Transfer-Learning-Based Approach for the Diagnosis of Lung Diseases from Chest X-ray Images
Abstract
:1. Introduction
- We built image classification models using pretrained networks;
- We preprocessed the data including data augmentation of the ChestX-ray8 dataset and dealt with the class imbalance problem;
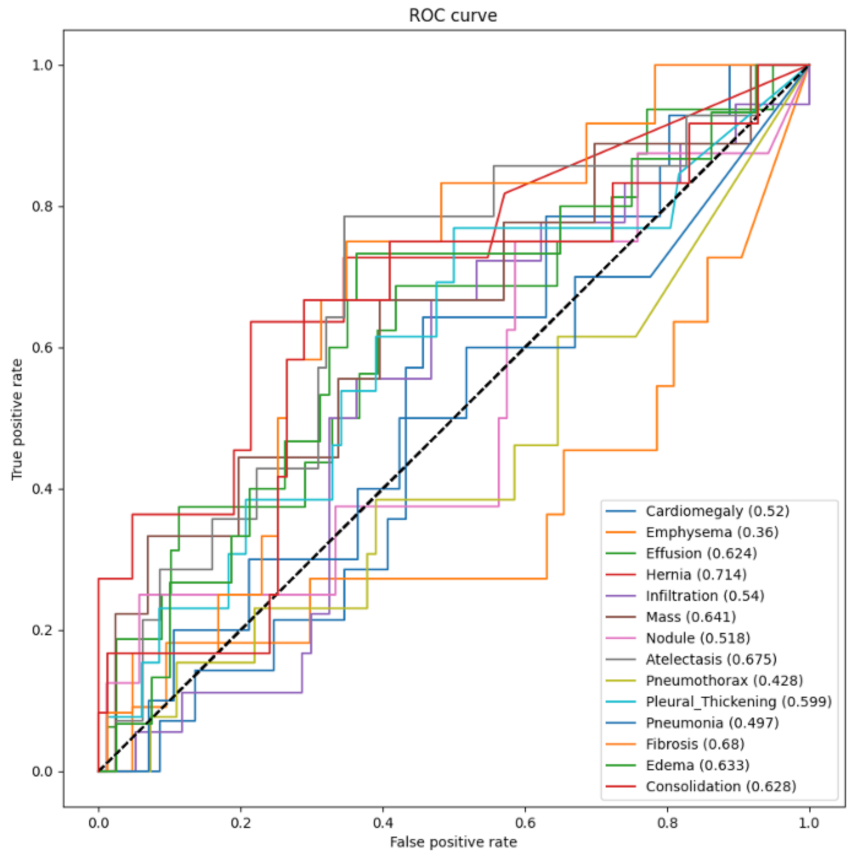
- We trained, validated, and tested the model using pretrained networks and compared the performance of each model using the ROC curves. We visualized the classification decision using Grad-CAM.
2. Proposed Transfer Learning Method
2.1. Transfer Learning with a Data Augmentation Approach
2.2. Evaluation Methods
2.3. Visualization Using Class Activation Maps
3. Simulation Results
3.1. Data Preprocessing
- Batch size. The batch size, the number of samples for one training, influences the optimization degree and speed. Since the network was trained on a GPU (2×Tesla V100)-equipped machine, matches the GPU’s performance;
- Resolution. The original images provided in [24] have a size of 1024 × 1024, which is relatively too big to be processed. With the help of a Python generator in Keras, the images were scaled to 400 × 400, the value of which was chosen to balance the accuracy and learning speed.
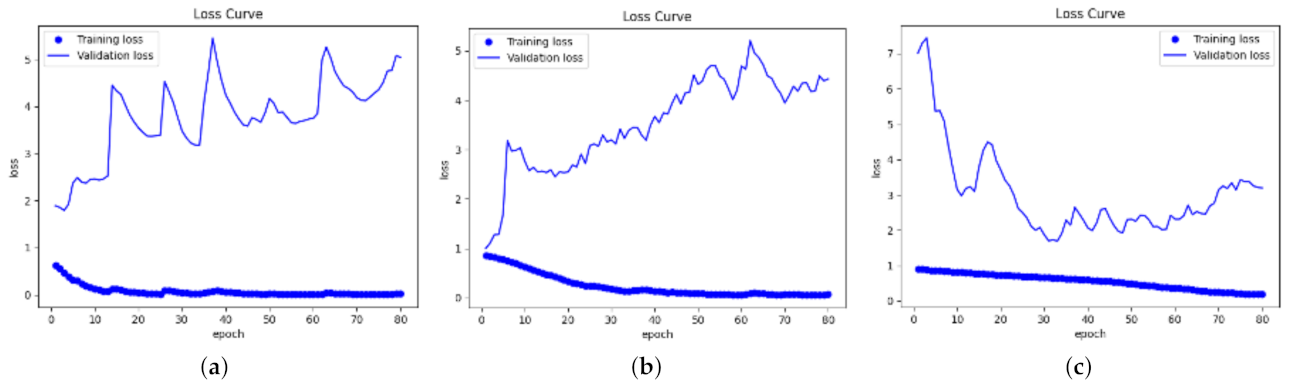
3.2. Training
- Steps per epoch means the number of steps for each epoch. Data in its batch size were the input from the generator to the network for each step. The relationship between this parameter and the batch size was (# steps per epoch) × batch size = # total training samples. Since the batch size was set to 16 because of the GPU performance and the total samples for training were 402, the steps per epoch should be 25;
- The value of the validation steps needs to be assigned, after the steps per epoch are determined. The validation steps were the total number of steps in the validation dataset. The validation steps should be two, since there were forty images for validation, and (# validation steps) × batch size = # total validation samples;
- The value of the epoch decides the total number of training samples. In each epoch, the network learns the features from all of the input images. In this work, the epoch was set to 80. The reason was that the plots with 80 epochs could clearly show the variation tendency of the accuracy and loss, and also, overfitting might occur if the network is trained for too many epochs. Early stopping was also used by stopping training if the accuracy did not increase for 10 epochs, which can help mitigate the overfitting problem to some extent.
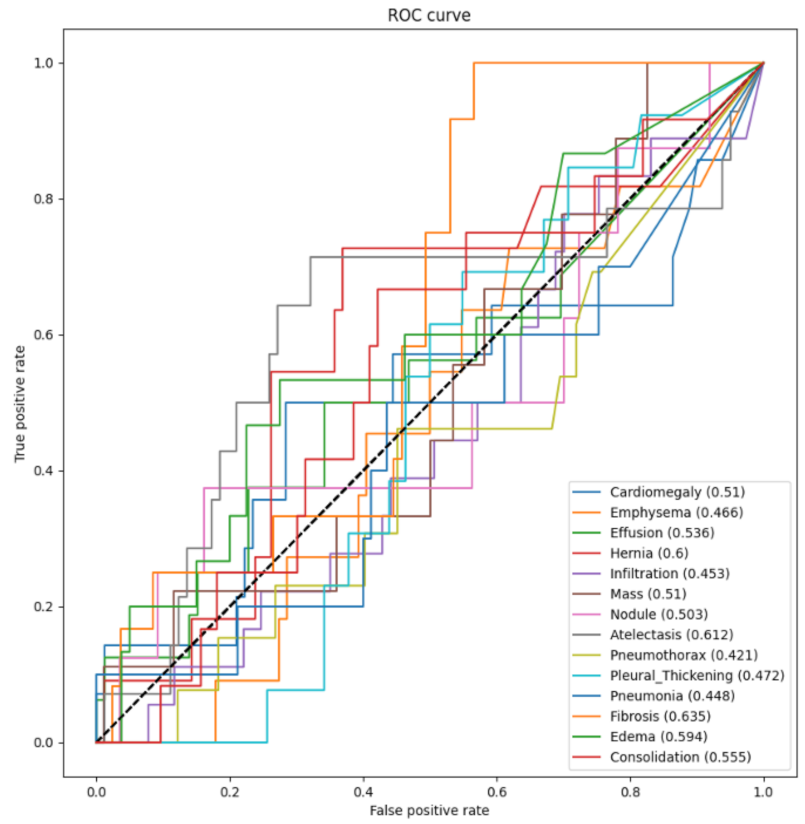
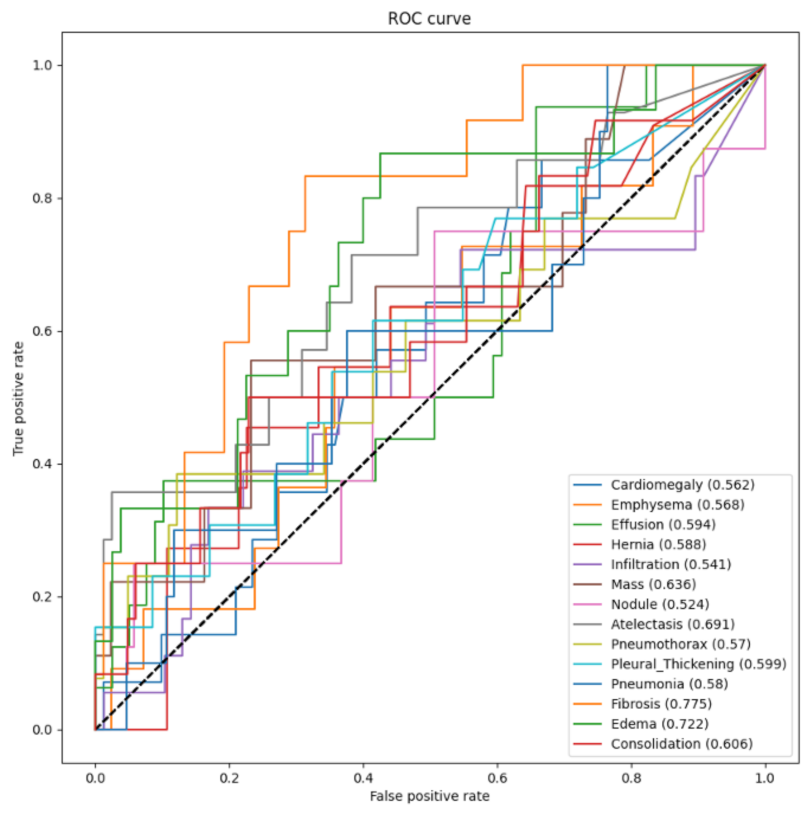
3.3. Testing and Evaluation
3.4. Visualization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fancourt, N.; Knoll, M.; Barger-Kamate, B.; de Campo, J.; Diallo, M.; Ebruke, B.E.; Feikin, D.; Gleeson, F.; Gong, W.; Hammitt, L.; et al. Standardized Interpretation of Chest Radiographs in Cases of Pediatric Pneumonia From the PERCH Study. Clin. Infect. Dis. 2017, 64, S253–S261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popper, H. Pathology of Lung Disease: Morphology—Pathogenesis—Etiology; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Liu, N.; Wan, L.; Zhang, Y.; Zhou, T.; Huo, H.; Fang, T. Exploiting Convolutional Neural Networks with Deeply Local Description for Remote Sensing Image Classification. IEEE Access 2018, 6, 11215–11228. [Google Scholar] [CrossRef]
- Yin, X.; Han, J.; Yang, J.; Yu, P.S. Efficient classification across multiple database relations: A CrossMine approach. IEEE Trans. Knowl. Data Eng. 2006, 18, 770–783. [Google Scholar] [CrossRef] [Green Version]
- Bhandary, A.; Prabhu, G.A.; Rajinikanth, V.; Thanaraj, K.P.; Satapathy, S.C.; Robbins, D.E.; Shasky, C.; Zhang, Y.; Tavares, J.M.; Raja, N.S.M. Deep-learning framework to detect lung abnormality—A study with chest X-Ray and lung CT scan images. Pattern Recognit. Lett. 2020, 129, 271–278. [Google Scholar] [CrossRef]
- Heidari, M.; Mirniaharikandehei, S.; Khuzani, A.Z.; Danala, G.; Qiu, Y.; Zheng, B. Improving the performance of CNN to predict the likelihood of COVID-19 using chest X-ray images with preprocessing algorithms. Int. J. Med. Inform. 2020, 144, 104284. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.L.; Ren, Y.; Feng, D.; Guo, Y.; Yang, H.; Li, X.; Fang, J.; Li, Q.; Ye, J.; Han, L.; et al. A promising approach for screening pulmonary hypertension based on frontal chest radiographs using deep learning: A retrospective study. PLoS ONE 2020, 15, e0236378. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, K.; Huang, L.; Zhao, B.; Zhang, X.; Guo, X.; Li, M.; Gu, Z.; Fu, G.; Hu, M.; et al. Detection of peripherally inserted central catheter (PICC) in chest X-ray images: A multi-task deep learning model. Comput. Methods Programs Biomed. 2020, 197, 105674. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Tiwari, P.; Kumar, S.; Gupta, D.; Khanna, A.; Rodrigues, J.J.P.C. Identifying pneumonia in chest X-rays: A deep learning approach. Meas. J. Int. Meas. Confed. 2019, 145, 511–518. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, X. Class Noise Handling for Effective Cost-Sensitive Learning by Cost-Guided Iterative Classification Filtering. IEEE Trans. Knowl. Data Eng. 2006, 18, 1435–1440. [Google Scholar]
- Pan, S.J.; Tsang, I.W.; Kwok, J.T.; Yang, Q. Domain Adaptation via Transfer Component Analysis. IEEE Trans. Neural Netw. 2011, 22, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minaee, S.; Kafieh, R.; Sonka, M.; Yazdani, S.; Soufi, G.J. Deep-COVID: Predicting COVID-19 from chest X-ray images using deep transfer learning. Med. Image Anal. 2020, 65, 101794. [Google Scholar] [CrossRef] [PubMed]
- Sufian, A.; Ghosh, A.; Sadiq, A.S.; Smarandache, F. A Survey on Deep Transfer Learning to Edge Computing for Mitigating the COVID-19 Pandemic. J. Syst. Archit. 2020, 108, 101830. [Google Scholar] [CrossRef]
- Ravishankar, H.; Sudhakar, P.; Venkataramani, R.; Thiruvenkadam, S.; Annangi, P.; Babu, N.; Vaidya, V. Understanding the Mechanisms of Deep Transfer Learning for Medical Images; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 188–196. [Google Scholar]
- Alzubaidi, L.; Fadhel, M.; Al-Shamma, O.; Zhang, J.; Santamaria, J.; Duan, Y.; Oleiwi, S. Towards a Better Understanding of Transfer Learning for Medical Imaging: A Case Study. Appl. Sci. 2020, 10, 4523. [Google Scholar] [CrossRef]
- Ahmed, S.E.; Amiri, S.; Doksum, K. Ensemble linear subspace analysis of high-dimensional data. Entropy 2021, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wong, A.; Kamel, M. Classification of imbalanced data: A review. Int. J. Pattern Recognit. Artif. Intell. 2009, 23, 687–719. [Google Scholar] [CrossRef]
- Somasundaram, A.; Reddy, S. Data Imbalance: Effects and Solutions for Classification of Large and Highly Imbalanced Data. In Proceedings of the 1st International Conference on Research in Engineering, Computers and Technology(ICRECT), Tiruchirappalli, India, 8–10 September 2016. [Google Scholar]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
- Chollet, F. Deep Learning with Python; Manning: Shelter Island, NY, USA, 2017. [Google Scholar]
- Brumback, L.C.; Pepe, M.S.; Alonzo, T.A. Using the ROC curve for gauging treatment effect in clinical trials. Stat. Med. 2006, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Int. J. Comput. Vis. 2020, 128, 336–359. [Google Scholar] [CrossRef] [Green Version]
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In Proceedings of the 32nd International Conference on International Conference on Machine Learning, Lille, France, 6–11 July 2015. [Google Scholar]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R.M. ChestX-ray8: Hospital-scale Chest X-ray Database and Benchmarks on Weakly-Supervised Classification and Localization of Common Thorax Diseases. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 3462–3471. [Google Scholar]



| Networks | Type of Data Processing | Training Accuracy |
|---|---|---|
| DenseNet121 | Without data augmentation | 0.89 |
| With data augmentation | 0.92 | |
| ResNet50 | With data augmentation | 0.84 |
| Networks | Type of Data Processing | Testing Accuracy |
|---|---|---|
| DenseNet121 | Without data augmentation | 0.82 |
| With data augmentation | 0.84 | |
| ResNet50 | With data augmentation | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Bu, S. Transfer-Learning-Based Approach for the Diagnosis of Lung Diseases from Chest X-ray Images. Entropy 2022, 24, 313. https://doi.org/10.3390/e24030313
Fan R, Bu S. Transfer-Learning-Based Approach for the Diagnosis of Lung Diseases from Chest X-ray Images. Entropy. 2022; 24(3):313. https://doi.org/10.3390/e24030313
Chicago/Turabian StyleFan, Rong, and Shengrong Bu. 2022. "Transfer-Learning-Based Approach for the Diagnosis of Lung Diseases from Chest X-ray Images" Entropy 24, no. 3: 313. https://doi.org/10.3390/e24030313
APA StyleFan, R., & Bu, S. (2022). Transfer-Learning-Based Approach for the Diagnosis of Lung Diseases from Chest X-ray Images. Entropy, 24(3), 313. https://doi.org/10.3390/e24030313







