — | — | — | — | [85] |
| WNbMoTaZr0.5 | 2021 | BCC1 + BCC2 | VAM | — | — | 1461 ± 89 | — | — | — | — | [85] |
| WNbMoTaZr | 2021 | BCC1 + BCC2 | VAM | — | — | 1589 ± 79 | — | 15.8 ± 3.2 | 555 ± 47 (1000 °C) | — | [85] |
| WNbMoTaTi | 2022 | BCC | SEBM | — | 511 ± 2 | 1047 | 1312 | 8.9 | — | — | [70] |
| (TiVCr)84W16 | 2022 | BCC | MS | — | 7.2 GPa | — | — | — | — | — | [143] |
| WNbMoTaZr0.1 | 2022 | BCC | VAM | — | 516 ± 7.6 | 1223 ± 20.1 | 1421 ± 38.3 | 6.4 ± 0.66 | — | — | [74] |
| WNbMoTaZr0.3 | 2022 | BCC1 + BCC2 | VAM | — | 518 ± 10.4 | 1448 ± 31.7 | 1819 ± 68.4 | 9.9 ± 1.12 | — | — | [74] |
| WNbMoTaZr0.5 | 2022 | BCC1 + BCC2 | VAM | — | 546 ± 9.4 | 1575 ± 28.2 | 2107 ± 46.9 | 12.6 ± 0.68 | — | — | [74] |
| WNbMoTaZr1.0 | 2022 | BCC1 + BCC2 | VAM | — | 557 ± 12.6 | 1618 ± 14.2 | 2439 ± 34.9 | 20 ± 0.60 | — | — | [74] |
| WNbMoTaVZr0.1 | 2022 | BCC | VAM | — | 582 ± 6 | 1294 ± 16 | 1422 ± 26 | 1.99 ± 0.20 | — | — | [75] |
| WNbMoTaVZr0.25 | 2022 | BCC1 + BCC2 | VAM | — | 579 ± 9 | 1439 ± 25 | 1671 ± 37 | 3.79 ± 0.53 | — | — | [75] |
| WNbMoTaVZr0.5 | 2022 | BCC1 + BCC2 | VAM | — | 588 ± 11 | 1548 ± 39 | 1788 ± 37 | 3.85 ± 0.31 | — | — | [75] |
| WNbMoTaVZr0.75 | 2022 | BCC1 + BCC2 | VAM | — | 584 ± 12 | 1637 ± 8 | 1919 ± 28 | 2.63 ± 0.14 | — | — | [75] |
| WNbMoTaVZr1.0 | 2022 | BCC1 + BCC2 | VAM | — | 595 ± 12 | 1680 ± 34 | 1913 ± 70 | 2.10 ± 0.45 | — | — | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Qi, C.; Liu, J.; Zhang, J.; Wu, Y. Recent Advances in W-Containing Refractory High-Entropy Alloys—An Overview. Entropy 2022, 24, 1553. https://doi.org/10.3390/e24111553
Chen S, Qi C, Liu J, Zhang J, Wu Y. Recent Advances in W-Containing Refractory High-Entropy Alloys—An Overview. Entropy. 2022; 24(11):1553. https://doi.org/10.3390/e24111553
Chicago/Turabian StyleChen, Shunhua, Chen Qi, Jiaqin Liu, Jingsai Zhang, and Yucheng Wu. 2022. "Recent Advances in W-Containing Refractory High-Entropy Alloys—An Overview" Entropy 24, no. 11: 1553. https://doi.org/10.3390/e24111553





